Inflammatory Forms of Cardiomyocyte Cell Death in the Rat Model of Isoprenaline-Induced Takotsubo Syndrome
Abstract
:1. Introduction
2. Methods
2.1. Ovariectomy and Sham Operation
2.2. Induction of Takotsubo Syndrome
2.3. Echocardiographic Examinations, Blood Collection, and Tissue Harvesting
2.4. Study Groups
- TTSO—ovariectomized Sprague Dawley female rats with ISO-induced TTS (n = 10);
- TTSP—sham-operated Sprague Dawley female rats with ISO-induced TTS (n = 10);
- CO—control ovariectomized Sprague Dawley female rats injected with 0.9% NaCl (n = 8);
- CP—control sham-operated Sprague Dawley female rats injected with 0.9% NaCl (n = 8).
2.5. Blood Collection and Heart Harvesting
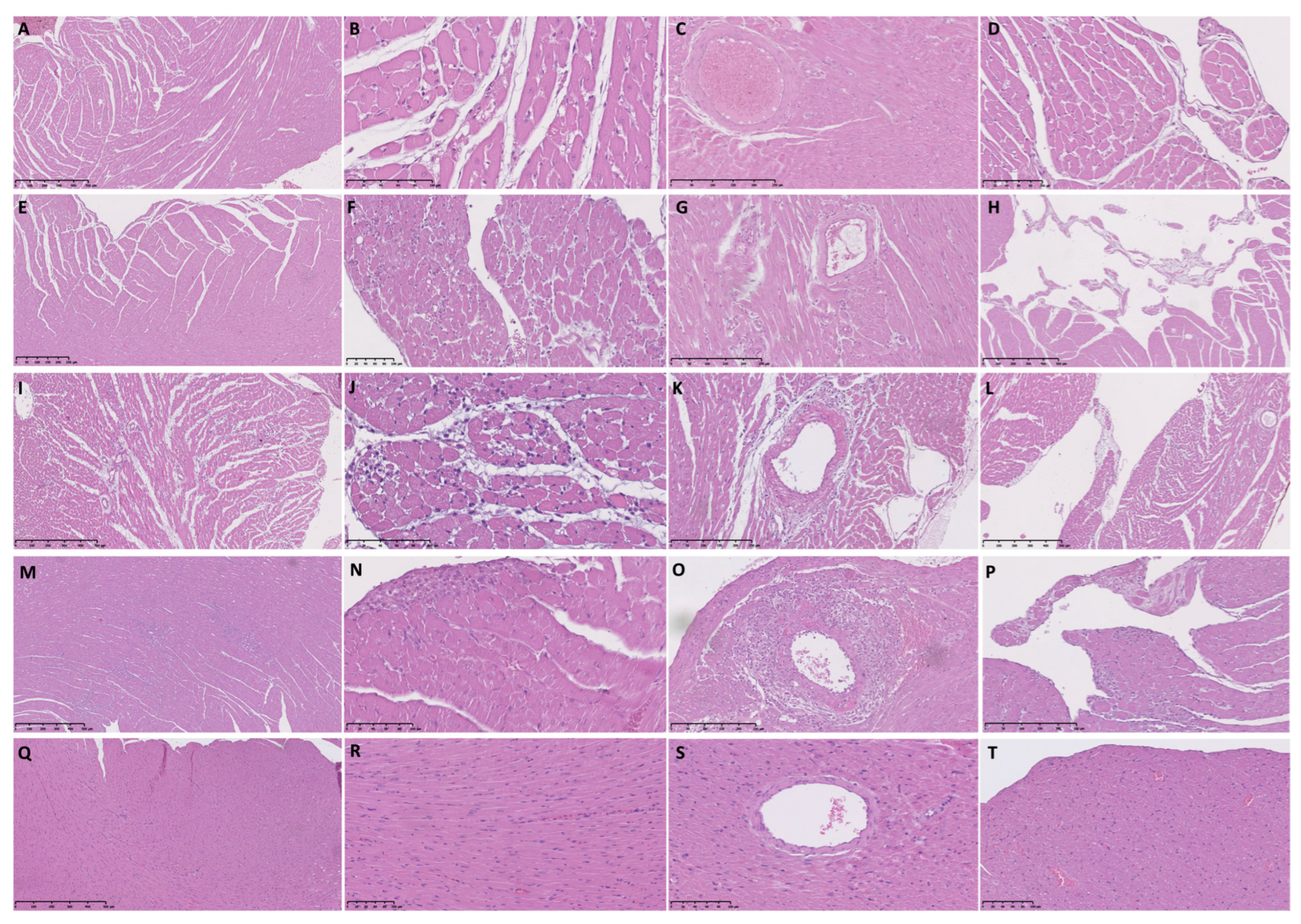
2.6. Histopathological Analysis
2.7. ELISA Analysis
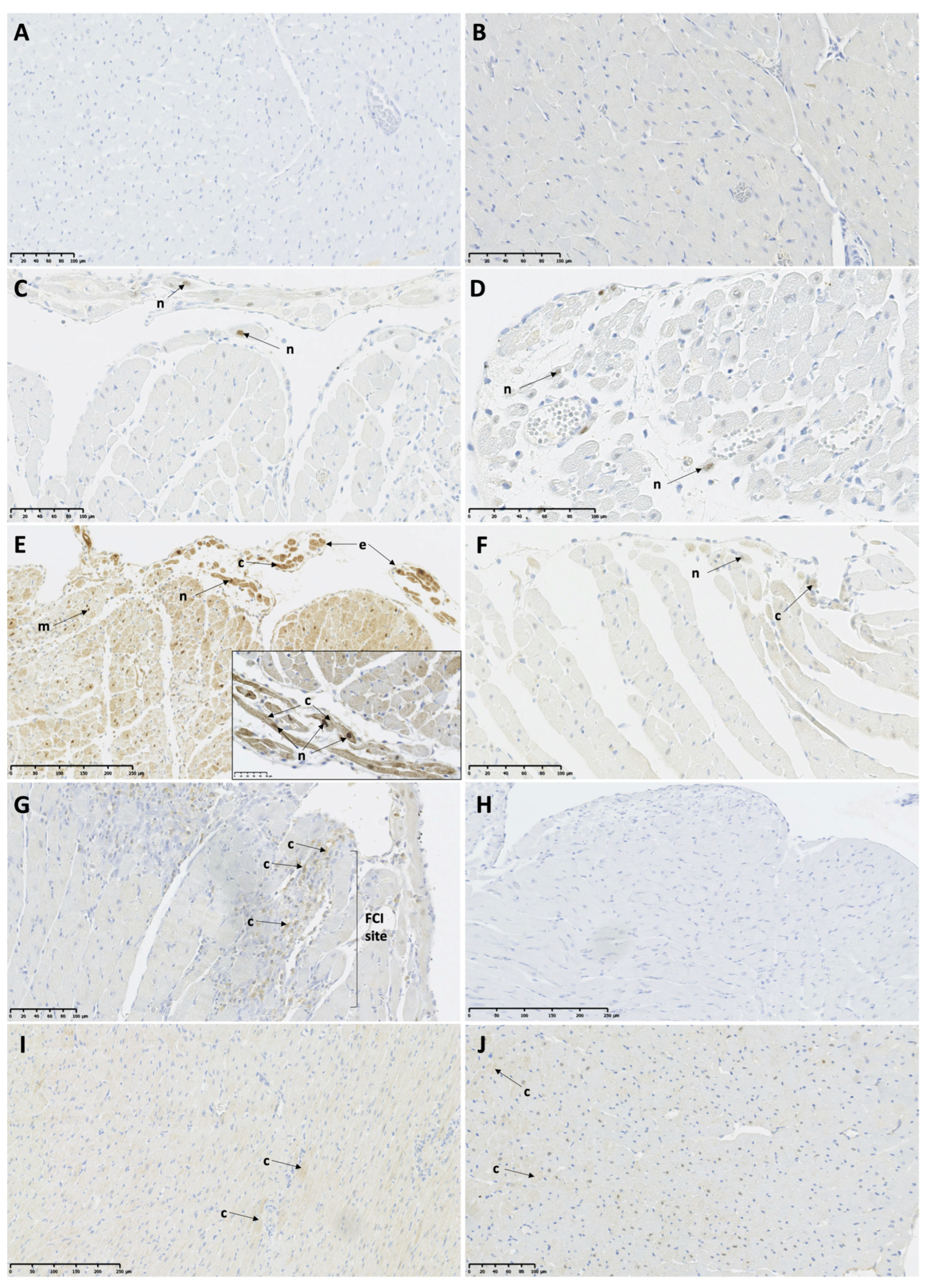
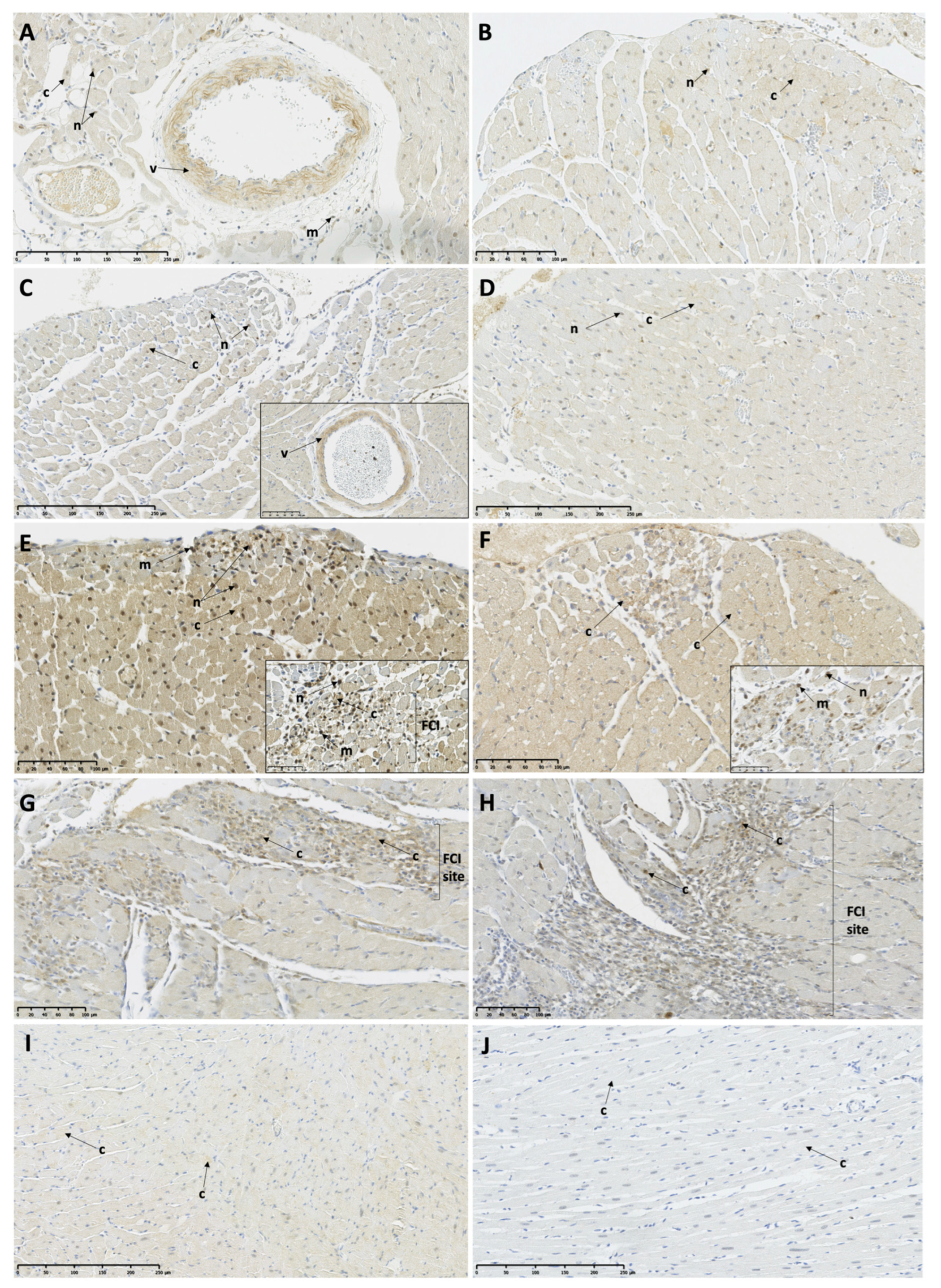
2.8. Immunohistochemistry
2.9. Statistical Analysis
3. Results
3.1. Echocardiographic Analysis
3.2. Histopathological Analysis of Female Rat Hearts
3.3. Plasma Concentrations of TnI and NT-proBNP in the TTSO and TTSP Rats
3.4. Plasma Concentrations of Necroptosis and Pyroptosis-Associated Proteins in the TTSO and TTSP Rats
3.5. Myocardial Expression of Necroptosis and Pyroptosis-Associated Proteins in the TTSO and TTSP Rats
3.5.1. Necroptosis-Associated Protein Expression
3.5.2. Pyroptosis-Associated Protein Expression
4. Discussion
5. Conclusions
6. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, P.K.; Adameova, A.; Hill, J.A.; Baines, C.P.; Kang, P.M.; Downey, J.M.; Narula, J.; Takahashi, M.; Abbate, A.; Piristine, H.C.; et al. Guidelines for evaluating myocardial cell death. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H891–H922. [Google Scholar] [CrossRef] [PubMed]
- Del Re, D.P.; Amgalan, D.; Linkermann, A.; Liu, Q.; Kitsis, R.N. Fundamental Mechanisms of Regulated Cell Death and Implications for Heart Disease. Physiol. Rev. 2019, 99, 1765–1817. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.E.; Price, D.R.; Ryter, S.W.; Choi, A.M.K. Necroptosis: A crucial pathogenic mediator of human disease. JCI Insight 2019, 4, e128834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Zhaolin, Z.; Guohua, L.; Shiyuan, W.; Zuo, W. Role of pyroptosis in cardiovascular disease. Cell Prolif. 2019, 52, e12563. [Google Scholar] [CrossRef] [Green Version]
- Borodzicz, S.; Czarzasta, K.; Opolski, G.; Cudnoch-Jędrzejewska, A. Autonomic nervous system in Takotsubo syndrome. Heart Fail. Rev. 2018, 24, 101–108. [Google Scholar] [CrossRef]
- Budnik, M.; Piatkowski, R.; Kochanowski, J.; Glowczynska, R.; Gorko, D.; Kowalik, R.; Pietrasik, A.; Opolski, G. The oldest patient with takotsubo cardiomyopathy. J. Geriatr. Cardiol. 2015, 12, 588–589. [Google Scholar] [CrossRef]
- Lyon, A.R.; Bossone, E.; Schneider, B.; Sechtem, U.; Citro, R.; Underwood, S.R.; Sheppard, M.N.; Figtree, G.A.; Parodi, G.; Akashi, Y.J.; et al. Current state of knowledge on Takotsubo syndrome: A Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 8–27. [Google Scholar] [CrossRef] [Green Version]
- Opolski, G.; Kochanowski, J.; Torbicki, A.; Scislo, P.; Kowalik, R.; Piotrowska-Kownacka, D.; Zarebinski, M.; Pruszczyk, P.; Kalarus, Z. The recurrence after ten years—“Mother in-law variant” of tako-tsubo syndrome. Kardiol. Pol. 2010, 68, 557–561. [Google Scholar]
- Deshmukh, A.; Kumar, G.; Pant, S.; Rihal, C.; Murugiah, K.; Mehta, J.L. Prevalence of Takotsubo cardiomyopathy in the United States. Am. Heart J. 2012, 164, 66–71.e1. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Zhou, C.; Chong, J.; Fu, L.; Zhang, L.; Sun, D.; Hou, H.; Zhang, Y.; Li, D.; Sun, H. Estrogen resisted stress-induced cardiomyopathy through increasing the activity of β2AR–Gαs signal pathway in female rats. Int. J. Cardiol. 2015, 187, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Kolodzinska, A.; Czarzasta, K.; Szczepankiewicz, B.; Glowczynska, R.; Fojt, A.; Ilczuk, T.; Budnik, M.; Krasuski, K.; Folta, M.; Cudnoch-Jedrzejewska, A.; et al. Toll-like receptor expression and apoptosis morphological patterns in female rat hearts with takotsubo syndrome induced by isoprenaline. Life Sci. 2018, 199, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Redfors, B.; Ali, A.; Shao, Y.; Lundgren, J.; Gan, L.-M.; Omerovic, E. Different catecholamines induce different patterns of takotsubo-like cardiac dysfunction in an apparently afterload dependent manner. Int. J. Cardiol. 2014, 174, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Redfors, B.; Stahlman, M.; Tang, M.S.; Miljanovic, A.; Mollmann, H.; Troidl, C.; Szardien, S.; Hamm, C.; Nef, H.; et al. A mouse model reveals an important role for catecholamine-induced lipotoxicity in the pathogenesis of stress-induced cardiomyopathy. Eur. J. Heart Fail. 2013, 15, 9–22. [Google Scholar] [CrossRef]
- Winogradow, J.; Geppert, G.; Reinhard, W.; Resch, M.; Radke, P.; Hengstenberg, C. Tako-tsubo cardiomyopathy after administration of intravenous epinephrine during an anaphylactic reaction. Int. J. Cardiol. 2011, 147, 309–311. [Google Scholar] [CrossRef]
- Ciutac, A.M.; Dawson, D. The role of inflammation in stress cardiomyopathy. Trends Cardiovasc. Med. 2021, 31, 225–230. [Google Scholar] [CrossRef]
- Scally, C.; Abbas, H.; Ahearn, T.; Srinivasan, J.; Mezincescu, A.; Rudd, A.; Spath, N.; Yucel-Finn, A.; Yuecel, R.; Oldroyd, K.; et al. Myocardial and Systemic Inflammation in Acute Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2019, 139, 1581–1592. [Google Scholar] [CrossRef]
- Scally, C.; Rudd, A.; Mezincescu, A.; Wilson, H.; Srivanasan, J.; Horgan, G.; Broadhurst, P.; Newby, D.E.; Henning, A.; Dawson, D.K. Persistent Long-Term Structural, Functional, and Metabolic Changes After Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2018, 137, 1039–1048. [Google Scholar] [CrossRef]
- Lachmet-Thebaud, L.; Marchandot, B.; Matsushita, K.; Sato, C.; Dagrenat, C.; Greciano, S.; De Poli, F.; Leddet, P.; Peillex, M.; Hess, S.; et al. Impact of residual inflammation on myocardial recovery and cardiovascular outcome in Takotsubo patients. ESC Heart Fail. 2021, 8, 259–269. [Google Scholar] [CrossRef]
- Sachdeva, J.; Dai, W.; Kloner, R.A. Functional and Histological Assessment of an Experimental Model of Takotsubo9s Cardiomyopathy. J. Am. Heart Assoc. 2014, 3, e000921. [Google Scholar] [CrossRef] [Green Version]
- Surikow, S.Y.; Nguyen, T.H.; Stafford, I.; Chapman, M.; Chacko, S.; Singh, K.; Licari, G.; Raman, B.; Kelly, D.J.; Zhang, Y.; et al. Nitrosative Stress as a Modulator of Inflammatory Change in a Model of Takotsubo Syndrome. JACC Basic Transl. Sci. 2018, 3, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Koebele, S.V.; Bimonte-Nelson, H.A. Modeling menopause: The utility of rodents in translational behavioral endocrinology research. Maturitas 2016, 87, 5–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nef, H.M.; Möllmann, H.; Hilpert, P.; Troidl, C.; Voss, S.; Rolf, A.; Behrens, C.B.; Weber, M.; Hamm, C.W.; Elsässer, A. Activated cell survival cascade protects cardiomyocytes from cell death in Tako-Tsubo cardiomyopathy. Eur. J. Heart Fail. 2009, 11, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Egami, H.; Uchida, Y.; Sakurai, T.; Kanai, M.; Shirai, S.; Nakagawa, O.; Oshima, T. Possible participation of endothelial cell apoptosis of coronary microvessels in the genesis of Takotsubo cardiomyopathy. Clin. Cardiol. 2010, 33, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Kolodzinska, A.; Czarzasta, K.; Szczepankiewicz, B.; Budnik, M.; Glowczynska, R.; Fojt, A.; Ilczuk, T.; Krasuski, K.; Borodzicz, S.; Cudnoch-Jedrzejewska, A.; et al. Isoprenaline induced Takotsubo syndrome: Histopathological analyses of female rat hearts. Cardiol. J. 2020, 29, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Omerovic, E.; Citro, R.; Bossone, E.; Redfors, B.; Backs, J.; Bruns, B.; Ciccarelli, M.; Couch, L.S.; Dawson, D.; Grassi, G.; et al. Pathophysiology of Takotsubo Syndrome—A joint scientific statement from the Heart Failure Association Takotsubo Syndrome Study Group and Myocardial Function Working Group of the European Society of Cardiology—Part 1: Overview and the central role for catecholamines and sympathetic nervous system. Eur. J. Heart Fail. 2021, 24, 257–273. [Google Scholar] [CrossRef]
- Matsushita, K.; Lachmet-Thébaud, L.; Marchandot, B.; Trimaille, A.; Sato, C.; Dagrenat, C.; Greciano, S.; De Poli, F.; Leddet, P.; Peillex, M.; et al. Incomplete Recovery From Takotsubo Syndrome Is a Major Determinant of Cardiovascular Mortality. Circ. J. 2021, 85, 1823–1831. [Google Scholar] [CrossRef]
- Wilson, H.M.; Cheyne, L.; Brown, P.A.J.; Kerr, K.; Hannah, A.; Srinivasan, J.; Duniak, N.; Horgan, G.; Dawson, D.K. Characterization of the Myocardial Inflammatory Response in Acute Stress-Induced (Takotsubo) Cardiomyopathy. JACC. Basic. Transl. Sci. 2018, 3, 766–778. [Google Scholar] [CrossRef]
- Tank, A.W.; Lee Wong, D. Peripheral and central effects of circulating catecholamines. Compr. Physiol. 2015, 5, 1–15. [Google Scholar] [CrossRef]
- Culling, W.; Penny, W.J.; Lewis, M.J.; Middleton, K.; Sheridan, D.J. Effects of myocardial catecholamine depletion on cellular electrophysiology and arrhythmias during ischaemia and reperfusion. Cardiovasc. Res. 1984, 18, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.; Zhang, S.; Fan, Y.; Tan, Y.; Guo, Z.; Chen, L.; Bai, L.; Jiang, D.; Hao, X.; Li, X.; et al. The Expression of microRNA in Adult Rat Heart with Isoproterenol-Induced Cardiac Hypertrophy. Cells 2020, 9, 1173. [Google Scholar] [CrossRef]
- Haines, D.D.; Juhasz, B.; Tosaki, A. Management of multicellular senescence and oxidative stress. J. Cell Mol. Med. 2013, 17, 936–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heather, L.C.; Catchpole, A.F.; Stuckey, D.J.; Cole, M.A.; Carr, C.A.; Clarke, K. Isoproterenol induces in vivo functional and metabolic abnormalities: Similar to those found in the infarcted rat heart. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2009, 60, 31–39. [Google Scholar]
- Li, H.; Shen, J.; Zhang, Y.; Hu, L.; Luo, W. 6-Shogaol protects against isoproterenol-induced cardiac injury in rats through attenutating oxidative stress, inflammation, apoptosis and activating nuclear respiratory factor-2/heme oxygenase-1 signaling pathway. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2022, 73, 737–744. [Google Scholar] [CrossRef]
- Li, L.; Ma, H.; Zhang, Y.; Jiang, H.; Xia, B.; Sberi, H.A.; Elhefny, M.A.; Lokman, M.S.; Kassab, R.B. Protocatechuic acid reverses myocardial infarction mediated by β-adrenergic agonist via regulation of Nrf2/HO-1 pathway, inflammatory, apoptotic, and fibrotic events. J. Biochem. Mol. Toxicol. 2023, 37, e23270. [Google Scholar] [CrossRef]
- Liaudet, L.; Calderari, B.; Pacher, P. Pathophysiological mechanisms of catecholamine and cocaine-mediated cardiotoxicity. Heart Fail. Rev. 2014, 19, 815–824. [Google Scholar] [CrossRef] [Green Version]
- Mikaelian, I.; Coluccio, D.; Morgan, K.T.; Johnson, T.; Ryan, A.L.; Rasmussen, E.; Nicklaus, R.; Kanwal, C.; Hilton, H.; Frank, K.; et al. Temporal gene expression profiling indicates early up-regulation of interleukin-6 in isoproterenol-induced myocardial necrosis in rat. Toxicol. Pathol. 2008, 36, 256–264. [Google Scholar] [CrossRef] [Green Version]
- Sethi, R.; Dhalla, N.S. Inotropic responses to isoproterenol in congestive heart failure subsequent to myocardial infarction in rats. J. Card. Fail. 1995, 1, 391–399. [Google Scholar] [CrossRef]
- Todd, G.L.; Baroldi, G.; Pieper, G.M.; Clayton, F.C.; Eliot, R.S. Experimental catecholamine-induced myocardial necrosis. II. Temporal development of isoproterenol-induced contraction band lesions correlated with ECG, hemodynamic and biochemical changes. J. Mol. Cell Cardiol. 1985, 17, 647–656. [Google Scholar] [CrossRef]
- Tosaki, A.; Woodward, B.; Yamamoto, F.; Hearse, D.J. Isoproterenol and the genesis of reperfusion-induced arrhythmias in isolated rat heart: Adrenoceptor or free radical-mediated mechanisms? J. Cardiovasc. Pharmacol. 1990, 15, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Cai, M.; Liu, J.; Wang, X. Catecholamine Surges Cause Cardiomyocyte Necroptosis via a RIPK1-RIPK3-Dependent Pathway in Mice. Front. Cardiovasc. Med. 2021, 8, 740839. [Google Scholar] [CrossRef]
- Sahu, B.D.; Anubolu, H.; Koneru, M.; Kumar, J.M.; Kuncha, M.; Rachamalla, S.S.; Sistla, R. Cardioprotective effect of embelin on isoproterenol-induced myocardial injury in rats: Possible involvement of mitochondrial dysfunction and apoptosis. Life Sci. 2014, 107, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Todd, G.L.; Baroldi, G.; Pieper, G.M.; Clayton, F.C.; Eliot, R.S. Experimental catecholamine-induced myocardial necrosis. I. Morphology, quantification and regional distribution of acute contraction band lesions. J. Mol. Cell Cardiol. 1985, 17, 317–338. [Google Scholar] [CrossRef]
- Van Vliet, P.D.; Burchell, H.B.; Titus, J.L. Focal myocarditis associated with pheochromocytoma. N. Engl. J. Med. 1966, 274, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Nichtova, Z.; Novotova, M.; Kralova, E.; Stankovicova, T. Morphological and functional characteristics of models of experimental myocardial injury induced by isoproterenol. Gen. Physiol. Biophys. 2012, 31, 141–151. [Google Scholar] [CrossRef]
- Bell, J.R.; Raaijmakers, A.J.; Curl, C.L.; Reichelt, M.E.; Harding, T.W.; Bei, A.; Ng, D.C.; Erickson, J.R.; Vila Petroff, M.; Harrap, S.B.; et al. Cardiac CaMKIIδ splice variants exhibit target signaling specificity and confer sex-selective arrhythmogenic actions in the ischemic-reperfused heart. Int. J. Cardiol. 2015, 181, 288–296. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, W.T.; Wu, S.; Wong, T.M. Oestrogen confers cardioprotection by suppressing Ca2+/calmodulin-dependent protein kinase II. Br. J. Pharmacol. 2009, 157, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Zhang, Y.; Cui, M.; Jin, L.; Wang, Y.; Lv, F.; Liu, Y.; Zheng, W.; Shang, H.; Zhang, J.; et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat. Med. 2016, 22, 175–182. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Wang, S.Q.; Chakir, K.; Yang, D.; Zhang, T.; Brown, J.H.; Devic, E.; Kobilka, B.K.; Cheng, H.; Xiao, R.P. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J. Clin. Investig. 2003, 111, 617–625. [Google Scholar] [CrossRef] [Green Version]
- Couchonnal, L.F.; Anderson, M.E. The role of calmodulin kinase II in myocardial physiology and disease. Physiology 2008, 23, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, H.; Li, H.; Wang, J.J.; Zhang, J.S.; Shen, J.; An, X.B.; Zhang, C.C.; Wu, J.M.; Song, Y.; Wang, X.Y.; et al. IL-18 cleavage triggers cardiac inflammation and fibrosis upon β-adrenergic insult. Eur. Heart J. 2018, 39, 60–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Wu, J.M.; Hu, G.M.; Li, M.Z.; Cong, W.W.; Feng, Y.N.; Wang, S.X.; Li, Z.J.; Xu, M.; Dong, E.D.; et al. Membrane nanotubes facilitate the propagation of inflammatory injury in the heart upon overactivation of the β-adrenergic receptor. Cell Death Dis. 2020, 11, 958. [Google Scholar] [CrossRef]
- Iacucci, I.; Carbone, I.; Cannavale, G.; Conti, B.; Iampieri, I.; Rosati, R.; Sardella, G.; Frustaci, A.; Fedele, F.; Catalano, C.; et al. Myocardial oedema as the sole marker of acute injury in Takotsubo cardiomyopathy: A cardiovascular magnetic resonance (CMR) study. Radiol. Med. 2013, 118, 1309–1323. [Google Scholar] [CrossRef]
- Elsokkari, I.; Cala, A.; Khan, S.; Hill, A. Takotsubo cardiomyopathy: Not always innocent or predictable: A unique post mortem insight. Int. J. Cardiol. 2013, 167, e46-8. [Google Scholar] [CrossRef]
- Sato, T.; Hagiwara, K.; Nishikido, A.; Miyamoto, S.; Komiyama, K.; Matsuno, H.; Hashida, H.; Kobayakawa, N.; Akiyama, O. Takotsubo (ampulla-shaped) cardiomyopathy associated with microscopic polyangiitis. Intern. Med. 2005, 44, 251–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akashi, Y. Reversible ventricular dysfunction takotsubo (ampulla-shaped) cardiomyopathy. Intern. Med. 2005, 44, 175–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abanador-Kamper, N.; Kamper, L.; Wolfertz, J.; Vorpahl, M.; Haage, P.; Seyfarth, M. Temporarily increased stroke rate after Takotsubo syndrome: Need for an anticoagulation? BMC Cardiovasc. Disord. 2018, 18, 117. [Google Scholar] [CrossRef] [Green Version]
- Heckle, M.R.; McCoy, C.W.; Akinseye, O.A.; Khouzam, R.N. Stress-induced thrombus: Prevalence of thromboembolic events and the role of anticoagulation in Takotsubo cardiomyopathy. Ann. Transl. Med. 2018, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Otani, Y.; Tokunaga, K.; Kawauchi, S.; Inoue, S.; Watanabe, K.; Kiriyama, H.; Sakane, K.; Maekawa, K.; Date, I.; Matsumoto, K. Cerebral Infarction Arising from Takotsubo Cardiomyopathy: Case Report and Literature Review. NMC Case Rep. J. 2016, 3, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Y-Hassan, S.; Shahgaldi, K. Thrombo-embolic renal infarction in a case of mid-ventricular takotsubo syndrome. Intern. Med. 2011, 50, 2175–2178. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.; Gamble, D.; Mezincescu, A.; Abbas, H.; Rudd, A.; Dawson, D. A systematic review of biomarkers in Takotsubo syndrome: A focus on better understanding the pathophysiology. Int. J. Cardiol. Heart Vasc. 2021, 34, 100795. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Redfors, B.; Täng, M.S.; Möllmann, H.; Troidl, C.; Szardien, S.; Hamm, C.; Nef, H.; Borén, J.; Omerovic, E. Novel rat model reveals important roles of β-adrenoreceptors in stress-induced cardiomyopathy. Int. J. Cardiol. 2013, 168, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Hammarsten, O.; Mair, J.; Möckel, M.; Lindahl, B.; Jaffe, A.S. Possible mechanisms behind cardiac troponin elevations. Biomarkers 2018, 23, 725–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, E.; Knapton, A.; Rosen, E.; Zhang, J.; Estis, J.; Agee, S.J.; Lu, Q.A.; Todd, J.A.; Lipshultz, S.E. Baseline serum cardiac troponin I concentrations in Sprague-Dawley, spontaneous hypertensive, Wistar, Wistar-Kyoto, and Fisher rats as determined with an ultrasensitive immunoassay. Toxicol. Pathol. 2011, 39, 653–663. [Google Scholar] [CrossRef] [PubMed]


| Analyzed Parameter | TTSO | CO | p-Value * | TTSP | CP | p-Value ** | p-Value *** |
|---|---|---|---|---|---|---|---|
| Left ventricular dimensions and systolic function | |||||||
| LVEDD (cm) | 0.65 ± 0.02 | 0.51 ± 0.04 | <0.01 | 0.58 ± 0.01 | 0.45 ± 0.03 | <0.01 | <0.01 |
| LVEDA (cm2) | 0.63 ± 0.01 | 0.52 ± 0.04 | <0.05 | 0.54 ± 0.02 | 0.52 ± 0.03 | NS | <0.01 |
| LVESA (cm2) | 0.17 ± 0.01 | 0.19 ± 0.02 | NS | 0.2 ± 0.02 | 0.16 ± 0.01 | NS | NS |
| FAC (%) | 72.23 ± 2.33 | 64.3 ± 1.54 | <0.05 | 63.94 ± 2.82 | 68.85 ± 2.33 | NS | <0.05 |
| FS (%) | 54.49 ± 3.07 | 48.59 ± 4.95 | NS | 51.2 ± 1.42 | 49.45 ± 2.18 | NS | NS |
| Plasma concentration of cardiac biomarkers | |||||||
| TnI (ng/L) | 120.01 ± 6.18 | 135.6 ± 10.59 | NS | 222.56 ± 15.03 | 178.26 ± 4.73 | NS | <0.001 |
| NT-proBNP (ng/L) | 1015.17 ± 27.75 | 894.19 ± 31.05 | NS | 173.42 ± 5.60 | 478.77 ± 30.53 | <0.001 | <0.001 |
| 6 h Post-ISO | 12 h Post-ISO | 24 h Post-ISO | 72 h Post-ISO | 24 h Post-0.9% NaCl | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TTSO | TTSP | TTSO | TTSP | TTSO | TTSP | TTSO | TTSP | CO | CP | |||||||||||
| LV | RV | LV | RV | LV | RV | LV | RV | LV | RV | LV | RV | LV | RV | LV | RV | LV | RV | LV | RV | |
| Number of FCI | 0–10 | numerous/ some/only a few (3–5) | 10–50 | 1–8 | 2–35 | 3–5 | 0–6 | 0–4 | 8–47 | 9–27 | 3–16 | 1–13 | 3–52 # | 2–11 # | 7–40 # | 1–3 # | – | – | – | – |
| Mean number of FCI | 6 ± 5 | – | 26 ± 19 | 5 ± 2 | 15 ± 18 | – | – | – | 22 ± 13 | 14 ± 6 | 8 ± 4 | 6 ± 4 | 24 ± 15 | 6 ± 3 | 20 ± 17 | 2 ± 1 | – | – | – | – |
| Cardiomyocyte vacuolization | + | + | + | + | + | – | + | – | – | – | + | – | – | – | – | – | -/+ | -/+ | -/+ | -/+ |
| Diffuse inflammatory infiltration | + | + | + | + | + | + | + | + | ++ | + | + | + | + | + | + | + | -/+ | -/+ | -/+ | -/+ |
| Interstitial edema | + | + | + | + | + | + | + | + | ++ | ++ | + | + | + | + | + | + | -/+ | -/+ | -/+ | -/+ |
| Cardiomyocyte edema | – | – | – | – | – | – | – | – | – | – | + | + | + | + | + | + | – | – | -/+ | -/+ |
| Endocardial edema | + | + | + | – | + | + | + | + | ++ | ++ | + | + | + | + | – | – | -/+ | -/+ | -/+ | -/+ |
| Endocardial protrusions | + | + | + | + | + | + | + | – | + | + | + | + | + | + | + | + | – | – | – | – |
| Perivascular edema | + | + | + | – | + | – | – | – | + | – | + | – | + | – | + | + | – | – | -/+ | -/+ |
| Analyzed Protein | TTSO | TTSP | CO | CP | p-Value |
|---|---|---|---|---|---|
| Necroptosis-related proteins | |||||
| CAMKIIδ (ng/mL) | 4 (3.88–5) | 19 (18.16–19) * | 2 (1.88–2) ### | 19 (18.87–19) | <0.001 |
| RIP3 (ng/mL) | 0.75 (0.59–0.88) | 0.99 (0.93–1.02) | 0.95 (0.9–1.3) | 1 (0.89–1.08) | NS |
| MLKL (ng/L) | 359 (323–393) | 744 (712–835) *** | 357 (332–385) | 585 (560–599) | <0.001 |
| Pyroptosis-related proteins | |||||
| Casp-1 (ng/mL) | 5.12 (4.93–5.38) | 2.59 (2.40–2.80) *** | 5.10 (5.00–5.21) # | 2.54 (2.40–2.74) | <0.001 |
| IL-1β (pg/L) | 2016 ± 108 | 2778 ± 215 ###,** | 1133 ± 197 ** | 1000 ± 63 | <0.001 |
| IL-18 (pg/mL) | 39 (36–46) | 44 (41–45) | 45 (44–47) | 47 (43–49) | NS |
| CAMKIIδ | MLKL | RIP3 | Casp-1 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TTSO | TTSP | CO | CP | TTSO | TTSP | CO | CP | TTSO | TTSP | CO | CP | TTSO | TTSP | CO | CP | |||
| 6 h post-ISO | FCI-no RM-no V-no | FCI-no RM-no V-no | FCI-absent RM-low V-low | FCI-absent RM-low V-low | FCI-no RM-low V-high | FCI-no RM-low V-low | FCI-absent RM-low V-no | FCI-absent RM-no V-no | FCI-no RM-no V-no | FCI-no RM-no V-no | FCI-absent RM-no V-no | FCI-absent RM-no V-no | FCI-low RM-low V-low | FCI-low RM-low V-no | FCI-absent RM-low V-no | FCI-absent RM-high V-high | ||
| 12 h post-ISO | FCI-low RM-low V-no | FCI-low RM-low V-no | FCI-no RM-low V-high | FCI-no RM-low V-low | FCI-no RM-no V-no | FCI-no RM-no V-no | FCI-low RM-low V-low | FCI-low RM-low V-low | ||||||||||
| 24 h post-ISO | FCI-high RM-high V-high | FCI-low RM-low V-no | FCI-high RM-high V-high | FCI-high RM-high V-high | FCI-no RM-no V-no | FCI-no RM-no V-no | FCI-high RM-high V-high | FCI-high RM-high V-low | ||||||||||
| 72 h post-ISO | FCI-low RM-low V-no | FCI-no RM-no V-no | FCI-high RM-low V-low | FCI-high RM-low V-low | FCI-no RM-no V-no | FCI-no RM-no V-no | FCI-low RM-low V-low | FCI-low RM-low V-low | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borodzicz-Jażdżyk, S.; Kołodzińska, A.; Czarzasta, K.; Wojciechowska, M.; Główczyńska, R.; Szczepankiewicz, B.; Puchalska, L.; Opolski, G.; Cudnoch-Jędrzejewska, A. Inflammatory Forms of Cardiomyocyte Cell Death in the Rat Model of Isoprenaline-Induced Takotsubo Syndrome. Biomedicines 2023, 11, 2060. https://doi.org/10.3390/biomedicines11072060
Borodzicz-Jażdżyk S, Kołodzińska A, Czarzasta K, Wojciechowska M, Główczyńska R, Szczepankiewicz B, Puchalska L, Opolski G, Cudnoch-Jędrzejewska A. Inflammatory Forms of Cardiomyocyte Cell Death in the Rat Model of Isoprenaline-Induced Takotsubo Syndrome. Biomedicines. 2023; 11(7):2060. https://doi.org/10.3390/biomedicines11072060
Chicago/Turabian StyleBorodzicz-Jażdżyk, Sonia, Agnieszka Kołodzińska, Katarzyna Czarzasta, Małgorzata Wojciechowska, Renata Główczyńska, Benedykt Szczepankiewicz, Liana Puchalska, Grzegorz Opolski, and Agnieszka Cudnoch-Jędrzejewska. 2023. "Inflammatory Forms of Cardiomyocyte Cell Death in the Rat Model of Isoprenaline-Induced Takotsubo Syndrome" Biomedicines 11, no. 7: 2060. https://doi.org/10.3390/biomedicines11072060





