Relationship between Diabetic Nephropathy and Development of Diabetic Macular Edema in Addition to Diabetic Retinopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standard Protocol Approvals, Registrations, and Patient Consents
2.2. Subjects and Examination
2.3. Data Processing and Analysis of Risk Factors for Development and Severity of DR
2.4. Analysis of Risk Factors for Developing DME
2.5. Data Availability
3. Results
3.1. Our Patients with Type 2 Diabetes
3.2. Kolmogorov-Smirnov Test
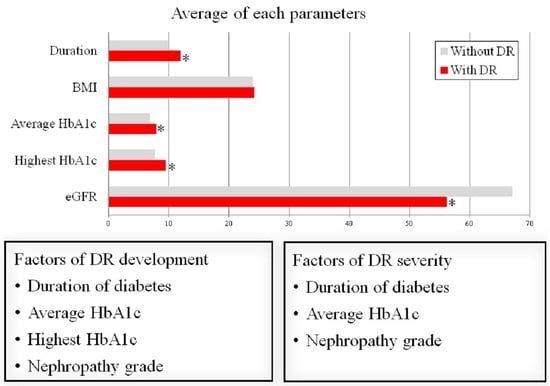
3.3. Risk Factors for the Development of DR
3.4. Risk Factors for Severity of DR
3.5. Risk Factors for the Development of DME
3.6. Risk Factors for Severity of DME
3.7. DME Prevalence by DR Grade
4. Discussion
4.1. Pathophysiology of DR
4.2. Pathophysiology of DN
4.3. Pathophysiology of DME
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas 2019, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; Available online: http://diabetesatlas.org (accessed on 12 April 2023).
- Jameson, J.L.; Fauci, A.S.; Kasper, D.L.; Hauser, S.L.; Longo, D.L.; Loscalzo, J. Harrison’s Principles of Internal Medicine, 20th ed.; McGraw Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Lozano, M.; Salinas, P. Diabetic retinopathy. Nutr. Hosp. 2013, 28 (Suppl. S2), 53–56. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Gubitosi-Klug, R.A.; Channa, R.; Wolf, R.M. Pediatric Diabetic Retinopathy: Updates in Prevalence, Risk Factors, Screening, and Management. Curr. Diabetes Rep. 2021, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Jeong, J.S.; Kim, M.K.; Kwon, H.S.; Baek, K.H.; Ko, S.H.; Ahn, Y.B. Discordance in risk factors for the progression of diabetic retinopathy and diabetic nephropathy in patients with type 2 diabetes mellitus. J. Diabetes Investig. 2019, 10, 745–752. [Google Scholar] [CrossRef]
- Lu, J.; Ma, X.; Zhang, L.; Mo, Y.; Ying, L.; Lu, W.; Zhu, W.; Bao, Y.; Zhou, J. Glycemic variability assessed by continuous glucose monitoring and the risk of diabetic retinopathy in latent autoimmune diabetes of the adult and type 2 diabetes. J. Diabetes Investig. 2019, 10, 753–759. [Google Scholar] [CrossRef]
- Fardeau, C.; Champion, E.; Massamba, N.; LeHoang, P. Uveitic macular edema. Eye 2016, 30, 1277–1292. [Google Scholar] [CrossRef]
- Iijima, H. Mechanisms of vision loss in eyes with macular edema associated with retinal vein occlusion. Jpn. J. Ophthalmol. 2018, 62, 265–273. [Google Scholar] [CrossRef]
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015, 2, 17. [Google Scholar] [CrossRef]
- Coscas, G.; Cunha-Vaz, J.; Soubrane, G. Macular edema: Definition and basic concepts. Dev. Ophthalmol. 2017, 58, 1–10. [Google Scholar] [CrossRef]
- Ding, J.; Wong, T.Y. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr. Diabetes Rep. 2012, 12, 346–354. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 2. Ophthalmology 1987, 94, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Browning, D.J.; Stewart, M.W.; Lee, C. Diabetic macular edema: Evidence-based management. Indian J. Ophthalmol. 2018, 66, 1736–1750. [Google Scholar] [CrossRef] [PubMed]
- Nagib, A.M.; Matter, Y.E.; Gheith, O.A.; Refaie, A.F.; Othman, N.F.; Al-Otaibi, T. Diabetic Nephropathy Following Posttransplant Diabetes Mellitus. Exp. Clin. Transplant. 2019, 17, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Deckert, T.; Feldt-Rasmussen, B.; Borch-Johnsen, K.; Jensen, T.; Kofoed-Enevoldsen, A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989, 32, 219–226. [Google Scholar] [CrossRef]
- Ochodnicky, P.; Henning, R.; van Dokkum, R.P.E.; de Zeeuw, D. Microalbuminuria and endothelial dysfunction: Emerging targets for primary prevention of end-organ damage. J. Cardiovasc. Pharmacol. 2006, 47 (Suppl. S2), S151–S162, discussion S72–S76. [Google Scholar] [CrossRef]
- Jeng, C.J.; Hsieh, Y.T.; Yang, C.M.; Yang, C.H.; Lin, C.L.; Wang, I.J. Diabetic Retinopathy in Patients with Diabetic Nephropathy: Development and Progression. PLoS ONE 2016, 11, e0161897. [Google Scholar] [CrossRef]
- Guo, M.F.; Dai, Y.J.; Gao, J.R.; Chen, P.J. Uncovering the Mechanism of Astragalus membranaceus in the Treatment of Diabetic Nephropathy Based on Network Pharmacology. J. Diabetes Res. 2020, 2020, 5947304. [Google Scholar] [CrossRef]
- Miyamoto, K.; Khosrof, S.; Bursell, S.-E.; Moromizato, Y.; Aiello, L.P.; Ogura, Y.; Adamis, A.P. Vascular endothelial growth factor (VEGF)-induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1). Am. J. Pathol. 2000, 156, 1733–1739. [Google Scholar] [CrossRef]
- Li, W.; Cheng, Z.; Song, Y.; Fang, Y.; Yang, M.; Zhang, M. Is diabetic retinopathy affected by diabetes type? A retrospective study using electronic medical record data from patients with latent autoimmune diabetes in adults, type 1 diabetes, and type 2 diabetes. Acta Diabetol. 2021, 58, 1503–1511. [Google Scholar] [CrossRef]
- Matuszewski, W.; Baranowska-Jurkun, A.; Stefanowicz-Rutkowska, M.M.; Modzelewski, R.; Pieczyński, J.; Bandurska-Stankiewicz, E. Prevalence of Diabetic Retinopathy in Type 1 and Type 2 Diabetes Mellitus Patients in North-East Poland. Medicina 2020, 56, 164. [Google Scholar] [CrossRef]
- Fukuda, M. Classification and treatment of diabetic retinopathy. Diabetes Res. Clin. Pract. 1994, 24, S171–S176. [Google Scholar] [CrossRef] [PubMed]
- De Faria, J.M.L.; Jalkh, A.E.; Trempe, C.L.; Mcmeel, J.W. Diabetic macular oedema: Risk factors and concomitants. Acta Ophthalmol. Scand. 1999, 77, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.K.; Chou, Y.B.; Lin, T.C.; Yang, H.Y.; Kao, Z.K.; Kao, C.L.; Yang, Y.P.; Chen, S.J.; Hsu, C.C.; Jheng, Y.C. Optical coherence tomography–based diabetic macula edema screening with artificial intelligence. J. Chin. Med. Assoc. 2020, 83, 1034–1038. [Google Scholar] [CrossRef]
- Wada, T.; Haneda, M.; Furuichi, K.; Babazono, T.; Yokoyama, H.; Iseki, K.; Araki, S.I.; Ninomiya, T.; Hara, S.; Suzuki, Y.; et al. The Research Group of Diabetic Nephropathy, Ministry of Health, Labour, and Welfare of Japan. Clinical impact of albuminuria and glomerular filtration rate on renal and cardiovascular events, and all-cause mortality in Japanese patients with type 2 diabetes. Clin. Exp. Nephrol. 2014, 18, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Bek, T. Diameter changes of retinal vessels in diabetic retinopathy. Curr. Diabetes Rep. 2017, 17, 82. [Google Scholar] [CrossRef]
- Ejaz, S.; Chekarova, I.; Ejaz, A.; Sohail, A.; Lim, C.W. Importance of pericytes and mechanisms of pericyte loss during diabetes retinopathy. Diabetes Obes. Metab. 2008, 10, 53–63. [Google Scholar] [CrossRef]
- Beltramo, E.; Porta, M. Pericyte loss in diabetic retinopathy: Mechanisms and consequences. Curr. Med. Chem. 2013, 20, 3218–3225. [Google Scholar] [CrossRef]
- Huang, H.; He, J.; Johnson, D.; Wei, Y.; Liu, Y.; Wang, S.; Lutty, G.A.; Duh, E.J.; Semba, R.D. Deletion of placental growth factor prevents diabetic retinopathy and is associated with Akt activation and HIF1alpha-VEGF pathway inhibition. Diabetes 2015, 64, 200–212. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Barber, A.J.; Hollinger, L.A.; Wolpert, E.B.; Gardner, T.W. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J. Biol. Chem. 1999, 274, 23463–23467. [Google Scholar] [CrossRef]
- Rousseau, S.; Houle, F.; Landry, J.; Huot, J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene 1997, 15, 2169–2177. [Google Scholar] [CrossRef]
- Adamis, A.P.; Miller, J.W.; Bernal, M.T.; D’Amico, D.J.; Folkman, J.; Yeo, T.K.; Yeo, K.T. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am. J. Ophthalmol. 1994, 118, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, A.; Kawai, K.; Motohashi, S.; Saito, K.; Kodama, S.; Yachi, Y.; Hirasawa, R.; Shimano, H.; Yamazaki, K.; Sone, H. HbA (1c) variability and the development of microalbuminuria in T2DM: Tsukuba Kawai Diabetes Registry 2. Diabetologia 2012, 55, 2128–2131. [Google Scholar] [CrossRef] [PubMed]
- Ihnat, M.A.; Thorpe, J.E.; Ceriello, A. Hypothesis: The ‘metabolic memory’, the new challenge of diabetes. Diabet. Med. 2007, 24, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.P.; Colette, C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with T2DM. J. Am. Med. Assoc. 2006, 295, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Russell, N.D.; Cooper, M.E. 50 years forward: Mechanisms of hyperglycaemia driven diabetic complications. Diabetologia 2015, 58, 1708–1714. [Google Scholar] [CrossRef]
- Ceriello, A. The emerging challenge in diabetes: The “metabolic memory”. Vasc. Pharmacol. 2012, 57, 133–138. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Abbas, S.N.; Odenbach, S. Reversal of hyperglycemia and diabetic nephropathy: Effect of reinstitution of good metabolic control on oxidative stress in the kidney of diabetic rats. J. Diabetes Complicat. 2004, 18, 282–288. [Google Scholar] [CrossRef]
- Ceriello, A.; Esposito, K.; Ihnat, M.; Thorpe, J.; Giugliano, D. Long-term glycemic control influences the long-lasting effect of hyperglycemia on endothelial function in type 1 diabetes. J. Clin. Endocrinol. Metab. 2009, 94, 2751–2756. [Google Scholar] [CrossRef]
- Quagliaro, L.; Piconi, L.; Assaloni, R.; Martinelli, L.; Motz, E.; Ceriero, A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: The role of protein kinase C and NAD(P)H-oxidase activation. Diabetes 2003, 52, 2795–2804. [Google Scholar] [CrossRef]
- Horvath, E.M.; Benko, R.; Kiss, L.; Murányi, M.; Pék, T.; Fekete, K.; Bárány, T.; Somlai, A.; Csordás, A.; Szabo, C. Rapid ‘glycaemic swings’ induce nitrosative stress, activate poly(ADP-ribose) polymerase and impair endothelial function in a rat model of diabetes mellitus. Diabetologia 2009, 52, 952–961. [Google Scholar] [CrossRef]
- Wang, X.N.; Cai, X.; Li, T.T.; Long, D.; Wu, Q. Peripapillary vessel density and retinal nerve fiber layer thickness changes in early diabetes retinopathy. Int. J. Ophthalmol. 2022, 15, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Yang, D.; Yu, H.; Xie, J.; Zeng, Y.; Wang, J.; Zhang, L. Optic nerve head perfusion changes preceding peripapillary retinal nerve fibre layer thinning in preclinical diabetic retinopathy. Clin. Exp. Ophthalmol. 2019, 47, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Wat, N.; Wong, R.L.; Wong, I.Y. Associations between diabetic retinopathy and systemic risk factors. Hong Kong Med. J. 2016, 22, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Lee, H.B. Reactive oxygen species amplify glucose signaling in renal cells cultured under high glucose and in diabetic nephropathy. Nephrology 2005, 10, S7–S10. [Google Scholar] [CrossRef]
- Lee, H.B.; Yu, M.I.-R.A.; Yang, Y.; Jiang, Z.; Ha, H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J. Am. Soc. Nephrol. 2003, 14 (Suppl. S3), S241–S245. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E.; Oldfield, M.D.; Thomas, M.C. Role of advanced glycation end products in diabetic nephropathy. J. Am. Soc. Nephrol. 2003, 14 (Suppl. S3), S254–S258. [Google Scholar] [CrossRef]
- Thallas-Bonke, V.; Lindschau, C.; Rizkalla, B.; Bach, L.A.; Boner, G.; Meier, M.; Haller, H.; Cooper, M.E.; Forbes, J.M. Attenuation of extracellular matrix accumulation in diabetic nephropathy by advanced glycation end product cross-link breaker ALT-711 via a PKC-α-dependent pathway. Diabetes 2004, 53, 2921–2930. [Google Scholar] [CrossRef]
- Cao, Z.; Cooper, M.E. Pathogenesis of diabetic nephropathy. J. Diabetes Investig. 2011, 2, 243–247. [Google Scholar] [CrossRef]
- Funatsu, H.; Yamashita, H.; Nakanishi, Y.; Hori, S. Angiotensin II and vascular endothelial growth factor in the vitreous fluid of patients with proliferative diabetic retinopathy. Br. J. Ophthalmol. 2002, 86, 311–315. [Google Scholar] [CrossRef]
- Tapp, R.J.; Shaw, J.E.; Zimmet, P.Z.; Balkau, B.; Chadban, S.J.; Tonkin, A.M.; Welborn, T.A.; Atkins, R.C. Albuminuria is evident in the early stages of diabetes onset: Results from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Am. J. Kidney Dis. 2004, 44, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, J.; Eliasson, B.; Nilsson, P.M.; Weiss, L.; Gudbjörnsdottir, S. Steering Committee of the Swedish National Diabetes Register. Microalbuminuria and risk factors in type 1 and type 2 diabetic patients. Diabetes Res. Clin. Pract. 2005, 67, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Gambaro, G.; Bax, G.; Fusaro, M.; Normanno, M.; Manani, S.M.; Zanella, M.; Dangelo, A.; Fedele, D.; Favaro, S. Cigarette smoking is a risk factor for nephropathy and its progression in type 2 diabetes mellitus. Diabetes Nutr. Metab. 2001, 14, 337–342. [Google Scholar] [PubMed]
- De Boer, I.H.; Rue, T.C.; Cleary, P.A.; Lachin, J.M.; Molitch, M.E.; Steffes, M.W.; Sun, W.; Zinman, B.; Brunzell, J.D.; White, N.H.; et al. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: An analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch. Intern. Med. 2011, 171, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Chang, Y.H.; Yang, S.Y.; Wu, K.D.; Chu, T.S. Update of pathophysiology and management of diabetic kidney disease. J. Formos. Med. Assoc. 2018, 117, 662–675. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.H.; Elwali, E.S.; Awadalla, H.; Almobarak, A.O. The relationship between diabetic retinopathy and nephropathy in Sudanese adult with diabetes: Population based study. Diabetes Metab. Syndr. 2017, 11 (Suppl. S1), S333–S336. [Google Scholar] [CrossRef]
- Saini, D.C.; Kochar, A.; Poonia, R. Clinical correlation of diabetic retinopathy with nephropathy and neuropathy. Indian J. Ophthalmol. 2021, 69, 3364. [Google Scholar] [CrossRef]
- Kotlarsky, P.; Bolotin, A.; Dorfman, K.; Knyazer, B.; Lifshitz, T.; Levy, J. Link between retinopathy and nephropathy caused by complications of diabetes mellitus type 2. Int. Ophthalmol. 2015, 35, 59–66. [Google Scholar] [CrossRef]
- Acan, D.; Calan, M.; Er, D.; Arkan, T.; Kocak, N.; Bayraktar, F.; Kaynak, S. The prevalence and systemic risk factors of diabetic macular edema: A cross-sectional study from Turkey. BMC Ophthalmol. 2018, 18, 91. [Google Scholar] [CrossRef]
- Koo, N.K.; Jin, H.C.; Kim, K.S.; Kim, Y.C. Relationship between the morphology of diabetic macular edema and renal dysfunction in diabetes. Korean J. Ophthalmol. 2013, 27, 98–102. [Google Scholar] [CrossRef]
- Temkar, S.; Karuppaiah, N.; Takkar, B.; Bhowmik, D.; Tripathi, M.; Ramakrishnan, S.; Sharma, Y.R.; Vohra, R.; Chawla, R.; Venkatesh, P. Impact of estimated glomerular filtration rate on diabetic macular edema. Int. Ophthalmol. 2018, 38, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Takamura, Y.; Matsumura, T.; Ohkoshi, K.; Takei, T.; Ishikawa, K.; Shimura, M.; Ueda, T.; Sugimoto, M.; Hirano, T.; Takayama, K.; et al. Functional and anatomical changes in diabetic macular edema after hemodialysis initiation: One-year follow-up multicenter study. Sci. Rep. 2020, 10, 7788. [Google Scholar] [CrossRef] [PubMed]
- Theodossiadis, P.G.; Theodoropoulou, S.; Neamonitou, G.; Grigoropoulos, V.; Liarakos, V.; Triantou, E.; Theodossiadis, G.P.; Vlahakos, D.V. Hemodialysis-induced alterations in macular thickness measured by optical coherence tomography in diabetic patients with end-stage renal disease. Ophthalmologica 2012, 227, 90–94. [Google Scholar] [CrossRef]
- Tatsumi, T.; Oshitari, T.; Takatsuna, Y.; Ishibashi, R.; Koshizaka, M.; Shiko, Y.; Baba, T.; Yokote, K.; Yamamoto, S. Sodium-Glucose Co-Transporter 2 Inhibitors Reduce Macular Edema in Patients with Diabetes mellitus. Life 2022, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Wakisaka, M.; Nagao, T. Sodium glucose cotransporter 2 in mesangial cells and retinal pericytes and its implications for diabetic nephropathy and retinopathy. Glycobiology 2017, 27, 691–695. [Google Scholar] [CrossRef]
- Querques, G.; Bandello, F.; Souied, E.H. Abnormal deep retinal capillary networking and microaneurysms in the outer nuclear layer of diabetic eyes. Ophthalmology 2014, 121, 803–840. [Google Scholar] [CrossRef]
- Browning, D.J. (Ed.) Diabetic macular edema. In Diabetic Retinopathy, Evidence-Based Management, 1st ed.; Springer Inc.: New York, NY, USA, 2010. [Google Scholar]
- Nagaoka, T.; Kitaya, N.; Sugawara, R.; Yokota, H.; Mori, F.; Hikichi, T.; Fujio, N.; Yoshida, A. Alteration of choroidal circulation in the foveal region in patients with type 2 diabetes. Br. J. Ophthalmol. 2004, 88, 1060–1063. [Google Scholar] [CrossRef]
- Stefánsson, E. Ocular oxygenation and the treatment of diabetic retinopathy. Surv. Ophthalmol. 2006, 51, 364–380. [Google Scholar] [CrossRef]
- Lund-Andersen, H. Mechanisms for monitoring changes in retinal status following therapeutic intervention in diabetic retinopathy. Surv. Ophthalmol. 2002, 47 (Suppl. S2), S270–S277. [Google Scholar] [CrossRef]
- Dvorak, H.F.; Brown, L.F.; Detmar, M.; Dvorak, A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995, 146, 1029–1039. [Google Scholar]
- Murata, T.; Nakagawa, K.; Khalil, A.; Ishibashi, T.; Inomata, H.; Sueishi, K. The relation between expression of vascular endothelial growth factor and breakdown of the blood-retinal barrier in diabetic rat retinas. Lab. Investig. 1996, 74, 819–825. [Google Scholar] [PubMed]
- Funatsu, H.; Yamashita, H.; Ikeda, T.; Mimura, T.; Eguchi, S.; Hori, S. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology 2003, 110, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Funatsu, H.; Yamashita, H.; Noma, H.; Mimura, T.; Yamashita, T.; Hori, S. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am. J. Ophthalmol. 2002, 133, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, T.A.; Amador, A.G.; Zinman, B. Diabetic retinopathy and diabetic macularoedema: Pathophysiology, screening, and novel therapies. Diabetes Care 2003, 26, 2653–2664. [Google Scholar] [CrossRef]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef]
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef]
- Cheloni, R.; Gandolfi, S.A.; Signorelli, C.; Odone, A. Global prevalence of diabetic retinopathy: Protocol for a systematic review and meta-analysis. BMJ Open 2019, 9, e022188. [Google Scholar] [CrossRef]
- Song, P.; Yu, J.; Chan, K.Y.; Theodoratou, E.; Rudan, I. Prevalence, risk factors and burden of diabetic retinopathy in China: A systematic review and meta-analysis. J. Glob. Health 2018, 8, 010803. [Google Scholar] [CrossRef]
- Brar, A.S.; Sahoo, J.; Behera, U.C.; Jonas, J.B.; Sivaprasad, S.; Das, T. Prevalence of diabetic retinopathy in urban and rural India: A systematic review and meta-analysis. Indian J. Ophthalmol. 2022, 70, 1945. [Google Scholar] [CrossRef]
- Heiran, A.; Azarchehry, S.P.; Dehghankhalili, S.; Afarid, M.; Shaabani, S.; Mirahmadizadeh, A. Prevalence of diabetic retinopathy in the Eastern Mediterranean Region: A systematic review and meta-analysis. J. Int. Med. Res. 2022, 50, 3000605221117134. [Google Scholar] [CrossRef]

| With DR | Without DR | p Value | |
|---|---|---|---|
| Male:Female | 70:57 | 73:61 | 0.9 |
| Average year | 68.9 ± 10.8 | 71.2 ± 9.4 | 0.8 |
| Diabetes duration | 12.0 ± 2.7 | 9.8 ± 2.0 | <0.0000001 |
| DME | 64 (50.4%) | 0 (0%) | <0.0000001 |
| Body mass index | 24.2 ± 4.8 | 24.0 ± 4.5 | 0.9 |
| Hypertension | 61 (48.0%) | 51 (38.1%) | 0.1 |
| Average HbA1c | 7.9 ± 1.3 | 6.9 ± 0.7 | <0.0000001 |
| Highest HbA1c | 9.5 ± 2.0 | 7.7 ± 1.3 | <0.0000001 |
| Serum LDL | 117.3 ± 34.8 | 115.9 ± 31.7 | 0.4 |
| Serum triglyceride | 151.6 ± 137.3 | 139.8 ± 69.3 | 0.4 |
| eGFR | 56.2 ± 26.4 | 67.1 ± 17.0 | 0.00008 |
| DN stage | 2.4 ± 1.2 | 1.4 ± 0.6 | <0.0000001 |
| Ischemic heart disease | 9 (7.1%) | 5 (3.7%) | 0.2 |
| RAS inhibitors | 22 (17.3%) | 38 (28.4%) | 0.04 |
| SGLT-2 inhibitors | 20 (15.7%) | 23 (17.2%) | 0.8 |
| Factor | Odds Ratio | 95% CI | p Value |
|---|---|---|---|
| Gender (male = 1, female = 0) | 2.77 | 0.43, 17.9 | 0.3 |
| Diabetes duration | 1.65 | 1.14, 2.39 | 0.009 |
| Body mass index | 1.08 | 0.87, 1.32 | 0.5 |
| Hypertension | 0.42 | 0.09, 2.03 | 0.3 |
| Average HbA1c | 5.60 | 1.36, 23.1 | 0.02 |
| Highest HbA1c | 2.46 | 1.12, 5.38 | 0.02 |
| Serum LDL | 1.02 | 0.99, 1.04 | 0.2 |
| Serum triglyceride | 0.99 | 0.98, 1.00 | 0.07 |
| DN stage | 7.62 | 2.63, 22.1 | 0.0002 |
| History of ischemic heart disease | 0.55 | 0.03, 8.82 | 0.7 |
| RAS inhibitors prescription | 1.27 | 0.26, 6.18 | 0.8 |
| SGLT-2 inhibitors prescription | 1.57 | 0.30, 8.15 | 0.6 |
| Factor | Odds Ratio | 95% CI | p Value |
|---|---|---|---|
| eGFR | 0.98 | 0.97, 0.99 | 0.0001 |
| Albuminuria | 5.23 | 3.14, 8.71 | <0.00001 |
| History of ischemic heart disease | 0.55 | 0.03, 8.82 | 0.7 |
| RAS inhibitors prescription | 0.77 | 0.42, 1.40 | 0.4 |
| SGLT-2 inhibitors prescription | 1.55 | 0.79, 3.03 | 0.2 |
| Factor | t-Statistic | 95% CI | p Value |
|---|---|---|---|
| Gender (male = 1, female = 0) | –1.35 | –0.85, 0.16 | 0.4 |
| Diabetes duration | 4.97 | 0.15, 0.36 | <0.00001 |
| Body mass index | –0.32 | –0.08, 0.05 | 0.8 |
| Hypertension | –1.99 | –1.01, 0.005 | 0.06 |
| Average HbA1c | 3.25 | 0.14, 1.02 | 0.002 |
| Highest HbA1c | –0.87 | –0.43, 0.17 | 0.4 |
| Serum LDL | 1.60 | –0.002, 0.02 | 0.1 |
| Serum triglyceride | –0.26 | –0.002, 0.002 | 0.8 |
| DN stage | 4.32 | 0.33, 0.89 | 0.00004 |
| History of ischemic heart disease | 1.30 | –0.35, 1.68 | 0.2 |
| RAS inhibitors prescription | 0.71 | –0.39, 0.82 | 0.5 |
| SGLT-2 inhibitors prescription | 0.66 | –0.45, 0.89 | 0.5 |
| Factor | t-Statistic | 95% CI | p Value |
|---|---|---|---|
| eGFR | –4.96 | –0.04, –0.02 | <0.00001 |
| Albuminuria | 0.19 | 1.24, 1.99 | <0.00001 |
| History of ischemic heart disease | 1.67 | –0.16, 1.91 | 0.1 |
| RAS inhibitors prescription | 0.27 | –0.68, 0.39 | 0.6 |
| SGLT-2 inhibitors prescription | 1.55 | 0.79, 3.03 | 0.5 |
| Factor | Odds Ratio | 95% CI | p Value |
|---|---|---|---|
| Gender (male = 1, female = 0) | 0.63 | 0.15, 2.61 | 0.5 |
| Duration of diabetes | 1.33 | 1.01, 1.75 | 0.04 |
| Body mass index | 0.95 | 0.79, 1.15 | 0.6 |
| Hypertension | 0.52 | 0.13, 2.04 | 0.3 |
| Average HbA1c | 5.52 | 1.27, 24.1 | 0.02 |
| Highest HbA1c | 0.75 | 0.35, 1.64 | 0.5 |
| Serum LDL | 1.01 | 0.93, 1.03 | 0.6 |
| Serum triglyceride | 1.00 | 0.99, 1.00 | 0.1 |
| DN stage | 2.80 | 1.37, 5.72 | 0.005 |
| History of ischemic heart disease | 2.63 | 0.27, 25.6 | 0.4 |
| RAS inhibitors prescription | 1.70 | –0.27, 0.35 | 0.2 |
| SGLT-2 inhibitors prescription | –0.12 | –0.47, 0.22 | 0.2 |
| Factor | Odds Ratio | 95% CI | p Value |
|---|---|---|---|
| eGFR | 0.98 | 0.97, 0.99 | 0.009 |
| Albuminuria | 4.05 | 2.30, 7.11 | <0.00001 |
| RAS inhibitors prescription | 0.76 | 0.36, 1.59 | 0.1 |
| SGLT-2 inhibitors prescription | 1.54 | 0.70, 3.37 | 0.3 |
| Factor | t-Statistic | 95% CI | p Value |
|---|---|---|---|
| Gender (male = 1, female = 0) | –0.53 | –0.37, 0.21 | 0.6 |
| Diabetes duration | 2.57 | 0.02, 0.13 | 0.001 |
| Body mass index | –0.21 | –0.04, 0.03 | 0.8 |
| Hypertension | –1.07 | –0.44, 0.13 | 0.3 |
| Average HbA1c | 2.54 | 0.08, 0.68 | 0.01 |
| Highest HbA1c | –0.64 | –0.22, 0.11 | 0.5 |
| Serum LDL | 0.95 | –0.005, 0.005 | 0.07 |
| Serum triglyceride | –1.92 | –0.002, 0.0004 | 0.06 |
| DN stage | 2.48 | 0.04, 0.36 | 0.002 |
| History of ischemic heart disease | –0.08 | –0.56, 0.52 | 0.9 |
| RAS inhibitors prescription | 0.25 | –0.27, 0.35 | 0.8 |
| SGLT-2 inhibitors prescription | –0.70 | –0.47, 0.22 | 0.5 |
| Factor | t-Statistic | 95% CI | p Value |
|---|---|---|---|
| eGFR | –2.44 | –0.009, –0.001 | 0.02 |
| Albuminuria | 5.36 | 0.26, 0.55 | <0.00001 |
| RAS inhibitors prescription | –0.86 | –0.30, 0.12 | 0.4 |
| SGLT-2 inhibitors prescription | 0.30 | –0.19, 0.26 | 0.8 |
| Fukuda Classification | Patients (Male/Female) | DME (Male/Female) | Prevalence |
|---|---|---|---|
| A0 | 134 (73/61) | 0 (0/0) | 0% |
| A1 | 3 (3/0) | 0 (0/0) | 0% |
| A2 | 49 (26/23) | 16 (8/8) | 32.7% |
| B1 | 34 (21/13) | 20 (10/10) | 58.8% |
| B2 | 7 (4/3) | 5 (2/3) | 71.4% |
| B3 | 0 (0/0) | 0 (0/0) | - |
| B4 | 23 (11/12) | 20 (9/11) | 87.0% |
| B5 | 3 (2/1) | 3 (2/1) | 100% |
| Report | DR Prevalence | Region | Year |
|---|---|---|---|
| Teo et al., 59 studies | 22.3% | Global | 2020 |
| Yau et al., 35 studies | 34.6% | Global | 1980–2008 |
| Cheloni et al., 10 studies | 34.6% | Global | 2008–2018 |
| Song et al., 31 studies | 18.5% | China | 1990–2017 |
| Brar et al., 10 studies | 16.1% | India | 1990–2021 |
| Heiran et al., 109 studies | 31% | Eastern Mediterranean | 2019–2020 |
| Present study | 48.7% | Japan | 2019–2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, Y.; Kiyosawa, M. Relationship between Diabetic Nephropathy and Development of Diabetic Macular Edema in Addition to Diabetic Retinopathy. Biomedicines 2023, 11, 1502. https://doi.org/10.3390/biomedicines11051502
Suzuki Y, Kiyosawa M. Relationship between Diabetic Nephropathy and Development of Diabetic Macular Edema in Addition to Diabetic Retinopathy. Biomedicines. 2023; 11(5):1502. https://doi.org/10.3390/biomedicines11051502
Chicago/Turabian StyleSuzuki, Yukihisa, and Motohiro Kiyosawa. 2023. "Relationship between Diabetic Nephropathy and Development of Diabetic Macular Edema in Addition to Diabetic Retinopathy" Biomedicines 11, no. 5: 1502. https://doi.org/10.3390/biomedicines11051502