Decreased MARCKS Protein Expression in Kidney Cortex Membrane Fractions of Cathepsin B Knockout Mice Is Associated with Reduced Lysophosphatidylcholine and Protein Kinase C Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Tissue Lysate Preparation, BCA Assay and Western Blotting
2.3. Immunoprecipitation Studies
2.4. Lipid Extraction from Kidney Cortex Membrane Fractions
2.5. LC-MS/MS Conditions
2.6. Lipid Quantification
2.7. PKC Kinase Activity Assay
2.8. CAMKII Beta Assay
2.9. Phosopholipase D Assay
2.10. Data Analysis and Statistics
3. Results
3.1. Reduced MARCKS Protein Expression in Cathepsin B Knockout Mice Compared to C57B6 Wild-Type Mice
3.2. Decrease Abundance of the Actin Cytoskeleton Protein Spectrin in Kidney Cortex Lysates of Cathepsin B Knockout Mice
3.3. Interaction between MARCKS and Spectrin Proteins in the Kidney
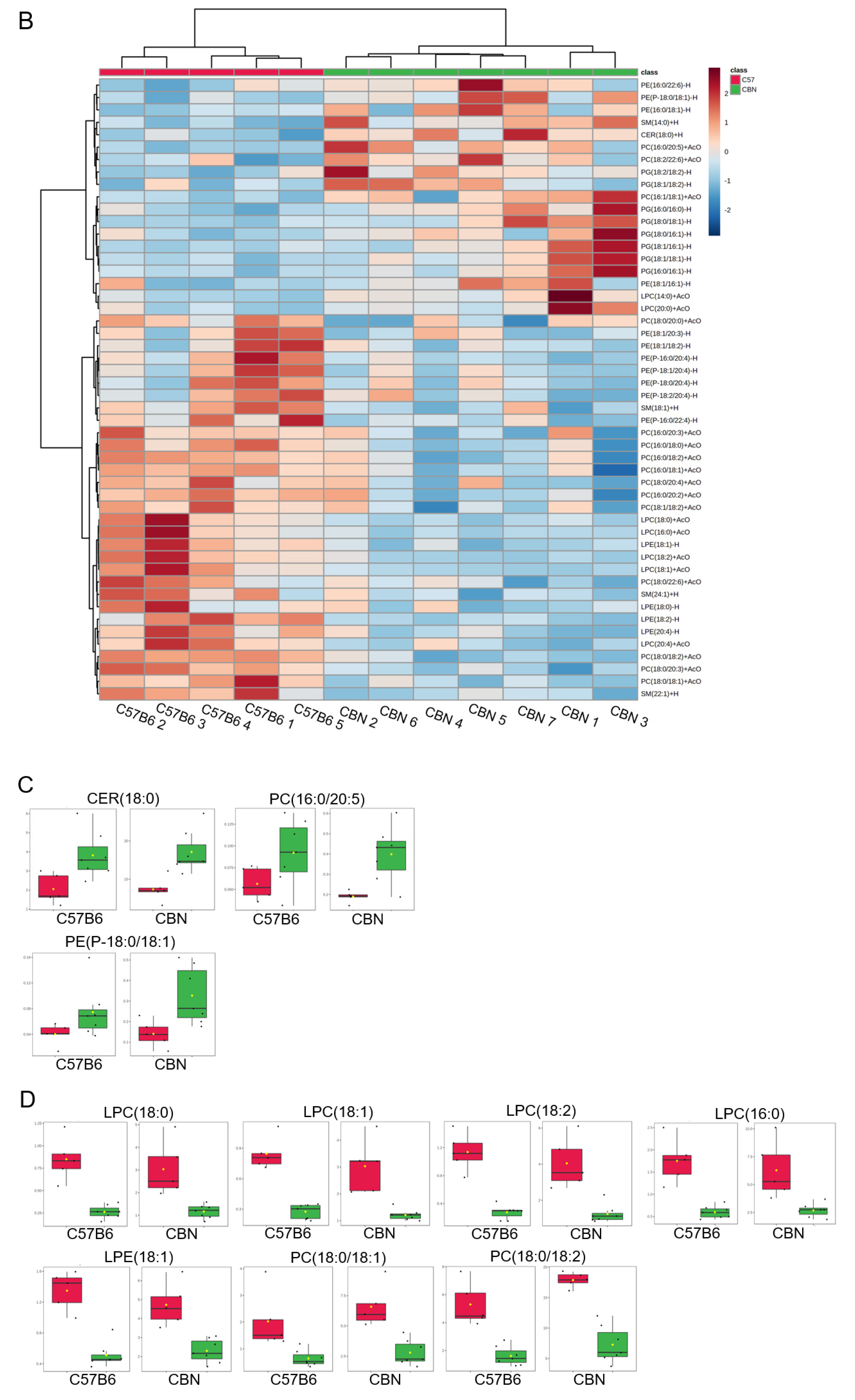
3.4. Differential Expression of Lipids in Kidney Cortex Membrane Fractions of C57B6 Wild-Type and Cathepsin B Knockout (CBN) Mice
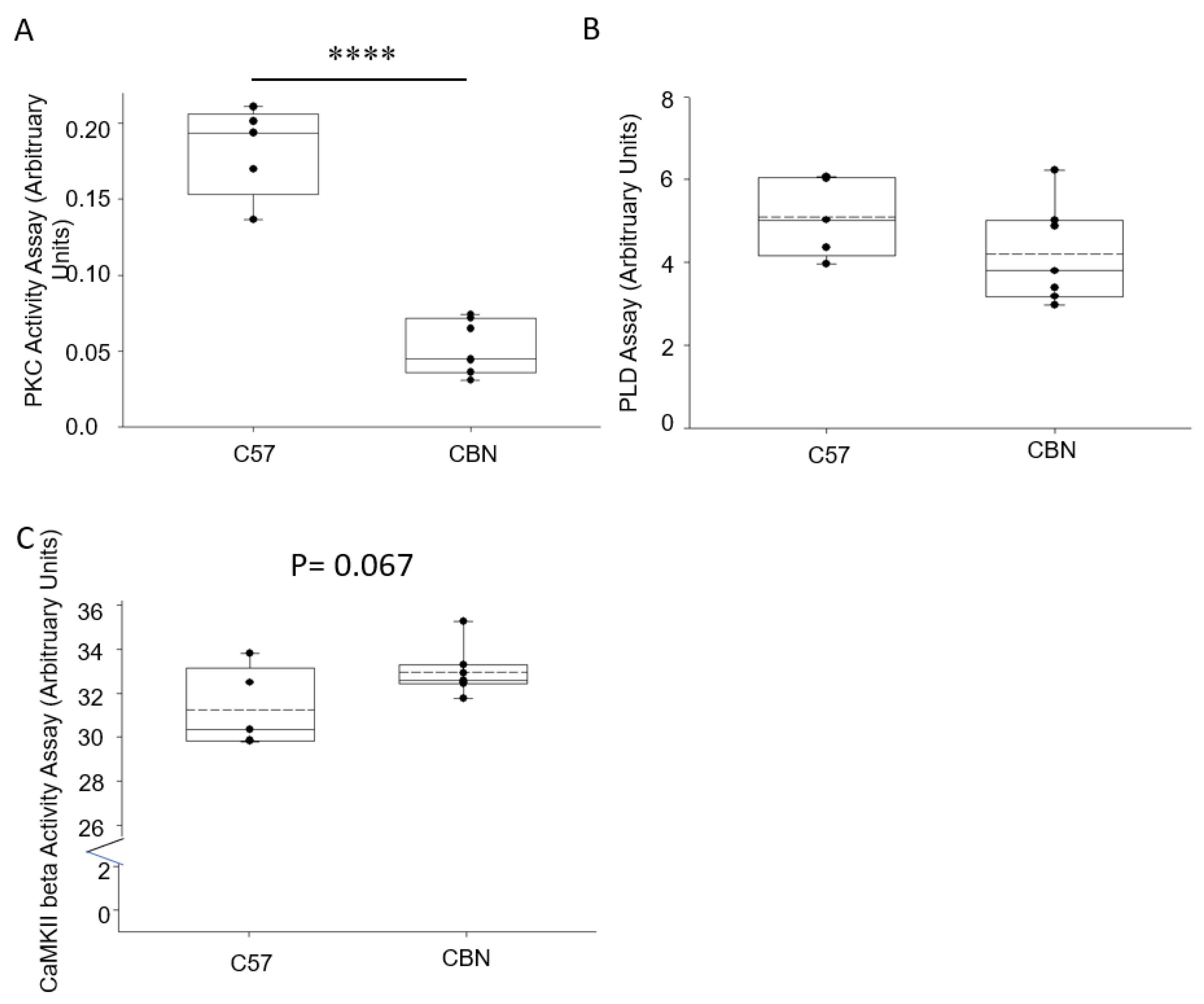
3.5. Basal Activity of Proteases and Enzymes in the Kidney Cortex of Cathepsin B Knockout and C57B6 Wild-Type Mice
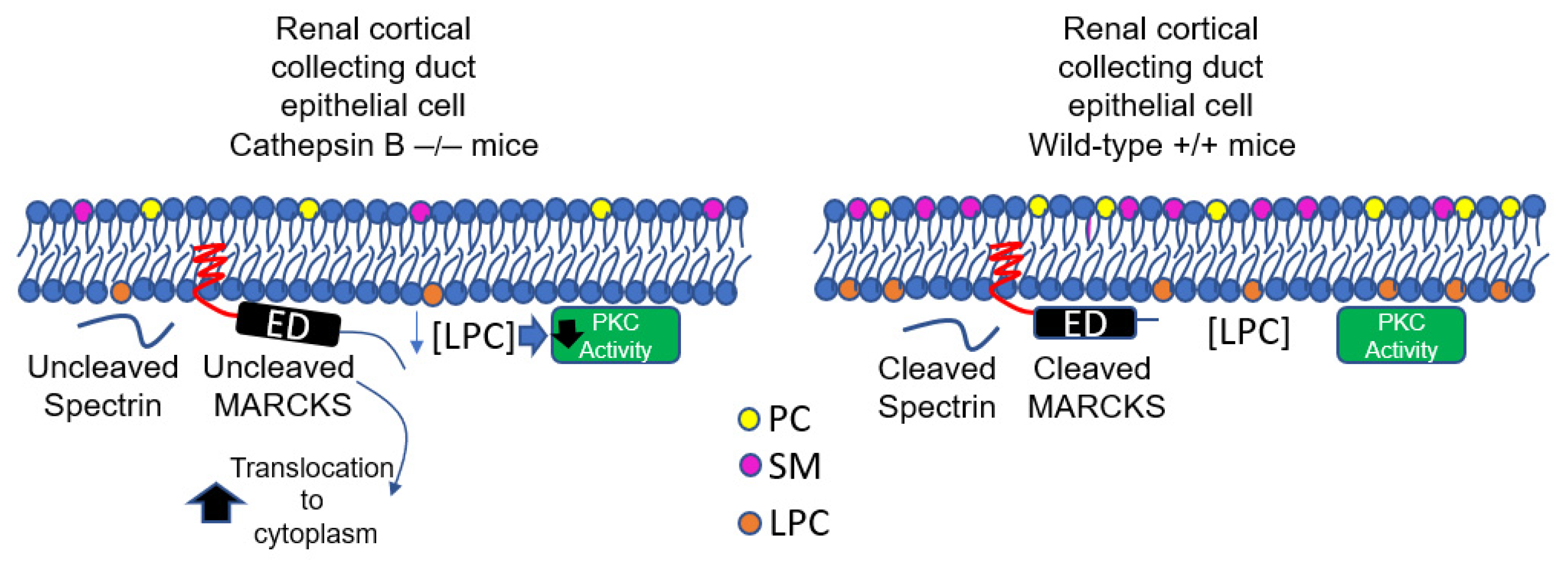
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagakannan, P.; Islam, I.; Conrad, M.; Eftekharpour, E. Cathepsin B is an executioner of ferroptosis. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118928. [Google Scholar] [CrossRef] [PubMed]
- Kuang, F.; Liu, J.; Li, C.; Kang, R.; Tang, D. Cathepsin B is a mediator of organelle-specific initiation of ferroptosis. Biochem. Biophys. Res. Commun. 2020, 533, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.; Sloane, B.F. Cathepsin B: Multiple roles in cancer. Proteom. Clin. Appl. 2014, 8, 427–437. [Google Scholar] [CrossRef]
- Thibeaux, S.; Siddiqi, S.; Zhelyabovska, O.; Moinuddin, F.; Masternak, M.M.; Siddiqi, S.A. Cathepsin B regulates hepatic lipid metabolism by cleaving liver fatty acid–binding protein. J. Biol. Chem. 2018, 293, 1910–1923. [Google Scholar] [CrossRef]
- Chevriaux, A.; Pilot, T.; Derangère, V.; Simonin, H.; Martine, P.; Chalmin, F.; Ghiringhelli, F.; Rébé, C. Cathepsin B Is Required for NLRP3 Inflammasome Activation in Macrophages, Through NLRP3 Interaction. Front. Cell Dev. Biol. 2020, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Lindkvist, B.; Fajardo, I.; Pejler, G.; Borgström, A. Cathepsin B activates human trypsinogen 1 but not proelastase 2 or procarboxypeptidase B. Pancreatology 2006, 6, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Alli, A.A.; Song, J.Z.; Al-Khalili, O.; Bao, H.F.; Ma, H.P.; Alli, A.A.; Eaton, D.C. Cathepsin B is secreted apically from Xenopus 2F3 cells and cleaves the epithelial sodium channel (ENaC) to increase its activity. J. Biol. Chem. 2012, 287, 30073–30083. [Google Scholar] [CrossRef]
- Larionov, A.; Dahlke, E.; Kunke, M.; Rodriguez, L.Z.; Schiessl, I.M.; Magnin, J.; Kern, U.; Alli, A.A.; Mollet, G.; Schilling, O.; et al. Cathepsin B increases ENaC activity leading to hypertension early in nephrotic syndrome. J. Cell Mol. Med. 2019, 23, 6543–6553. [Google Scholar] [CrossRef]
- Downs, C.A.; Dang, V.D.; Johnson, N.M.; Denslow, N.D.; Alli, A.A. Hydrogen Peroxide Stimulates Exosomal Cathepsin B Regulation of the Receptor for Advanced Glycation End-Products (RAGE). J. Cell Biochem. 2018, 119, 599–606. [Google Scholar] [CrossRef]
- Kotaska, K.; Dusek, P.; Prusa, R.; Vesely, S.; Babjuk, M. Urine and Serum Cathepsin B Concentrations in the Transitional Cell Carcinoma of the Bladder. J. Clin. Lab. Anal. 2012, 26, 61–65. [Google Scholar] [CrossRef]
- Sundelöf, J.; Sundström, J.; Hansson, O.; Eriksdotter-Jönhagen, M.; Giedraitis, V.; Larsson, A.; Degerman-Gunnarsson, M.; Ingelsson, M.; Minthon, L.; Blennow, K.; et al. Higher cathepsin B levels in plasma in Alzheimer’s disease compared to healthy controls. J. Alzheimers Dis. 2010, 22, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Spizz, G.; Blackshear, P. Identification and Characterization of Cathepsin B as the Cellular MARCKS Cleaving Enzyme. J. Biol. Chem. 1997, 272, 23833–23842. [Google Scholar] [CrossRef] [PubMed]
- Reifenberger, M.S.; Yu, L.; Bao, H.-F.; Duke, B.J.; Liu, B.-C.; Ma, H.-P.; Alli, A.A.; Eaton, D.C.; Alli, A.A.; Tuna, K.M.; et al. Cytochalasin E alters the cytoskeleton and decreases ENaC activity in Xenopus 2F3 cells. Am. J. Physiol. Physiol. 2014, 307, F86–F95. [Google Scholar] [CrossRef]
- Lugo, C.I.; Liu, L.P.; Bala, N.; Morales, A.G.; Gholam, M.F.; Abchee, J.C.; Elmoujahid, N.; Elshikha, A.S.; Avdiaj, R.; Searcy, L.A.; et al. Human Alpha-1 Antitrypsin Attenuates ENaC and MARCKS and Lowers Blood Pressure in Hypertensive Diabetic db/db Mice. Biomolecules 2022, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Chacko, K.M.; Nouri, M.-Z.; Schramm, W.C.; Malik, Z.; Liu, L.P.; Denslow, N.D.; Alli, A.A. Tempol Alters Urinary Extracellular Vesicle Lipid Content and Release While Reducing Blood Pressure during the Development of Salt-Sensitive Hypertension. Biomolecules 2021, 11, 1804. [Google Scholar] [CrossRef]
- Zuckerman, J.B.; Chen, X.; Jacobs, J.D.; Hu, B.; Kleyman, T.R.; Smith, P.R. Association of the Epithelial Sodium Channel with Apx and α-Spectrin in A6 Renal Epithelial Cells. J. Biol. Chem. 1999, 274, 23286–23295. [Google Scholar] [CrossRef]
- Mizunoe, Y.; Kobayashi, M.; Hoshino, S.; Tagawa, R.; Itagawa, R.; Hoshino, A.; Okita, N.; Sudo, Y.; Nakagawa, Y.; Shimano, H.; et al. Cathepsin B overexpression induces degradation of perilipin 1 to cause lipid metabolism dysfunction in adipocytes. Sci. Rep. 2020, 10, 634. [Google Scholar] [CrossRef]
- Prokazova, N.V.; Zvezdina, N.D.; Korotaeva, A.A. Effect of lysophosphatidylcholine on transmembrane signal transduction. Biochemistry 1998, 63, 31–37. [Google Scholar]
- Ohara, Y.; Peterson, T.E.; Zheng, B.; Kuo, J.F.; Harrison, D.G. Lysophosphatidylcholine increases vascular superoxide anion production via protein kinase C activation. Arter. Thromb. A J. Vasc. Biol. 1994, 14, 1007–1013. [Google Scholar] [CrossRef]
- Sasaki, Y.; Asaoka, Y.; Nishizuka, Y. Potentiation of diacylglycerol-induced activation of protein kinase C by lysophospholipids: Subspecies difference. FEBS Lett. 1993, 320, 47–51. [Google Scholar] [CrossRef]
- Sugiyama, S.; Kugiyama, K.; Ohgushi, M.; Fujimoto, K.; Yasue, H. Lysophosphatidylcholine in oxidized low-density lipoprotein increases endothelial susceptibility to polymorphonuclear leukocyte-induced endothelial dysfunction in porcine coronary arteries. Role of protein kinase C. Circ. Res. 1994, 74, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Alli, A.A.; Bao, H.F.; Alli, A.A.; Aldrugh, Y.; Song, J.Z.; Ma, H.P.; Yu, L.; Al-Khalili, O.; Eaton, D.C. Phosphatidylinositol phosphate-dependent regulation of Xenopus ENaC by MARCKS protein. Am. J. Physiol. Renal. Physiol. 2012, 303, F800–F811. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Montgomery, D.S.; Yu, L.; Ghazi, Z.M.; Thai, T.L.; Al-Khalili, O.; Ma, H.-P.; Eaton, D.C.; Alli, A.A. ENaC activity is regulated by calpain-2 proteolysis of MARCKS proteins. Am. J. Physiol. Physiol. 2017, 313, C42–C53. [Google Scholar] [CrossRef]
- Spizz, G.; Blackshear, P. Protein Kinase C-mediated Phosphorylation of the Myristoylated Alanine-rich C-kinase Substrate Protects It from Specific Proteolytic Cleavage. J. Biol. Chem. 1996, 271, 553–562. [Google Scholar] [CrossRef]
- Zimmerman, U.-J.P.; Speicher, D.W.; Fisher, A.B. Secretagogue-induced proteolysis of lung spectrin in alveolar epithelial type II cells. Biochim. Biophys. Acta 1992, 1137, 127–134. [Google Scholar] [CrossRef]
- Cho, K.; Yoon, S.Y.; Choi, J.E.; Kang, H.J.; Jang, H.Y.; Kim, D.-H. CA-074Me, a cathepsin B inhibitor, decreases APP accumulation and protects primary rat cortical neurons treated with okadaic acid. Neurosci. Lett. 2013, 548, 222–227. [Google Scholar] [CrossRef]
- Taylor, J.; Patio, K.; De Rubis, G.; Morris, M.B.; Evenhuis, C.; Johnson, M.; Bebawy, M. Membrane to cytosol redistribution of αII-spectrin drives extracellular vesicle biogenesis in malignant breast cells. Proteomics 2021, 21, 2000091. [Google Scholar] [CrossRef]
- Yoshioka, K.; Hirakawa, Y.; Kurano, M.; Ube, Y.; Ono, Y.; Kojima, K.; Iwama, T.; Kano, K.; Hasegawa, S.; Inoue, T.; et al. Lysophosphatidylcholine mediates fast decline in kidney function in diabetic kidney disease. Kidney Int. 2022, 101, 510–526. [Google Scholar] [CrossRef]
- Handa, R.K.; Buckalew, V.M. Effect of lysophosphatidylcholine on renal hemodynamics and excretory function in anesthetized rats. Life Sci. 1992, 51, 1571–1575. [Google Scholar] [CrossRef]
- Bassa, B.V.; Roh, D.D.; Vaziri, N.D.; Kirschenbaum, M.A.; Kamanna, V.S. Lysophosphatidylcholine activates mesangial cell PKC and MAP kinase by PLCgamma-1 and tyrosine kinase-Ras pathways. Am. J. Physiol. 1999, 277, F328–F337. [Google Scholar] [PubMed]
- Huwiler, A.; Staudt, G.; Kramer, R.M.; Pfeilschifter, J. Cross-talk between secretory phospholipase A2 and cytosolic phospholipase A2 in rat renal mesangial cells. Biochim Biophys Acta. 1997, 1348, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Kugiyama, K.; Ohgushi, M.; Sugiyama, S.; Murohara, T.; Fukunaga, K.; Miyamoto, E.; Yasue, H. Lysophosphatidylcholine inhibits surface receptor-mediated intracellular signals in endothelial cells by a pathway involving protein kinase C activation. Circ. Res. 1992, 71, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.A.; Arioka, M.; Jacobs, S.E. Lysophosphatidylcholine mediates melanocyte dendricity through PKCzeta activation. J. Invest. Dermatol. 2007, 127, 668–675. [Google Scholar] [CrossRef]
- Alapati, K.; Gopinath, S.; Malla, R.R.; Dasari, V.R.; Rao, J.S. uPAR and cathepsin B knockdown inhibits radiation-induced PKC integrated integrin signaling to the cytoskeleton of glioma-initiating cells. Int. J. Oncol. 2012, 41, 599–610. [Google Scholar] [CrossRef]
- Gholam, M.F.; Liu, L.P.; Searcy, L.A.; Denslow, N.D.; Alli, A.A. Dapagliflozin Treatment Augments Bioactive Phosphatidylethanolamine Concentrations in Kidney Cortex Membrane Fractions of Hypertensive Diabetic db/db Mice and Alters the Density of Lipid Rafts in Mouse Proximal Tubule Cells. Int. J. Mol. Sci. 2023, 24, 1408. [Google Scholar] [CrossRef]
- Alli, A.A.; Desai, D.; Elshika, A.; Conrad, M.; Proneth, B.; Clapp, W.; Atkinson, C.; Segal, M.; Searcy, L.A.; Denslow, N.D.; et al. Kidney tubular epithelial cell ferroptosis links glomerular injury to tubulointerstitial pathology in lupus nephritis. Clin. Immunol. 2023, 248, 109213. [Google Scholar] [CrossRef]
- Gonzalez, P.; Debnath, S.; Chen, Y.-A.; Hernandez, E.; Jha, P.; Dakanali, M.; Hsieh, J.-T.; Sun, X. A Theranostic Small-Molecule Prodrug Conjugate for Neuroendocrine Prostate Cancer. Pharmaceutics 2023, 15, 481. [Google Scholar] [CrossRef]
- Debnath, S.; Hao, G.; Guan, B.; Thapa, P.; Hao, J.; Hammers, H.; Sun, X. Theranostic Small-Molecule Prodrug Conjugates for Targeted Delivery and Controlled Release of Toll-like Receptor 7 Agonists. Int. J. Mol. Sci. 2022, 23, 7160. [Google Scholar] [CrossRef]




| Parameter | CBN | 129Sv Wild-Type | C57B6 Wild-Type |
|---|---|---|---|
| Body Weight | 26.14 +/− 1.76 | 26.33 +/− 1.8 | 26.6 +/− 1.5 |
| Kidney Weight | 0.26 +/− 0.01 | 0.27 +/− 0.01 | 0.26 +/− 0.02 |
| Assays | Manufacture | Cat. No. |
|---|---|---|
| CAMKII Beta Assay | My Biosource | MBS456030 |
| Phsopholipase D Assay Kit PKC Kinase Activity Assay Kit | Sigma Aldrich Abcam | MAK-137 ab139437 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawakibi, T.; Bala, N.; Liu, L.P.; Searcy, L.A.; Denslow, N.D.; Alli, A.A. Decreased MARCKS Protein Expression in Kidney Cortex Membrane Fractions of Cathepsin B Knockout Mice Is Associated with Reduced Lysophosphatidylcholine and Protein Kinase C Activity. Biomedicines 2023, 11, 1489. https://doi.org/10.3390/biomedicines11051489
Kawakibi T, Bala N, Liu LP, Searcy LA, Denslow ND, Alli AA. Decreased MARCKS Protein Expression in Kidney Cortex Membrane Fractions of Cathepsin B Knockout Mice Is Associated with Reduced Lysophosphatidylcholine and Protein Kinase C Activity. Biomedicines. 2023; 11(5):1489. https://doi.org/10.3390/biomedicines11051489
Chicago/Turabian StyleKawakibi, Tamim, Niharika Bala, Lauren P. Liu, Louis A. Searcy, Nancy D. Denslow, and Abdel A. Alli. 2023. "Decreased MARCKS Protein Expression in Kidney Cortex Membrane Fractions of Cathepsin B Knockout Mice Is Associated with Reduced Lysophosphatidylcholine and Protein Kinase C Activity" Biomedicines 11, no. 5: 1489. https://doi.org/10.3390/biomedicines11051489





