Factors Influencing the Yield of Progenitor Cells in Bone Marrow Aspiration Concentrate—A Retrospective Analysis of 58 Patients
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Age
3.2. Sex and Comorbidities
3.3. Aspiration Volume
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeyaraman, M.; Nallakumarasamy, A.; Jeyaraman, N. Industry 5.0 in Orthopaedics. JOIO 2022, 56, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Gianakos, A.L.; Sun, L.; Patel, J.N.; Adams, D.M.; Liporace, F.A. Clinical Application of Concentrated Bone Marrow Aspirate in Orthopaedics: A Systematic Review. World J. Orthop. 2017, 8, 491–506. [Google Scholar] [CrossRef]
- Chahla, J.; Alland, J.A.; Verma, N.N. Bone Marrow Aspirate Concentrate for Orthopaedic Use. Orthop. Nurs. 2018, 37, 379–381. [Google Scholar] [CrossRef]
- Mabuchi, Y.; Okawara, C.; Méndez-Ferrer, S.; Akazawa, C. Cellular Heterogeneity of Mesenchymal Stem/Stromal Cells in the Bone Marrow. Front. Cell Dev. Biol. 2021, 9, 689366. [Google Scholar] [CrossRef]
- Dragoo, J.L.; Guzman, R.A. Evaluation of the Consistency and Composition of Commercially Available Bone Marrow Aspirate Concentrate Systems. Orthop. J. Sports Med. 2020, 8, 2325967119893634. [Google Scholar] [CrossRef] [PubMed]
- Freitas, G.P.; Souza, A.T.P.; Lopes, H.B.; Trevisan, R.L.B.; Oliveira, F.S.; Fernandes, R.R.; Ferreira, F.U.; Ros, F.A.; Beloti, M.M.; Rosa, A.L. Mesenchymal Stromal Cells Derived from Bone Marrow and Adipose Tissue: Isolation, Culture, Characterization and Differentiation. Bio. Protoc. 2020, 10, e3534. [Google Scholar] [CrossRef]
- Cercone, M.; Greenfield, M.R.; Fortier, L.A. Bone Marrow Concentrate Mesenchymal Stromal Cells Do Not Correlate With Nucleated Cell Count or Colony Forming Units. J. Cartil. Jt. Preserv. 2021, 1, 100017. [Google Scholar] [CrossRef]
- Robert, A.W.; Marcon, B.H.; Dallagiovanna, B.; Shigunov, P. Adipogenesis, Osteogenesis, and Chondrogenesis of Human Mesenchymal Stem/Stromal Cells: A Comparative Transcriptome Approach. Front. Cell Dev. Biol. 2020, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Aboushady, I.M.; Salem, Z.A.; Sabry, D.; Mohamed, A. Comparative Study of the Osteogenic Potential of Mesenchymal Stem Cells Derived from Different Sources. J. Clin. Exp. Dent. 2018, 10, e7–e13. [Google Scholar] [CrossRef]
- Merryweather-Clarke, A.T.; Cook, D.; Lara, B.J.; Hua, P.; Repapi, E.; Ashley, N.; Lim, S.Y.; Watt, S.M. Does Osteogenic Potential of Clonal Human Bone Marrow Mesenchymal Stem/Stromal Cells Correlate with Their Vascular Supportive Ability? Stem Cell Res. Ther. 2018, 9, 351. [Google Scholar] [CrossRef]
- Fernandez-Moure, J.S.; Corradetti, B.; Chan, P.; Van Eps, J.L.; Janecek, T.; Rameshwar, P.; Weiner, B.K.; Tasciotti, E. Enhanced Osteogenic Potential of Mesenchymal Stem Cells from Cortical Bone: A Comparative Analysis. Stem Cell Res. Ther. 2015, 6, 203. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, H.; Muneta, T.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Comparison of Rat Mesenchymal Stem Cells Derived from Bone Marrow, Synovium, Periosteum, Adipose Tissue, and Muscle. Cell Tissue Res. 2007, 327, 449–462. [Google Scholar] [CrossRef]
- Everts, P.A.; Malanga, G.A.; Paul, R.V.; Rothenberg, J.B.; Stephens, N.; Mautner, K.R. Assessing Clinical Implications and Perspectives of the Pathophysiological Effects of Erythrocytes and Plasma Free Hemoglobin in Autologous Biologics for Use in Musculoskeletal Regenerative Medicine Therapies. A Review. Regen. Ther. 2019, 11, 56–64. [Google Scholar] [CrossRef]
- Ziegler, C.G.; Van Sloun, R.; Gonzalez, S.; Whitney, K.E.; DePhillipo, N.N.; Kennedy, M.I.; Dornan, G.J.; Evans, T.A.; Huard, J.; LaPrade, R.F. Characterization of Growth Factors, Cytokines, and Chemokines in Bone Marrow Concentrate and Platelet-Rich Plasma: A Prospective Analysis. Am. J. Sports Med. 2019, 47, 2174–2187. [Google Scholar] [CrossRef] [PubMed]
- Jahani, M.; Rezazadeh, D.; Mohammadi, P.; Abdolmaleki, A.; Norooznezhad, A.; Mansouri, K. Regenerative Medicine and Angiogenesis; Challenges and Opportunities. Adv. Pharm. Bull. 2020, 10, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Keeling, L.E.; Belk, J.W.; Kraeutler, M.J.; Kallner, A.C.; Lindsay, A.; McCarty, E.C.; Postma, W.F. Bone Marrow Aspirate Concentrate for the Treatment of Knee Osteoarthritis: A Systematic Review. Am. J. Sports Med. 2021, 50, 2315–2323. [Google Scholar] [CrossRef]
- Gs, T.; Gd, C.; Im, K.; Ia, G.; Ms, T.; Pj, P.; Od, S. Effectiveness of a Single Intra-Articular Bone Marrow Aspirate Concentrate (BMAC) Injection in Patients with Grade 3 and 4 Knee Osteoarthritis. Heliyon 2018, 4, e00871. [Google Scholar] [CrossRef] [Green Version]
- Cavallo, C.; Boffa, A.; Andriolo, L.; Silva, S.; Grigolo, B.; Zaffagnini, S.; Filardo, G. Bone Marrow Concentrate Injections for the Treatment of Osteoarthritis: Evidence from Preclinical Findings to the Clinical Application. Int. Orthop. 2021, 45, 525–538. [Google Scholar] [CrossRef]
- Gursoy, S.; Akkaya, M.; Simsek, M.E.; Bozkurt, M. Functional Outcomes of Bone Marrow Aspirate Concentrate Application in Osteoarthritis of the Knee. Int. J. Res. Med. Sci. 2019, 7, 587–592. [Google Scholar] [CrossRef]
- Vina, M.F.; Camozzi, L.B.; Cipitria, J. A Single Dose of Bone Marrow Mononuclear Cells (BMMNCs) and Bone Marrow Aspirate Concentrate (BMAC) for the Treatment of Knee OA. First 6 Months of Follow Up. Cytotherapy 2019, 21, S76–S77. [Google Scholar] [CrossRef]
- Shapiro, S.A.; Kazmerchak, S.E.; Heckman, M.G.; Zubair, A.C.; O’Connor, M.I. A Prospective, Single-Blind, Placebo-Controlled Trial of Bone Marrow Aspirate Concentrate for Knee Osteoarthritis. Am. J. Sports Med. 2017, 45, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.P.; Selvaratnam, L.; Abbas, A.A.; Kamarul, T. Factors Influencing the Successful Isolation and Expansion of Aging Human Mesenchymal Stem Cells. Open Life Sci. 2018, 13, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Scarpone, M.; Kuebler, D.; Chambers, A.; De Filippo, C.M.; Amatuzio, M.; Ichim, T.E.; Patel, A.N.; Caradonna, E. Isolation of Clinically Relevant Concentrations of Bone Marrow Mesenchymal Stem Cells without Centrifugation. J. Transl. Med. 2019, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; Bingi, S.K.; Muthu, S.; Jeyaraman, N.; Packkyarathinam, R.P.; Ranjan, R.; Sharma, S.; Jha, S.K.; Khanna, M.; Rajendran, S.N.S.; et al. Impact of the Process Variables on the Yield of Mesenchymal Stromal Cells from Bone Marrow Aspirate Concentrate. Bioengineering 2022, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Carp, D.M.; Liang, Y. Universal or Personalized Mesenchymal Stem Cell Therapies: Impact of Age, Sex, and Biological Source. Cells 2022, 11, 2077. [Google Scholar] [CrossRef]
- Zhou, S.; Greenberger, J.S.; Epperly, M.W.; Goff, J.P.; Adler, C.; Leboff, M.S.; Glowacki, J. Age-Related Intrinsic Changes in Human Bone-Marrow-Derived Mesenchymal Stem Cells and Their Differentiation to Osteoblasts. Aging Cell 2008, 7, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Beane, O.S.; Fonseca, V.C.; Cooper, L.L.; Koren, G.; Darling, E.M. Impact of Aging on the Regenerative Properties of Bone Marrow-, Muscle-, and Adipose-Derived Mesenchymal Stem/Stromal Cells. PLoS ONE 2014, 9, e115963. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, P.; El-Jawhari, J.J.; Giannoudis, P.V.; Burska, A.N.; Ponchel, F.; Jones, E.A. Age-Related Changes in Bone Marrow Mesenchymal Stromal Cells: A Potential Impact on Osteoporosis and Osteoarthritis Development. Cell Transplant. 2017, 26, 1520–1529. [Google Scholar] [CrossRef] [Green Version]
- Cavallo, C.; Boffa, A.; de Girolamo, L.; Merli, G.; Kon, E.; Cattini, L.; Santo, E.; Grigolo, B.; Filardo, G. Bone Marrow Aspirate Concentrate Quality Is Affected by Age and Harvest Site. Knee Surg. Sports Traumatol. Arthrosc. 2022. [Google Scholar] [CrossRef]
- Evans, C.H. Advances in Regenerative Orthopaedics. Mayo Clin. Proc. 2013, 88, 1323–1339. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wong, W.H.-S.; Chan, S.; San Chim, J.C.; Cheung, K.M.-C.; Lee, T.-L.; Au, W.-Y.; Ha, S.-Y.; Lie, A.K.-W.; Lau, Y.-L.; et al. Factors Affecting Mesenchymal Stromal Cells Yield from Bone Marrow Aspiration. Chin. J. Cancer Res. 2011, 23, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanikawa, S.; Sakamaki, H.; Mori, S.; Akiyama, H.; Miyamoto, H.; Tanaka, Y.; Yoshinaga, H.; Okamoto, R.; Maeda, Y.; Mukaiyama, T.; et al. Relationship between the presence of side-holes in bone marrow aspiration needle and the number of harvested bone marrow mononuclear cells. Rinsho Ketsueki 1997, 38, 1249–1253. [Google Scholar] [PubMed]
- El-Jawhari, J.J.; Ganguly, P.; Churchman, S.; Jones, E.; Giannoudis, P.V. The Biological Fitness of Bone Progenitor Cells in Reamer/Irrigator/Aspirator Waste. J. Bone Jt. Surg. Am. 2019, 101, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.A.; Wynn, R.F.; Jowitt, S.N.; Wraith, J.E.; Fairbairn, L.J.; Bellantuono, I. Study of Telomere Length Reveals Rapid Aging of Human Marrow Stromal Cells Following in Vitro Expansion. Stem Cells 2004, 22, 675–682. [Google Scholar] [CrossRef]
- Bellantuono, I.; Aldahmash, A.; Kassem, M. Aging of Marrow Stromal (Skeletal) Stem Cells and Their Contribution to Age-Related Bone Loss. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2009, 1792, 364–370. [Google Scholar] [CrossRef] [Green Version]
- D’Ippolito, G.; Schiller, P.C.; Ricordi, C.; Roos, B.A.; Howard, G.A. Age-Related Osteogenic Potential of Mesenchymal Stromal Stem Cells from Human Vertebral Bone Marrow. J. Bone Miner. Res. 1999, 14, 1115–1122. [Google Scholar] [CrossRef]
- Stenderup, K.; Justesen, J.; Eriksen, E.F.; Rattan, S.I.; Kassem, M. Number and Proliferative Capacity of Osteogenic Stem Cells Are Maintained during Aging and in Patients with Osteoporosis. J. Bone Miner. Res. 2001, 16, 1120–1129. [Google Scholar] [CrossRef]
- Justesen, J.; Stenderup, K.; Eriksen, E.F.; Kassem, M. Maintenance of Osteoblastic and Adipocytic Differentiation Potential with Age and Osteoporosis in Human Marrow Stromal Cell Cultures. Calcif. Tissue Int. 2002, 71, 36–44. [Google Scholar] [CrossRef]
- Nagao, T.; Yamauchi, K.; Komatsuda, M. Serial in Vitro Bone Marrow Fibroblast Culture in Human Leukemia. Blood 1983, 61, 589–592. [Google Scholar] [CrossRef]
- Katsuno, M.; Hirata, J.; Kaneko, S.; Nishimura, J.; Motomura, S.; Ibayashi, H. Serial Studies of Bone Marrow-Derived Fibroblastoid Colony-Forming Cells and Granulocyte/Macrophage Precursor Cells in Patients with Acute Leukemia. Acta Haematol. 1986, 76, 185–191. [Google Scholar] [CrossRef]
- Ganguly, P.; El-Jawhari, J.J.; Burska, A.N.; Ponchel, F.; Giannoudis, P.V.; Jones, E.A. The Analysis of In Vivo Aging in Human Bone Marrow Mesenchymal Stromal Cells Using Colony-Forming Unit-Fibroblast Assay and the CD45lowCD271+ Phenotype. Stem Cells Int. 2019, 2019, 5197983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choumerianou, D.M.; Martimianaki, G.; Stiakaki, E.; Kalmanti, L.; Kalmanti, M.; Dimitriou, H. Comparative Study of Stemness Characteristics of Mesenchymal Cells from Bone Marrow of Children and Adults. Cytotherapy 2010, 12, 881–887. [Google Scholar] [CrossRef]
- Beletskey, A.; Manderle, B.; Mirmonsef, P.; Gowd, A.K.; Chahla, J.; Yanke, A.; Forsythe, B.; Cole, B.J.; Verma, N.N. Age and Sex Influence the Mesenchymal Stem Cell composition of Bone Marrow Aspirate Concentrate (BMAC). Available online: https://www.sportssurgerychicago.com/2019/03/26/age-and-sex-influence-the-mesenchymal-stem-cell-composition-of-bone-marrow-aspirate-concentrate-bmac/ (accessed on 1 January 2023).
- Ghazeeri, G.; Abdullah, L.; Abbas, O. Immunological Differences in Women Compared with Men: Overview and Contributing Factors. Am. J. Reprod. Immunol. 2011, 66, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Beery, A.K.; Zucker, I. Sex Bias in Neuroscience and Biomedical Research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeyaraman, M.; Muthu, S.; Nischith, D.S.; Jeyaraman, N.; Nallakumarasamy, A.; Khanna, M. PRISMA-Compliant Meta-Analysis of Randomized Controlled Trials on Osteoarthritis of Knee Managed with Allogeneic vs Autologous MSCs: Efficacy and Safety Analysis. JOIO 2022, 56, 2042–2059. [Google Scholar] [CrossRef]
- Joswig, A.-J.; Mitchell, A.; Cummings, K.J.; Levine, G.J.; Gregory, C.A.; Smith, R.; Watts, A.E. Repeated Intra-Articular Injection of Allogeneic Mesenchymal Stem Cells Causes an Adverse Response Compared to Autologous Cells in the Equine Model. Stem Cell Res. Ther. 2017, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Babu, G.S.; Badrish, Y.; Oswal, V.M.; Jeyaraman, N.; Prajwal, G.S.; Jeyaraman, M.; Muthu, S.; Khanna, M. Immunomodulatory Actions of Mesenchymal Stromal Cells (MSCs) in Osteoarthritis of the Knee. Osteology 2021, 1, 209–224. [Google Scholar] [CrossRef]
- Muthu, S. Osteoarthritis, an old wine in a new bottle! World J. Orthop. 2023, 14, 1–5. [Google Scholar] [CrossRef]
- Schäfer, R.; DeBaun, M.R.; Fleck, E.; Centeno, C.J.; Kraft, D.; Leibacher, J.; Bieback, K.; Seifried, E.; Dragoo, J.L. Quantitation of Progenitor Cell Populations and Growth Factors after Bone Marrow Aspirate Concentration. J. Transl. Med. 2019, 17, 115. [Google Scholar] [CrossRef]
- Muthu, S.; Saravanakumar, T.P.; Ganie, P.A.; Yadav, V.; Baghel, P.K.; Jeyaraman, M. Thematic Trend Mapping and Hotspot Analysis in Bone Marrow Aspirate Concentrate Therapy: A Scientometric Literature Analysis and Advances in Osteoarthritis. Cytotherapy 2022, 24, 445–455. [Google Scholar] [CrossRef]
- Gothard, D.; Dawson, J.I.; Oreffo, R.O.C. Assessing the Potential of Colony Morphology for Dissecting the CFU-F Population from Human Bone Marrow Stromal Cells. Cell Tissue Res. 2013, 352, 237–247. [Google Scholar] [CrossRef] [PubMed]
- El-Jawhari, J.J.; Ganguly, P.; Jones, E.; Giannoudis, P.V. Bone Marrow Multipotent Mesenchymal Stromal Cells as Autologous Therapy for Osteonecrosis: Effects of Age and Underlying Causes. Bioengineering 2021, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; Muthu, S.; Jain, R.; Khanna, M. Autologous Bone Marrow Derived Mesenchymal Stem Cell Therapy for Osteonecrosis of Femoral Head: A Systematic Overview of Overlapping Meta-Analyses. J. Clin. Orthop. Trauma. 2021, 13, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Muthu, S.; Kartheek, R.R.; Jeyaraman, N.; Rajendran, R.L.; Khanna, M.; Jeyaraman, M.; Packkyarathinam, R.P.; Gangadaran, P.; Ahn, B.-C. Is Culture Expansion Necessary in Autologous Mesenchymal Stromal Cell Therapy to Obtain Superior Results in the Management of Knee Osteoarthritis?—Meta-Analysis of Randomized Controlled Trials. Bioengineering 2021, 8, 220. [Google Scholar] [CrossRef]
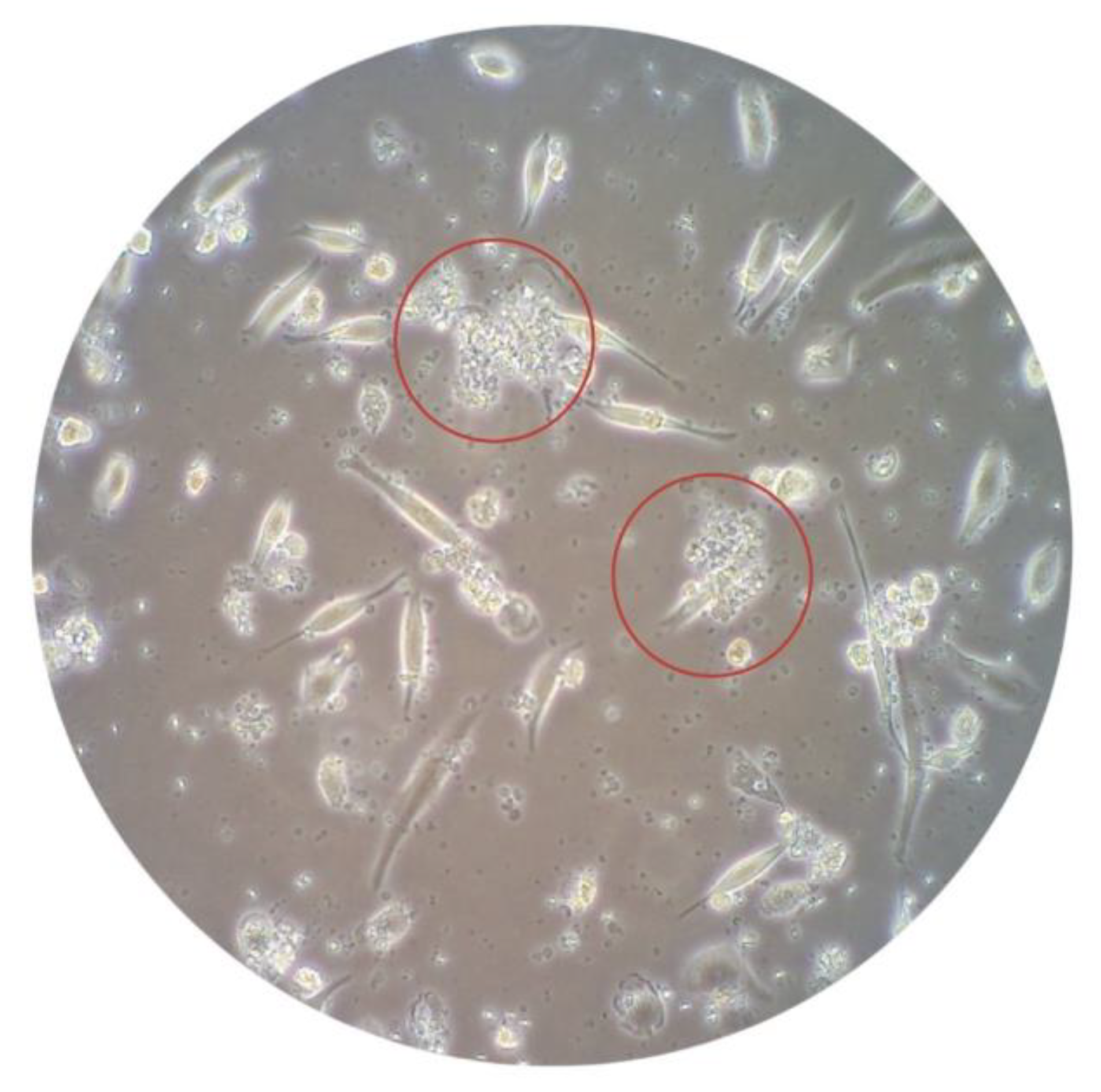


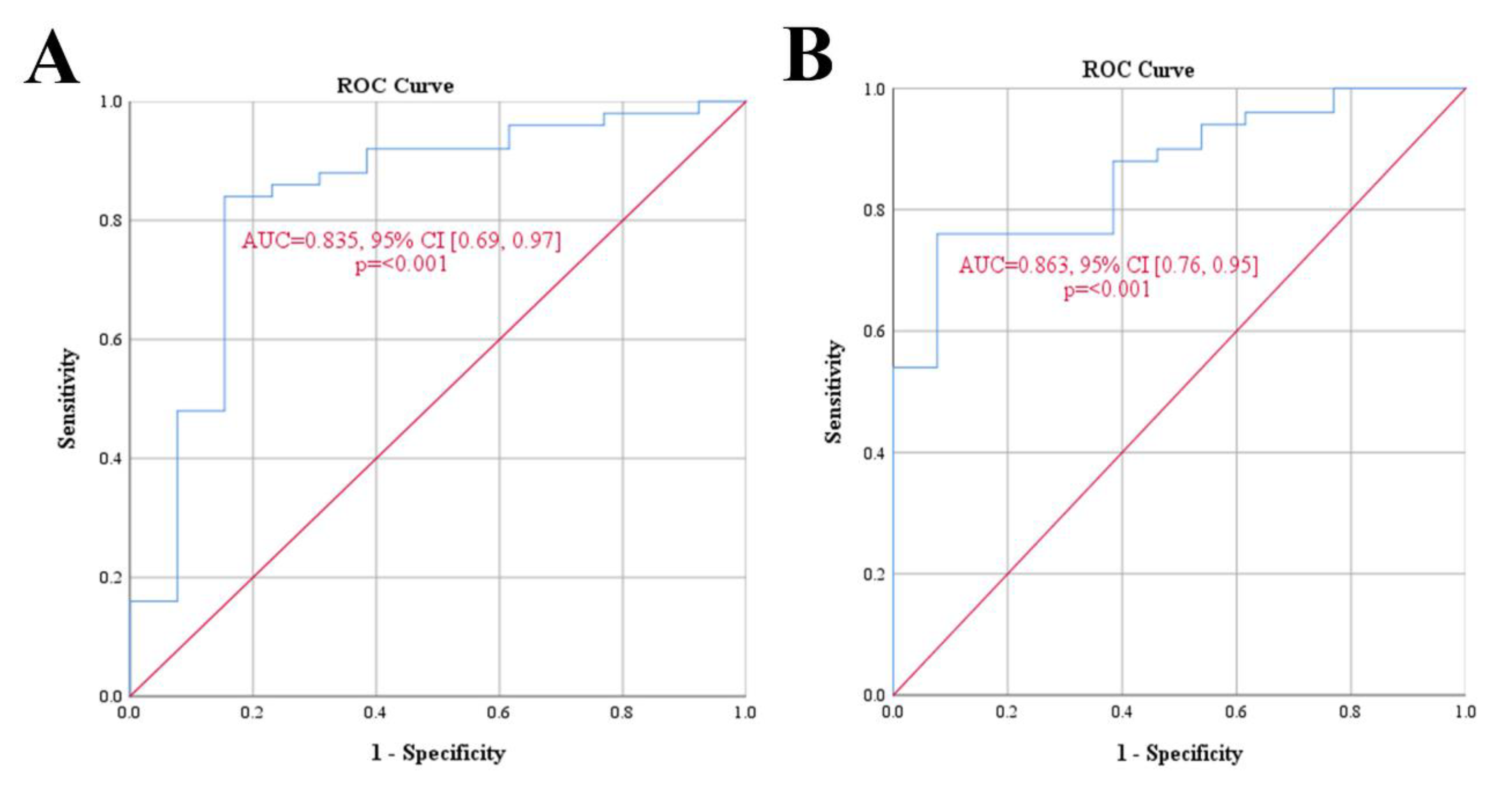


| Variables | Median (IQR) |
|---|---|
| Age | 60 (40–66) years |
| Sex | |
| Males | 31 |
| Females | 27 |
| Comorbidities | |
| Diabetes mellitus | 9 |
| Rheumatoid arthritis | 4 |
| No comorbid illness | 45 |
| Diagnosis | |
| Osteoarthritis | 32 |
| Diabetic foot ulcers | 8 |
| Rheumatoid arthritis | 4 |
| Others | 14 |
| BMA Characteritics | |
| Aspiration volume | 70 (50–80) mL |
| MNC count | 16.27 (11.17–20.66) × 106 cells/10 mL aspirate |
| CFU count | 7.33 (5–12.33) colonies/10 mL aspirate |
| S. No. | Comorbidity | MNC Count (×106 Cells) | X2 (df), p Value | OR (CI) | |
|---|---|---|---|---|---|
| <10 | ≥10 | ||||
| 1 | Present | 6 (60) | 7 (13.2) | 11.247 (1), 0.001 | 9.85 (2.21 to 43.92) |
| 2 | Absent | 4 (40) | 41 (86.8) | ||
| S.No. | Comorbidity | CFU | X2 (df), p Value | OR (CI) | |
|---|---|---|---|---|---|
| <3 | ≥3 | ||||
| 1 | Present | 3 (75) | 10 (16.9) | 7.708 (1), 0.005 | 14.70 (1.38 to 156.18) |
| 2 | Absent | 1 (25) | 44 (83.1) | ||
| Age | Sex | Aspiration Volume | Comorbidity | MNC | CFU | |
|---|---|---|---|---|---|---|
| Age | 1 | |||||
| Sex | 0.0728 | 1 | ||||
| Aspiration Volume | 0.4243 | 0.0825 | 1 | |||
| Comorbidity | 0.1922 | 0.1519 | 0.4877 | 1 | ||
| MNC | −0.6819 | −0.1634 | −0.7404 | −0.2872 | 1 | |
| CFU | −0.6938 | −0.1352 | −0.6299 | −0.2992 | 0.95 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthu, S.; Jeyaraman, M.; Narula, A.; Ravi, V.R.; Gandi, A.; Khanna, M.; Maffulli, N.; Gupta, A. Factors Influencing the Yield of Progenitor Cells in Bone Marrow Aspiration Concentrate—A Retrospective Analysis of 58 Patients. Biomedicines 2023, 11, 738. https://doi.org/10.3390/biomedicines11030738
Muthu S, Jeyaraman M, Narula A, Ravi VR, Gandi A, Khanna M, Maffulli N, Gupta A. Factors Influencing the Yield of Progenitor Cells in Bone Marrow Aspiration Concentrate—A Retrospective Analysis of 58 Patients. Biomedicines. 2023; 11(3):738. https://doi.org/10.3390/biomedicines11030738
Chicago/Turabian StyleMuthu, Sathish, Madhan Jeyaraman, Aditya Narula, V. R. Ravi, Avinash Gandi, Manish Khanna, Nicola Maffulli, and Ashim Gupta. 2023. "Factors Influencing the Yield of Progenitor Cells in Bone Marrow Aspiration Concentrate—A Retrospective Analysis of 58 Patients" Biomedicines 11, no. 3: 738. https://doi.org/10.3390/biomedicines11030738









