Lower Extremity Arterial Disease in Type 2 Diabetes Mellitus: Metformin Inhibits Femoral Artery Ultrastructural Alterations as well as Vascular Tissue Levels of AGEs/ET-1 Axis-Mediated Inflammation and Modulation of Vascular iNOS and eNOS Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Determination of Vascular and Blood Levels of AGEs, ET-1, TNF-α, TG, CHOL, LDL-C, HDL-C, HbA1c, and Glucose
2.4. Detection of eNOS and iNOS mRNAs by Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
2.5. Transmission Electron Microscopy
2.6. Statistical Analysis
3. Results
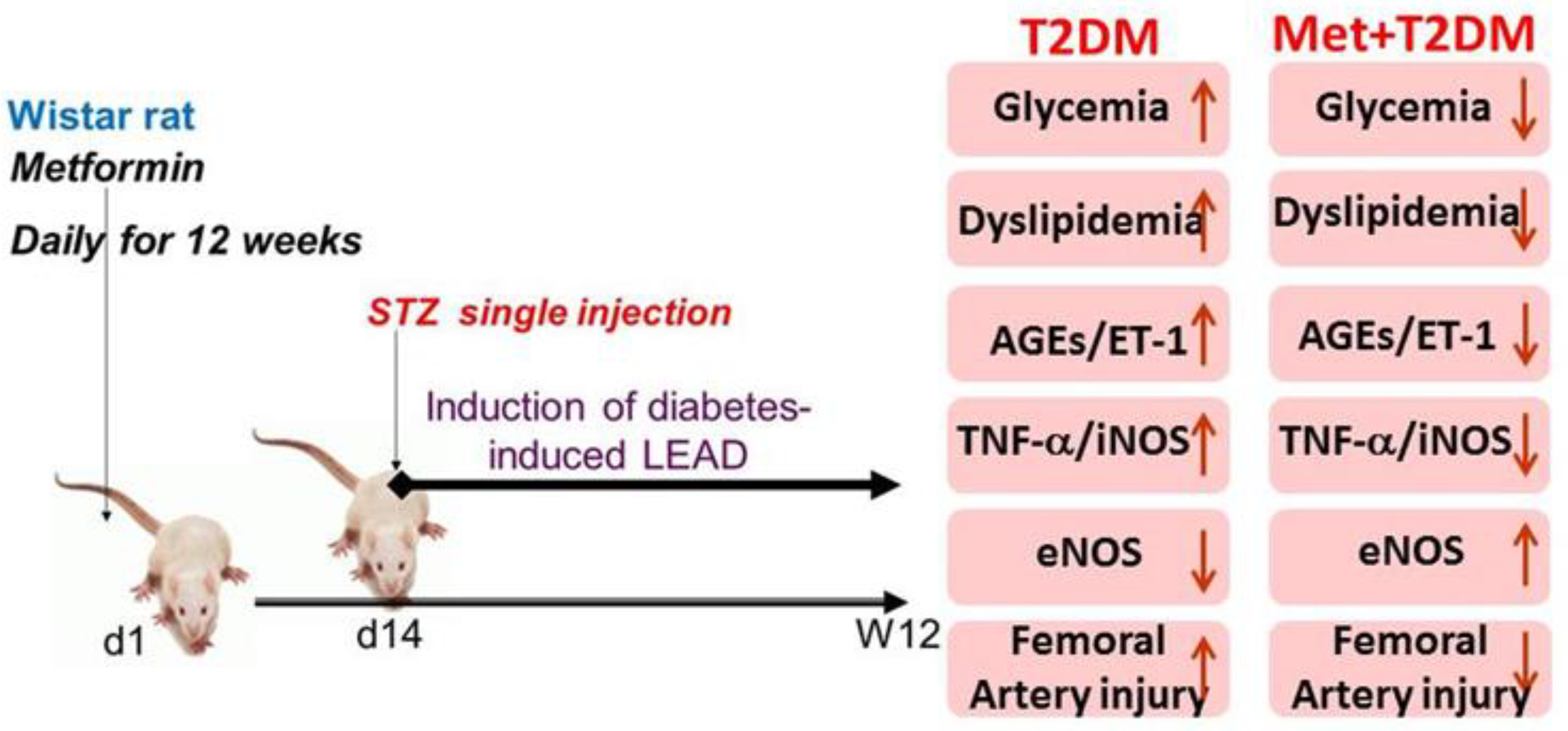
3.1. Induction of Lower Extremity Arterial Disease (LEAD) Secondary to Diabetes
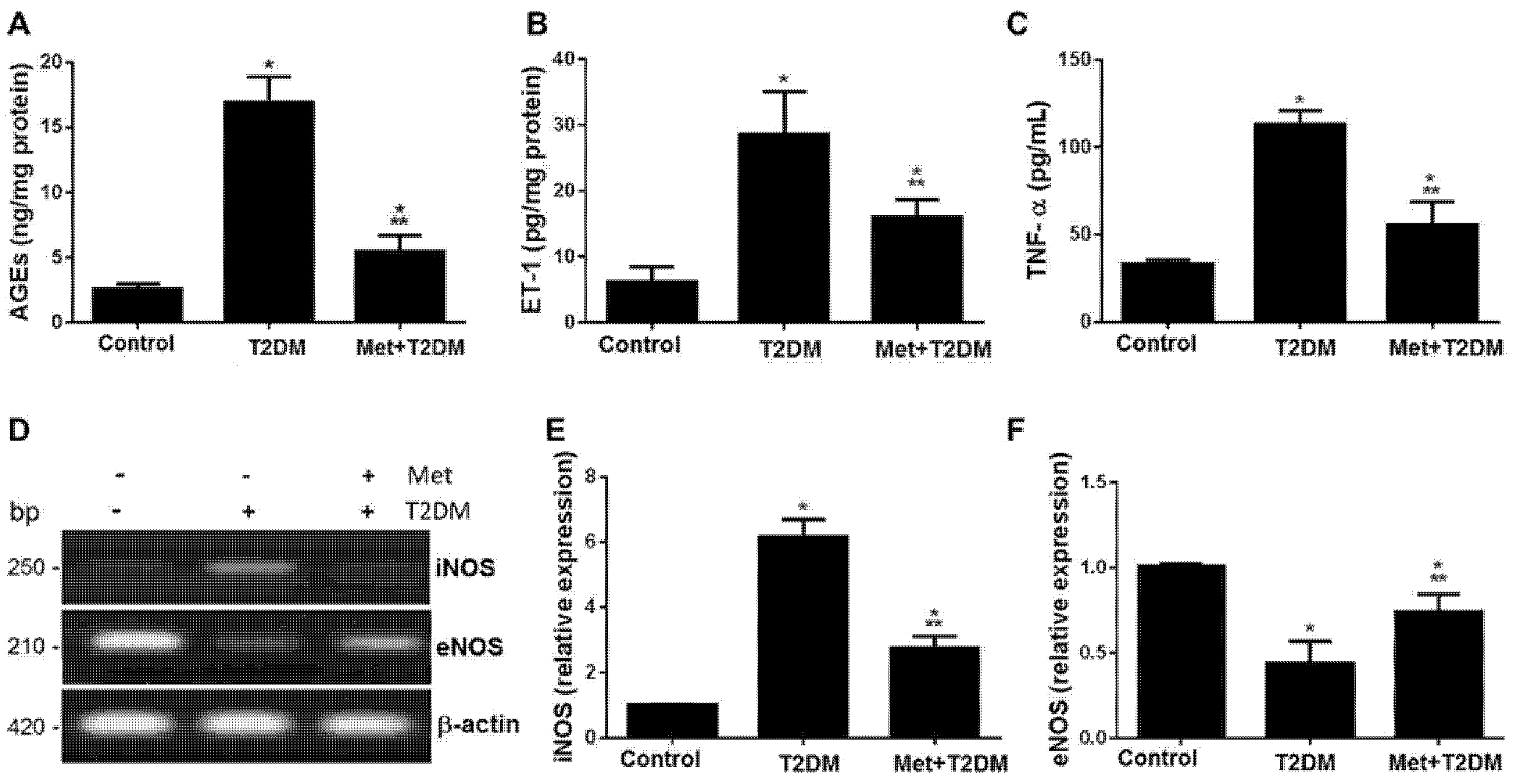
3.2. Metformin Inhibits Vascular and Blood Levels of AGEs, ET-1, iNOS, TNF-α, as well as Dyslipidemia and Glycemia Induced by Diabetes
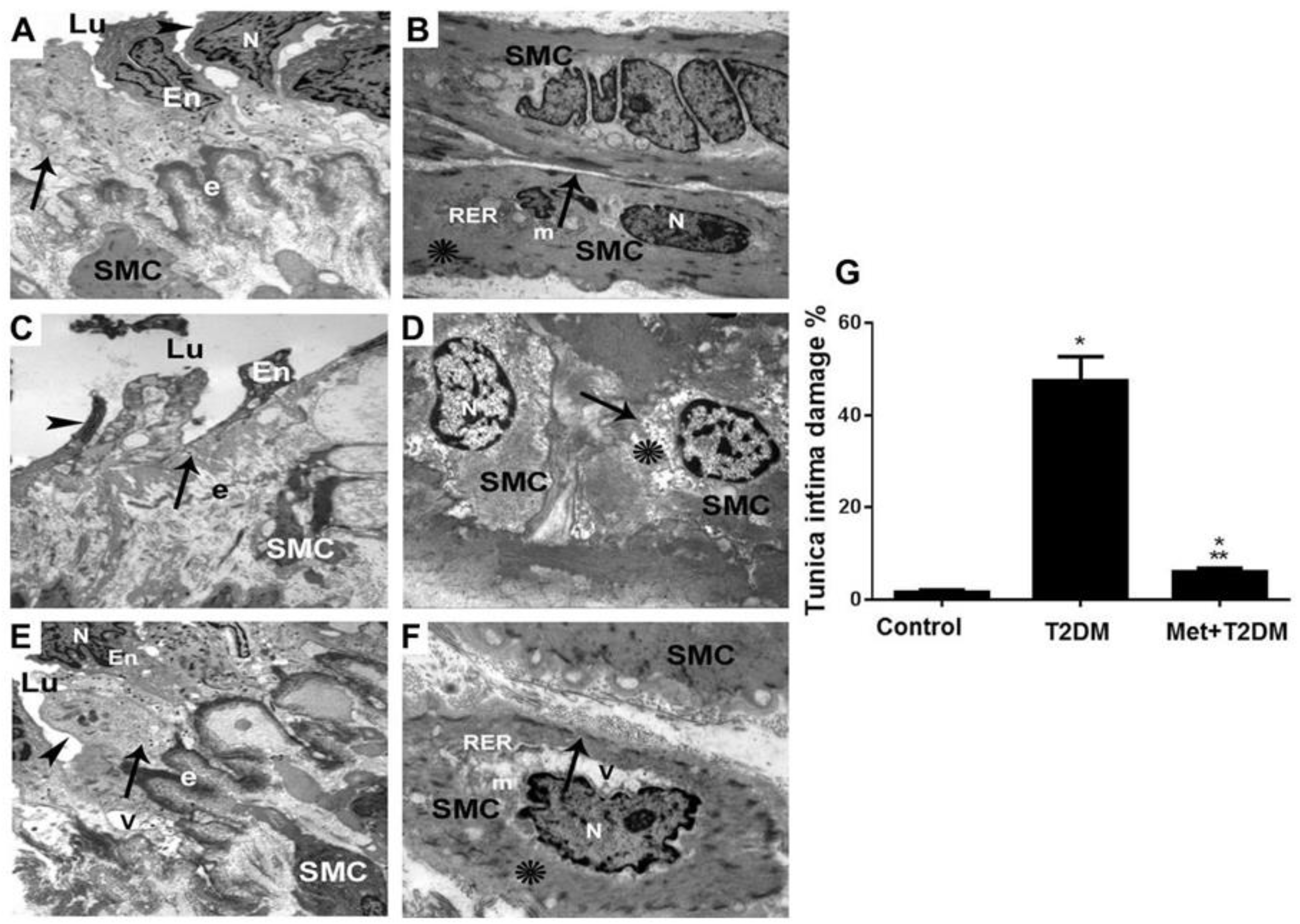
3.3. Metformin Is Associated with the Inhibition of Diabetes-Induced LEAD
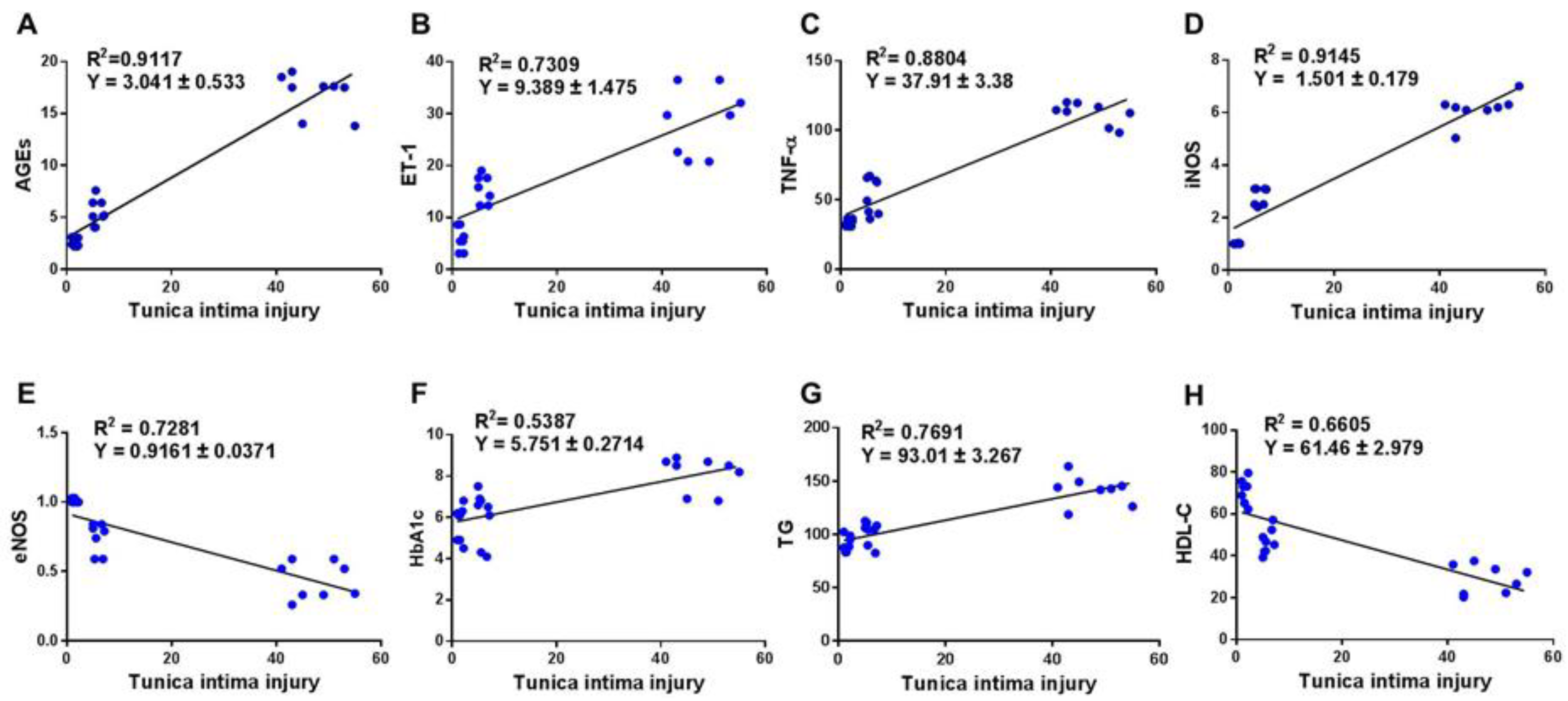
3.4. Correlation between Score of Tunica Intima Injury and AGEs/ET-1/TNF-α/NOS Axis and Biomarkers of Glycemia and Dyslipidemia
4. Discussion
Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Acierno, C.C.A.; Pafundi, P.C.; Nevola, R.; Adinolfi, L.E.; Sasso, F.C. Nonalcoholic fatty liver disease and type 2 diabetes: Patho-physiological mechanisms shared between the two faces of the same coin. Explor. Med. 2020, 1, 287–306. [Google Scholar] [CrossRef]
- Nyenwe, E.A.; Jerkins, T.W.; Umpierrez, G.E.; Kitabchi, A.E. Management of type 2 diabetes: Evolving strategies for the treatment of patients with type 2 diabetes. Metabolism 2011, 60, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Yakaryılmaz, F.D.; Öztürk, Z.A. Treatment of type 2 diabetes mellitus in the elderly. World J. Diabetes 2017, 8, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M. Cardiovascular disease in type 2 diabetes from population to man to mechanisms: The Kelly West Award Lecture 2008. Diabetes Care 2010, 33, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Finucane, M.M.; Lu, Y.; Singh, G.M.; Cowan, M.J.; Paciorek, C.J.; Lin, J.K.; Farzadfar, F.; Khang, Y.-H.; Stevens, G.A.; et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 2011, 378, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.A.; Eid, M.A.; Creager, M.A.; Goodney, P.P. Epidemiology and Risk of Amputation in Patients With Diabetes Mellitus and Peripheral Artery Disease. Arter. Thromb. Vasc. Biol. 2020, 40, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Zahorska-Markiewicz, B. Metabolic effects associated with adipose tissue distribution. Adv. Med. Sci. 2006, 51, 111–114. [Google Scholar] [PubMed]
- Takaguri, A. Elucidation of a New Mechanism of Onset of Insulin Resistance: Effects of Statins and Tumor Necrosis Factor-α on Insulin Signal Transduction. Yakugaku Zasshi 2018, 138, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.F.; Maarouf, A.; Alzamil, N.M.; Momenah, M.A.; Shati, A.A.; Bayoumy, N.M.; Kamar, S.S.; Haidara, M.A.; ShamsEldeen, A.M.; Yassin, H.Z.; et al. Metformin Is Associated with the In-hibition of Renal Artery AT1R/ET-1/iNOS Axis in a Rat Model of Diabetic Nephropathy with Suppression of Inflammation and Oxidative Stress and Kidney Injury. Biomedicines 2022, 10, 1644. [Google Scholar] [CrossRef]
- Wautier, M.-P.; Chappey, O.; Corda, S.; Stern, D.M.; Schmidt, A.M.; Wautier, J.-L. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E685–E694. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. The receptor for advanced glycation endproducts (RAGE) and cardiovascular disease. Expert Rev. Mol. Med. 2009, 11, e9. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Shoji, T.; Yokoyama, H.; Motoyama, K.; Mori, K.; Fukumoto, S.; Emoto, M.; Shoji, T.; Tamei, H.; Matsuki, H.; et al. Plasma Level of Endogenous Secretory RAGE Is Associated With Components of the Metabolic Syndrome and Atherosclerosis. Arter. Thromb. Vasc. Biol. 2005, 25, 2587–2593. [Google Scholar] [CrossRef]
- Kiuchi, K.; Nejima, J.; Takano, T.; Ohta, M.; Hashimoto, H. Increased serum concentrations of advanced glycation end products: A marker of coronary artery disease activity in type 2 diabetic patients. Heart 2001, 85, 87–91. [Google Scholar] [CrossRef]
- Schram, M.T.; Schalkwijk, C.G.; Bootsma, A.H.; Fuller, J.H.; Chaturvedi, N.; Stehouwer, C.D. Advanced glycation end products are associated with pulse pressure in type 1 diabetes: The EURODIAB Prospective Complications Study. Hypertension 2005, 46, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, S.K.; Zhao, J.; Wray, D.W.; Richardson, R.S. Vascular function and endothelin-1: Tipping the balance between vasodi-lation and vasoconstriction. J. Appl. Physiol. 2017, 122, 354–360. [Google Scholar] [CrossRef]
- Wedgwood, S.; Black, S.M. Endothelin-1 decreases endothelial NOS expression and activity through ETA receptor-mediated generation of hydrogen peroxide. Am. J. Physiol. Cell. Mol. Physiol. 2005, 288, L480–L487. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, J.; Kim, J.H.; Lee, D.K.; Park, W.; Park, M.; Kim, S.; Hwang, J.Y.; Won, M.-H.; Choi, Y.K.; et al. Carbon monoxide prevents TNF-α-induced eNOS downregulation by inhibiting NF-κB-responsive miR-155-5p biogenesis. Exp. Mol. Med. 2017, 49, e403. [Google Scholar] [CrossRef]
- Jenkins, A.; Welsh, P.; Petrie, J.R. Metformin, lipids and atherosclerosis prevention. Curr. Opin. Infect. Dis. 2018, 29, 346–353. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, X.; Chen, X. Liraglutide and Metformin alone or combined therapy for type 2 diabetes patients complicated with coronary artery disease. Lipids Health Dis. 2017, 16, 227. [Google Scholar] [CrossRef]
- Dziubak, A.; Wójcicka, G.; Wojtak, A.; Bełtowski, J. Metabolic Effects of Metformin in the Failing Heart. Int. J. Mol. Sci. 2018, 19, 2869. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Pafundi, P.C.; Morgillo, F.; Di Liello, R.; Galiero, R.; Nevola, R.; Marfella, R.; Monaco, L.; Rinaldi, L.; Adinolfi, L.E.; et al. Metformin: An old drug against old age and asso-ciated morbidities. Diabetes Res. Clin. Pract. 2020, 160, 108025. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.; Meszaros, K.; Entes, L.; Claypool, M.; Pinkett, J.; Gadbois, T.; Reaven, G. A new rat model of type 2 diabetes: The fat-fed, streptozotocin-treated rat. Metabolism 2000, 49, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Dallak, M.; Haidara, M.A.; Bin-Jaliah, I.; Eid, R.A.; Amin, S.N.; Latif, N.S.A.; Al-Ani, B. Metformin suppresses aortic ultrastrucural damage and hypertension induced by diabetes: A potential role of advanced glycation end products. Ultrastruct. Pathol. 2019, 43, 190–198. [Google Scholar] [CrossRef]
- Guo, X.; Shi, Y.; Huang, X.; Ye, M.; Xue, G.; Zhang, J. Features Analysis of Lower Extremity Arterial Lesions in 162 Diabetes Patients. J. Diabetes Res. 2013, 2013, 781360. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef]
- Luo, B.; Liu, L.; Tang, L.; Zhang, J.; Ling, Y.; Fallon, M.B. ET-1 and TNF-α in HPS: Analysis in prehepatic portal hypertension and biliary and nonbiliary cirrhosis in rats. Am. J. Physiol. Liver Physiol. 2004, 286, G294–G303. [Google Scholar] [CrossRef]
- Tang, J.; Xie, Q.; Ma, D.; Wang, W. Effects of ET-1 and TNF-α levels on the cardiac function and prognosis in rats with chronic heart failure. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 11004–11010. [Google Scholar]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Rinaldi, L.; Caturano, A.; Vetrano, E.; Aprea, C.; Albanese, G.; Di Martino, A.; Ricozzi, C.; et al. Can Metformin Exert as an Active Drug on En-dothelial Dysfunction in Diabetic Subjects? Biomedicines 2020, 9, 3. [Google Scholar] [CrossRef]
- Salvatore, T.; Galiero, R.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Coviello, F.; Di Martino, A.; Albanese, G.; Marfella, R.; Sardu, C.; et al. Effects of Metformin in Heart Failure: From Path-ophysiological Rationale to Clinical Evidence. Biomolecules 2021, 11, 1834. [Google Scholar] [CrossRef]
- Le, T.D.; Nguyen, N.P.T.; Nguyen, S.T.; Nguyen, H.T.; Tran, H.T.T.; Nguyen, T.H.L.; Nguyen, C.D.; Nguyen, G.T.; Nguyen, X.T.; Nguyen, B.D.; et al. The Association Between Femoral Artery In-tima-Media Thickness and Serum Glucagon-Like Peptide-1 Levels Among Newly Diagnosed Patients with Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2020, 13, 3561–3570. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tang, Y.; Jin, X.; Chen, C.; Lu, Y.; Liu, L.; Shen, C. Metformin Inhibits Advanced Glycation End Products-Induced Inflammatory Response in Murine Macrophages Partly through AMPK Activation and RAGE/NFκB Pathway Suppression. J. Diabetes Res. 2016, 2016, 4847812. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Spina, G.; Kouli, C.; Migdalis, I. Increased endothelin-1 levels in women with polycystic ovary syndrome and the beneficial effect of metformin therapy. J. Clin. Endocrinol. Metab. 2001, 86, 4666–4673. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.H.; Lu, L.; Wang, L.J.; Yan, X.X.; Chen, Q.J.; Zhang, Q.; Zhang, R.Y.; Shen, W.F. RAGE Gene Polymorphisms Are Associated with Circulating Levels of Endogenous Secretory RAGE but Not with Coronary Artery Disease in Chinese Patients with Type 2 Diabetes Mellitus. Arch. Med. Res. 2009, 40, 393–398. [Google Scholar] [CrossRef]
- Salem, M.A.S. Ultrastructural changes in peripheral arteries and nerves in diabetic ischemic lower limbs, by electron micro-scope. Alex. J. Med. 2017, 53, 372–381. [Google Scholar]
- Dawood, A.F.; Alzamil, N.M.; Hewett, P.W.; Momenah, M.A.; Dallak, M.; Kamar, S.S.; Kader, D.H.A.; Yassin, H.; Haidara, M.A.; Maarouf, A.; et al. Metformin Protects against Diabetic Car-diomyopathy: An Association between Desmin-Sarcomere Injury and the iNOS/mTOR/TIMP-1 Fibrosis Axis. Biomedicines 2022, 10, 984. [Google Scholar] [CrossRef]
- Conti, S.; Perico, N.; Novelli, R.; Carrara, C.; Benigni, A.; Remuzzi, G. Early and late scanning electron microscopy findings in diabetic kidney disease. Sci. Rep. 2018, 8, 4909. [Google Scholar] [CrossRef]
- Soma, P.; Pretorius, E. Interplay between ultrastructural findings and atherothrombotic complications in type 2 diabetes mellitus. Cardiovasc. Diabetol. 2015, 14, 96. [Google Scholar] [CrossRef]
- Creager, M.A.; Lüscher, T.F.; Cosentino, F.; Beckman, J.A. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 2003, 108, 1527–1532. [Google Scholar] [CrossRef]
- Nativel, M.; Potier, L.; Alexandre, L.; Baillet-Blanco, L.; Ducasse, E.; Velho, G.; Marre, M.; Roussel, R.; Rigalleau, V.; Mohammedi, K. Lower extremity arterial disease in patients with diabetes: A contemporary narrative review. Cardiovasc. Diabetol. 2018, 17, 138. [Google Scholar] [CrossRef]

| Animal Groups | Glucose (mg/dL) | HbA1c (%) | TG (mg/dL) | CHOL (mg/dL) | LDL-C (mg/dL) | HDL-C (mg/dL) |
|---|---|---|---|---|---|---|
| Control | 120.3 ± 20.6 | 5.8 ± 0.9 | 90.5 ± 6.7 | 135.0 ± 9.1 | 46.0 ± 10.97 | 70.8 ± 6.5 |
| T2DM | 264.0 ± 32.7 a | 8.5 ± 0.3 a | 140.1 ± 13.6 a | 238.7 ± 21.7 a | 182.3 ± 20.56 a | 28.5 ± 6.6 a |
| Met+T2DM | 158.9 ± 18.0 ab | 6.0 ± 1.4 b | 104.1 ± 11.4 ab | 192.3 ± 9.5 ab | 123.8 ± 11.8 ab | 47.8 ± 6.3 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shati, A.A.; Maarouf, A.; Dawood, A.F.; Bayoumy, N.M.; Alqahtani, Y.A.; A. Eid, R.; Alqahtani, S.M.; Abd Ellatif, M.; Al-Ani, B.; Albawardi, A. Lower Extremity Arterial Disease in Type 2 Diabetes Mellitus: Metformin Inhibits Femoral Artery Ultrastructural Alterations as well as Vascular Tissue Levels of AGEs/ET-1 Axis-Mediated Inflammation and Modulation of Vascular iNOS and eNOS Expression. Biomedicines 2023, 11, 361. https://doi.org/10.3390/biomedicines11020361
Shati AA, Maarouf A, Dawood AF, Bayoumy NM, Alqahtani YA, A. Eid R, Alqahtani SM, Abd Ellatif M, Al-Ani B, Albawardi A. Lower Extremity Arterial Disease in Type 2 Diabetes Mellitus: Metformin Inhibits Femoral Artery Ultrastructural Alterations as well as Vascular Tissue Levels of AGEs/ET-1 Axis-Mediated Inflammation and Modulation of Vascular iNOS and eNOS Expression. Biomedicines. 2023; 11(2):361. https://doi.org/10.3390/biomedicines11020361
Chicago/Turabian StyleShati, Ayed A., Amro Maarouf, Amal F. Dawood, Nervana M. Bayoumy, Youssef A. Alqahtani, Refaat A. Eid, Saeed M. Alqahtani, Mohamed Abd Ellatif, Bahjat Al-Ani, and Alia Albawardi. 2023. "Lower Extremity Arterial Disease in Type 2 Diabetes Mellitus: Metformin Inhibits Femoral Artery Ultrastructural Alterations as well as Vascular Tissue Levels of AGEs/ET-1 Axis-Mediated Inflammation and Modulation of Vascular iNOS and eNOS Expression" Biomedicines 11, no. 2: 361. https://doi.org/10.3390/biomedicines11020361
APA StyleShati, A. A., Maarouf, A., Dawood, A. F., Bayoumy, N. M., Alqahtani, Y. A., A. Eid, R., Alqahtani, S. M., Abd Ellatif, M., Al-Ani, B., & Albawardi, A. (2023). Lower Extremity Arterial Disease in Type 2 Diabetes Mellitus: Metformin Inhibits Femoral Artery Ultrastructural Alterations as well as Vascular Tissue Levels of AGEs/ET-1 Axis-Mediated Inflammation and Modulation of Vascular iNOS and eNOS Expression. Biomedicines, 11(2), 361. https://doi.org/10.3390/biomedicines11020361





