Adipose Tissue and Adipose-Tissue-Derived Cell Therapies for the Treatment of the Face and Hands of Patients Suffering from Systemic Sclerosis
Abstract
:1. Introduction
2. Biological Properties of ASCs and AD-SVF
- The proangiogenic effect of ASCs is based on both a powerful paracrine effect [17,18] and a capacity to differentiate into endothelial cells [19]. Indeed, ASCs secrete a large panel of proangiogenic molecules (VEGF, FGF-2 and HGF) that will intervene from the recruitment of endothelial cells to ischemic sites to the stabilization of the neovessels [20]. Furthermore, the crucial role of ASCs in promoting angiogenesis has been demonstrated in several animal models of limb and brain ischemia or myocardial infarction with encouraging results [21,22,23,24].
- The demonstration of the antifibrotic potential of ASCs is based almost exclusively on in vivo studies. The antifibrotic effect of ASCs was assessed in two preclinical models of SSc (bleomycin-induced model and scleroderma graft-versus-host disease model) with positive results [25]. These studies showed that intravenous injections of ASCs reduce inflammatory infiltrates as well as fibrosis markers such as collagen content. Maria et al. also reported the efficacy of ASCs in a hypochlorous acid-induced mouse preclinical model of SSc [26,27]. In this work, the administration of ASCs reduces fibrosis damage from a histological point of view. The authors show a disappearance of the ASCs seven days after the injection, whereas the peak of action was observed at 3 weeks post-treatment, suggesting that the effectiveness of the ASCs does not rely solely on the mechanisms of cell migration and differentiation. In total, ASCs seem to be able to act at each stage of the development of fibrosis [28]: they limit the early inflammation induced by hypochlorous acid, then reduce extracellular matrix deposits during the establishment of fibrosis and finally promote matrix remodelling by matrix metalloproteinases during the resolution of lesions. These results support the plasticity of ASCs and their ability to respond positively to different pathogenic stimuli.
- The immunomodulatory effect of ASCs has been demonstrated with the in vitro test called “mixed lymphocyte reaction”, showing an inhibitory effect on the proliferation of activated immune cells in co-culture models with ASCs [29]. The action of ASCs on the immune system is twofold, based on both a direct interaction with immune cells and a paracrine effect. ASCs interact with all the players of the innate (NK cells, macrophages, dendritic cells) and adaptive immune systems (T and B lymphocytes), resulting in a global modulation of immunity [30].
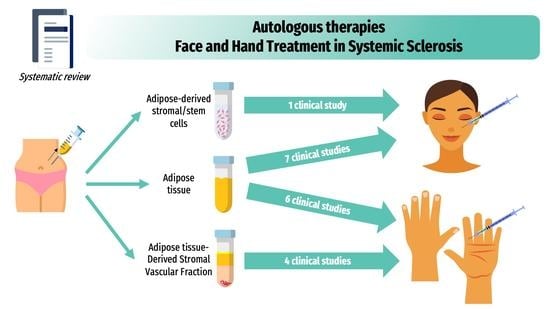
3. Clinical Use of Autologous Fat-Grafting and Adipose Tissue Cell-Based Therapies
4. Main Outcome Measures for the Face and Hands Used in Systemic Sclerosis Clinical Trials
4.1. Main Outcome Measures to Assess Facial Involvement
- (1)
- The mouth-related disability can be assessed using the MHISS (Mouth Handicap in Systemic Sclerosis) scale, which is the first mouth-specific disability outcome measure designed for SSc patients [4]. This scale evaluates three domains: reduced mouth opening, sicca syndrome, and aesthetic concerns, with 12 items (each scoring 0 to 4) and a total score ranging from 0 (no mouth disability) to 48 (severe mouth disability). The MHISS score was used to explain that facial disability contributes up to 36% of the variance of the global HAQ (Health Assessment Questionnaire) score, highlighting its burden on the patient’s life and the need for therapeutics [4].
- (2)
- Skin involvement is usually assessed using the modified Rodnan skin score (mRSS) [39]. This semi-quantitative score rates the severity of skin sclerosis from 0 (normal skin texture and thickness) to 3 (severe thickness with the inability to pinch the skin into a fold). Measurements can be made at several fixed facial and perioral sites making sure the facial muscles are relaxed. To assess skin hardness, the durometer is an objective, effective, and reliable method. Durometer-measured skin hardness correlates well with mRSS and ultrasound-measured skin thickness [40]. The cutometer is also a non-invasive biometrological method for monitoring skin stiffening [41].
- (3)
- Xerostomia can be easily measured using the sugar test (the time it takes for a sugar cube to melt on the tongue without crunching it) and the Xerostomia Inventory Index [42]. The Xerostomia Inventory index is an 11-item summated rating scale that results in a single continuous scale score representing the severity of chronic xerostomia with five levels of answers with responses from “Never” to “Always” (total score range: 11 to 55 extremely dry mouth) [42].
- (4)
- Mouth opening is assessed in centimeters by measuring the distance between the tips of the upper and lower incisors with calipers.
- (5)
- Standard or 3D photographs can also be used for assessing volumizing and aesthetic effects.
4.2. Main Outcome Measures for Hand Assessment
- (1)
- The Cochin Hand Function Scale (CHFS), a functional disability questionnaire about daily activities, has been validated for SSc patients. It includes 18 items with five subscales: kitchen, dressing, hygiene, office, and other. Each question is on a scale of 0 to 5. The total score is obtained by adding the scores of all the items (range 0 to 90). It was shown that hand-functional disability was the major component of global disability, contributing to 75% of global disability (HAQ) in SSc patients [5].
- (2)
- The mRSS applied to hands, which evaluates skin thickening on the dorsal aspect of the hand and the first and second phalanges of the most affected finger (score 0–18) [39],
- (3)
- A visual analog scale (VAS) of hand pain.
- (4)
- For global hand mobility: (a) the Kapandji score assessing opposition of the thumb [43]; (b) the Hand Mobility in Scleroderma index (HAMIS), a nine-item performance-based measure of impairment using different grips and movements to assess finger and wrist mobility or the modified HAMIS with only four items [44]; (c) the lateral range of motion of the fingers measured by the distance between the thumb and index finger (1st corner) and the distances between the four fingers (2nd, 3rd, and 4th corners) upon maximal stretch and (d) finger flexion by measuring the finger-to-palm distance in maximal active flexion.
- (5)
- The grip and pinch strength using a Jamar dynamometer under standard conditions.
- (6)
- Vascular components are assessed by Raynaud’s Condition Score (RCS) and Digital Ulcer (DUs) counts and aspects. The RCS is a daily self-assessment of Raynaud’s phenomenon activity, using a scale of 0 to 10 that incorporates the frequency, duration, severity, and impact of Raynaud’s phenomenon attacks [45]. Outcomes with DUs are not standardized. DU measurements can include the number of DUs, size, pain scale, healing or partial healing of DU (all or a cardinal ulcer). Nailfold capillaroscopy can also be considered as a fairly sensitive and highly specific test for detecting and monitoring scleroderma-spectrum disorders.
- (7)
- The Scleroderma Health Assessment Questionnaire (SHAQ) consists of the HAQ (a self-reported questionnaire in eight domains) and a continuous VAS regarding pain, global patient assessment, vascular, digital ulcers, lung involvement, and gastrointestinal involvement [46].
5. Particular Aspects of the Use of Autologous Fat-Grafting and Adipose-Tissue-Derived Cell Therapies for Patients Suffering from Systemic Sclerosis
- Lipoaspirate processing by centrifugation necessitated placing the fat in a centrifuge and spun at a specified speed for 3 min. This allows the separation of lipoaspirate into three layers: the superior oily layer, the middle fat layer and the inferior aqueous layer consisting of blood and infiltration liquid. In addition to the three layers, a pellet of cells and debris can be seen at the bottom of the syringes. Only the middle layer is used for grafting.
- Lipoaspirate processing by sedimentation (gravity separation) simply necessitates leaving the adipose decantate under gravity for approximately 10 to 15 min. This allows to naturally separate lipoaspirate into three layers, as described above. Similarly, the middle layer of the refined fat tissue is reserved for fat injection.
- Lipoaspirate processing by filtration allows for the removal of fluids, oil and debris faster than decantation and without using centrifugation.
6. Use of Autologous Fat-Grafting or Adipose-Tissue-Derived Cell Therapies for Facial Handicaps in Systemic Sclerosis
6.1. Autologous Fat-Grafting for the Face of Patients Suffering from SSc
6.2. ASC Injections in Facial Skin-Induced Lesions
6.3. Combined Platelet-Rich Plasma and Lipofilling in Facial Skin-Induced Lesions
7. Use of Autologous Fat-Grafting or Adipose-Tissue-Derived Cell Therapy for Hand Handicap in Systemic Sclerosis
7.1. Autologous Adipose Tissue Grafting in the Treatment of Hand Manifestations
7.2. Autologous AD-SVF in the Treatment of Hand Handicap in SSc
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef]
- Van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef] [Green Version]
- Maddali-Bongi, S.; Del Rosso, A.; Mikhaylova, S.; Francini, B.; Branchi, A.; Baccini, M.; Matucci-Cerinic, M. Impact of hand and face disabilities on global disability and quality of life in systemic sclerosis patients. Ann. Rheum. Dis. 2014, 32, S15–S20. [Google Scholar]
- Mouthon, L.; Rannou, F.; Berezne, A.; Pagnoux, C.; Arene, J.; Fois, E.; Cabane, J.; Guillevin, L.; Revel, M.; Fermanian, J.; et al. Development and validation of a scale for mouth handicap in systemic sclerosis: The Mouth Handicap in Systemic Sclerosis scale. Ann. Rheum. Dis. 2007, 66, 1651–1655. [Google Scholar] [CrossRef] [Green Version]
- Rannou, F.; Poiraudeau, S.; Berezné, A.; Baubet, T.; Le-Guern, V.; Cabane, J.; Guillevin, L.; Revel, M.; Fermanian, J.; Mouthon, L. Assessing disability and quality of life in systemic sclerosis: Construct validities of the Cochin Hand Function Scale, Health Assessment Questionnaire (HAQ), Systemic Sclerosis HAQ, and Medical Outcomes Study 36-Item Short Form Health Survey. Arthritis Rheum. 2007, 57, 94–102. [Google Scholar] [CrossRef]
- Coleman, S.R. Structural Fat Grafting: More Than a Permanent Filler. Plast. Reconstr. Surg. 2006, 118, 108S–120S. [Google Scholar] [CrossRef] [Green Version]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Nguyen, A.; Guo, J.; Banyard, D.A.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 1: Current concepts and review of the literature. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 170–179. [Google Scholar] [CrossRef]
- Griffin, M.; Ryan, C.M.; Pathan, O.; Abraham, D.; Denton, C.P.; Butler, P.E.M. Characteristics of human adipose derived stem cells in scleroderma in comparison to sex and age matched normal controls: Implications for regenerative medicine. Stem Cell Res. Ther. 2017, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Daumas, A.; Eraud, J.; Hautier, A.; Sabatier, F.; Magalon, G.; Granel, B. Interests and potentials of adipose tissue in scleroderma. La Rev. De Med. Interne 2013, 34, 763–769. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Abreu, S.C.; Weiss, D.J.; Rocco, P.R.M. Extracellular vesicles derived from mesenchymal stromal cells: A therapeutic option in respiratory diseases? Stem Cell Res. Ther. 2016, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 2016, 7, 125. [Google Scholar] [CrossRef] [Green Version]
- He, A.; Jiang, Y.; Gui, C.; Sun, Y.; Li, J.; Wang, J.-A. The antiapoptotic effect of mesenchymal stem cell transplantation on ischemic myocardium is enhanced by anoxic preconditioning. Can. J. Cardiol. 2009, 25, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.; Ki, S.M.; Park, S.E.; Kim, M.-J.; Hyung, B.; Lee, N.K.; Shim, S.; Choi, B.-O.; Na, D.L.; Lee, J.E.; et al. Anti-apoptotic Effects of Human Wharton’s Jelly-derived Mesenchymal Stem Cells on Skeletal Muscle Cells Mediated via Secretion of XCL1. Mol. Ther. 2016, 24, 1550–1560. [Google Scholar] [CrossRef] [Green Version]
- Imberti, B.; Morigi, M.; Tomasoni, S.; Rota, C.; Corna, D.; Longaretti, L.; Rottoli, D.; Valsecchi, F.; Benigni, A.; Wang, J.; et al. Insulin-Like Growth Factor-1 Sustains Stem Cell–Mediated Renal Repair. J. Am. Soc. Nephrol. 2007, 18, 2921–2928. [Google Scholar] [CrossRef] [Green Version]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef]
- Nakagami, H.; Maeda, K.; Morishita, R.; Iguchi, S.; Nishikawa, T.; Takami, Y.; Kikuchi, Y.; Saito, Y.; Tamai, K.; Ogihara, T.; et al. Novel Autologous Cell Therapy in Ischemic Limb Disease Through Growth Factor Secretion by Cultured Adipose Tissue–Derived Stromal Cells. Arter. Thromb. Vasc. Biol. 2005, 25, 2542–2547. [Google Scholar] [CrossRef]
- Planat-Benard, V.; Silvestre, J.-S.; Cousin, B.; Andreé, M.; Nibbelink, M.; Tamarat, R.; Clergue, M.; Manneville, C.; Saillan-Barreau, C.; Duriez, M.; et al. Plasticity of Human Adipose Lineage Cells Toward Endothelial Cells: Physiological and therapeutic perspectives. Circulation 2004, 109, 656–663. [Google Scholar] [CrossRef]
- Bronckaers, A.; Hilkens, P.; Martens, W.; Gervois, P.; Ratajczak, J.; Struys, T.; Lambrichts, I. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol. Ther. 2014, 143, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Liew, A.; O’Brien, T. Therapeutic potential for mesenchymal stem cell transplantation in critical limb ischemia. Stem Cell Res. Ther. 2012, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Miyahara, Y.; Nagaya, N.; Kataoka, M.; Yanagawa, B.; Tanaka, K.; Hao, H.; Ishino, K.; Ishida, H.; Shimizu, T.; Kangawa, K.; et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat. Med. 2006, 12, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Han, K.-H.; Kim, A.-K.; Kim, D.-I. Therapeutic Potential of Human Mesenchymal Stem Cells for Treating Ischemic Limb Diseases. Int. J. Stem Cells 2016, 9, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.-L.; Liu, K.-D.; Li, F.-C.; Jiang, X.-M.; Jiang, L.; Li, H.-L. Human mesenchymal stem cells increases expression of ?-tubulin and angiopoietin 1 and 2 in focal cerebral ischemia and reperfusion. Curr. Neurovascular Res. 2013, 10, 103–111. [Google Scholar] [CrossRef]
- Okamura, A.; Matsushita, T.; Komuro, A.; Kobayashi, T.; Maeda, S.; Hamaguchi, Y.; Takehara, K. Adipose-derived stromal/stem cells successfully attenuate the fibrosis of scleroderma mouse models. Int. J. Rheum. Dis. 2020, 23, 216–225. [Google Scholar] [CrossRef]
- Maria, A.T.; Toupet, K.; Maumus, M.; Fonteneau, G.; Le Quellec, A.; Jorgensen, C.; Guilpain, P.; Noël, D. Human adipose mesenchymal stem cells as potent anti-fibrosis therapy for systemic sclerosis. J. Autoimmun. 2016, 70, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Maria, A.T.J.; Toupet, K.; Bony, C.; Pirot, N.; Vozenin, M.-C.; Petit, B.; Roger, P.; Batteux, F.; Le Quellec, A.; Jorgensen, C.; et al. Antifibrotic, Antioxidant, and Immunomodulatory Effects of Mesenchymal Stem Cells in HOCl-Induced Systemic Sclerosis. Arthritis Rheumatol. 2016, 68, 1013–1025. [Google Scholar] [CrossRef] [Green Version]
- Maria, A.T.J.; Toupet, K.; Maumus, M.; Rozier, P.; Vozenin, M.-C.; Le Quellec, A.; Jorgensen, C.; Noël, D.; Guilpain, P. Fibrosis Development in HOCl-Induced Systemic Sclerosis: A Multistage Process Hampered by Mesenchymal Stem Cells. Front. Immunol. 2018, 9, 2571. [Google Scholar] [CrossRef] [Green Version]
- Bartholomew, A.; Sturgeon, C.; Siatskas, M.; Ferrer, K.; McIntosh, K.; Patil, S.; Hardy, W.; Devine, S.; Ucker, D.; Deans, R.; et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002, 30, 42–48. [Google Scholar] [CrossRef]
- Nauta, A.J.; Fibbe, W.E. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007, 110, 3499–3506. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, V.M.; Boyd, N.L. The Adipose Stromal Vascular Fraction as a Complex Cellular Source for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2018, 24, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Zakhari, J.S.; Zabonick, J.; Gettler, B.; Williams, S.K. Vasculogenic and angiogenic potential of adipose stromal vascular fraction cell populations in vitro. Vitr. Cell. Dev. Biol.-Anim. 2017, 54, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Nguyen, A.; Banyard, D.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 2: Mechanisms of regenerative action. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 180–188. [Google Scholar] [CrossRef]
- Domergue, S.; Bony, C.; Maumus, M.; Toupet, K.; Frouin, E.; Rigau, V.; Vozenin, M.-C.; Magalon, G.; Jorgensen, C.; Noël, D. Comparison between Stromal Vascular Fraction and Adipose Mesenchymal Stem Cells in Remodeling Hypertrophic Scars. PLoS ONE 2016, 11, e0156161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velier, M.; Mattei, A.; Simoncini, S.; Magalon, J.; Giraudo, L.; Arnaud, L.; Giovanni, A.; Dignat-George, F.; Sabatier, F.; Gugatschka, M.; et al. Paracrine Effects of Adipose-Derived Cellular Therapies in an in Vitro Fibrogenesis Model of Human Vocal Fold Scarring. J. Voice 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Serratrice, N.; Bruzzese, L.; Magalon, J.; Véran, J.; Giraudo, L.; Aboudou, H.; Ould-Ali, D.; Nguyen, P.S.; Bausset, O.; Daumas, A.; et al. New fat-derived products for treating skin-induced lesions of scleroderma in nude mice. Stem Cell Res. Ther. 2014, 5, 138. [Google Scholar] [CrossRef] [Green Version]
- Mattei, A.; Bertrand, B.; Jouve, E.; Blaise, T.; Philandrianos, C.; Grimaud, F.; Giraudo, L.; Aboudou, H.; Dumoulin, C.; Arnaud, L.; et al. Feasibility of First Injection of Autologous Adipose Tissue–Derived Stromal Vascular Fraction in Human Scarred Vocal Folds. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 355–363. [Google Scholar] [CrossRef]
- Wood, R.E.; Lee, P. Analysis of the oral manifestations of systemic sclerosis (scleroderma). Oral Surg. Oral Med. Oral Pathol. 1988, 65, 172–178. [Google Scholar] [CrossRef]
- Clements, P.J.; A Lachenbruch, P.; Seibold, J.R.; Zee, B.C.-Y.; Steen, V.D.; Brennan, P.; Silman, A.J.; Allegar, N.; Varga, J.; Massa, M. Skin thickness score in systemic sclerosis: An assessment of interobserver variability in 3 independent studies. J. Rheumatol. 1993, 20, 1892–1896. [Google Scholar]
- Kissin, E.; Schiller, A.M.; Gelbard, R.B.; Anderson, J.J.; Falanga, V.; Simms, R.; Korn, J.H.; Merkel, P.A. Durometry for the assessment of skin disease in systemic sclerosis. Arthritis Rheum. 2006, 55, 603–609. [Google Scholar] [CrossRef]
- E Piérard, G.; Hermanns-Lê, T.; Pierard-Franchimont, C. Scleroderma: Skin stiffness assessment using the stress–strain relationship under progressive suction. Expert. Opin. Med. Diagn. 2012, 7, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Thomson, W.M.; Chalmers, J.M.; Spencer, A.J.; Williams, S.M. The Xerostomia Inventory: A multi-item approach to measuring dry mouth. Community Dent Health 1999, 16, 12–17. [Google Scholar] [PubMed]
- Kapandji, A. Clinical test of apposition and counter-apposition of the thumb. Ann. Chir. Main 1986, 5, 67–73. [Google Scholar] [CrossRef]
- Sandqvist, G.; Nilsson, J.; Wuttge, D.M.; Hesselstrand, R. Development of a Modified Hand Mobility in Scleroderma (HAMIS) Test and its Potential as an Outcome Measure in Systemic Sclerosis. J. Rheumatol. 2014, 41, 2186–2192. [Google Scholar] [CrossRef] [PubMed]
- Merkel, P.A.; Herlyn, K.; Martin, R.W.; Anderson, J.J.; Mayes, M.D.; Bell, P.; Korn, J.H.; Simms, R.W.; Csuka, M.E.; Medsger, T.A.; et al. Measuring disease activity and functional status in patients with scleroderma and Raynaud’s phenomenon. Arthritis Rheum. 2002, 46, 2410–2420. [Google Scholar] [CrossRef]
- Pope, J. Measures of Systemic Sclerosis (Scleroderma): Health Assessment Questionnaire (HAQ) and Scleroderma HAQ (SHAQ), Physician- and Patient-Rated Global Assessments, Symptom Burden Index (SBI), University of California, Los Angeles, Scleroderma Clinical Trials Consortium Gastrointestinal Scale (UCLA SCTC GIT) 2.0, Baseline Dyspnea Index (BDI) and Transition Dyspnea Index (TDI) (Mahler’s Index), Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR), and Raynaud’s Condition Score (RCS). Arthritis Care Res. 2011, 63 (Suppl. 11), S98–S111. [Google Scholar] [CrossRef]
- Granel, B.; Daumas, A.; Jouve, E.; Harlé, J.-R.; Nguyen, P.-S.; Chabannon, C.; Colavolpe, N.; Reynier, J.-C.; Truillet, R.; Mallet, S.; et al. Safety, tolerability and potential efficacy of injection of autologous adipose-derived stromal vascular fraction in the fingers of patients with systemic sclerosis: An open-label phase I trial. Ann. Rheum. Dis. 2015, 74, 2175–2182. [Google Scholar] [CrossRef] [Green Version]
- Tsekouras, A.; Mantas, D.; Tsilimigras, I.D.; Moris, D.; Kontos, M.; Zografos, C.G. Comparison of the Viability and Yield of Adipose-Derived Stem Cells (ASCs) from Different Donor Areas. Vivo 2017, 31, 1229–1234. [Google Scholar] [CrossRef] [Green Version]
- Sautereau, N.; Daumas, A.; Truillet, R.M.; Jouve, E.M.; Magalon, J.; Veran, J.; Casanova, D.; Frances, Y.; Magalon, G.; Granel, B. Efficacy of Autologous Microfat Graft on Facial Handicap in Systemic Sclerosis Patients. Plast. Reconstr. Surg.-Glob. Open 2016, 4, e660. [Google Scholar] [CrossRef]
- V, D.; Ould-Ali, D.; Hautier, A.; Andrac-Meyer, L.; Bardin, N.; Magalon, G.; Granel, B. Bleomycin-induced Scleroderma in Nude Mice can be Reversed by Injection of Adipose Tissue: Evidence for a Novel Therapeutic Intervention in Systemic Sclerosis. J. Clin. Exp. Dermatol. Res. 2013, 3. [Google Scholar] [CrossRef]
- Alharbi, Z.; Opländer, C.; Almakadi, S.; Fritz, A.; Vogt, M.; Pallua, N. Conventional vs. micro-fat harvesting: How fat harvesting technique affects tissue-engineering approaches using adipose tissue-derived stem/stromal cells. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 1271–1278. [Google Scholar] [CrossRef]
- Del Papa, N.; Di Luca, G.; Sambataro, D.; Zaccara, E.; Maglione, W.; Gabrielli, A.; Fraticelli, P.; Moroncini, G.; Beretta, L.; Santaniello, A.; et al. Regional Implantation of Autologous Adipose Tissue-Derived Cells Induces a Prompt Healing of Long-Lasting Indolent Digital Ulcers in Patients with Systemic Sclerosis. Cell Transplant. 2015, 24, 2297–2305. [Google Scholar] [CrossRef]
- Del Papa, N.; Di Luca, G.; Andracco, R.; Zaccara, E.; Maglione, W.; Pignataro, F.; Minniti, A.; Vitali, C. Regional grafting of autologous adipose tissue is effective in inducing prompt healing of indolent digital ulcers in patients with systemic sclerosis: Results of a monocentric randomized controlled study. Arthritis Res. Ther. 2019, 21, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bank, J.; Fuller, S.M.; Henry, G.I.; Zachary, L.S. Fat Grafting to the Hand in Patients with Raynaud Phenomenon: A Novel Therapeutic Modality. Plast. Reconstr. Surg. 2014, 133, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Cohen, S.R.; Hicok, K.C.; Shanahan, R.K.; Strem, B.M.; Yu, J.C.; Arm, D.M.; Fraser, J.K. Comparison of Three Different Fat Graft Preparation Methods: Gravity Separation, Centrifugation, and Simultaneous Washing with Filtration in a Closed System. Plast. Reconstr. Surg. 2013, 131, 873–880. [Google Scholar] [CrossRef]
- Gerth, D.J.; King, B.; Rabach, L.; Glasgold, R.A.; Glasgold, M.J. Long-Term Volumetric Retention of Autologous Fat Grafting Processed with Closed-Membrane Filtration. Aesthetic Surg. J. 2014, 34, 985–994. [Google Scholar] [CrossRef]
- Condé-Green, A.; de Amorim, N.F.G.; Pitanguy, I. Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: A comparative study. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Magalon, J.; Velier, M.; Simoncini, S.; François, P.; Bertrand, B.; Daumas, A.; Benyamine, A.; Boissier, R.; Arnaud, L.; Lyonnet, L.; et al. Molecular profile and proangiogenic activity of the adipose-derived stromal vascular fraction used as an autologous innovative medicinal product in patients with systemic sclerosis. Ann. Rheum. Dis. 2019, 78, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Capelli, C.; Zaccara, E.; Cipriani, P.; Di Benedetto, P.; Maglione, W.; Andracco, R.; Di Luca, G.; Pignataro, F.; Giacomelli, R.; Introna, M.; et al. Phenotypical and Functional Characteristics of in Vitro-Expanded Adipose-Derived Mesenchymal Stromal Cells from Patients with Systematic Sclerosis. Cell Transplant. 2017, 26, 841–854. [Google Scholar] [CrossRef] [Green Version]
- Velier, M.; Simoncini, S.; Abellan, M.; Francois, P.; Eap, S.; Lagrange, A.; Bertrand, B.; Daumas, A.; Granel, B.; Delorme, B.; et al. Adipose-Derived Stem Cells from Systemic Sclerosis Patients Maintain Pro-Angiogenic and Antifibrotic Paracrine Effects In Vitro. J. Clin. Med. 2019, 8, 1979. [Google Scholar] [CrossRef] [Green Version]
- Di Benedetto, P.; Panzera, N.; Cipriani, P.; Mastroiaco, V.; Tessitore, A.; Liakouli, V.; Ruscitti, P.; Berardicurti, O.; Carubbi, F.; Guggino, G.; et al. Mesenchymal stem cells of Systemic Sclerosis patients, derived from different sources, show a profibrotic microRNA profiling. Sci. Rep. 2019, 9, 7144. [Google Scholar] [CrossRef] [Green Version]
- Rozier, P.; Maria, A.; Goulabchand, R.; Jorgensen, C.; Guilpain, P.; Noël, D. Mesenchymal Stem Cells in Systemic Sclerosis: Allogenic or Autologous Approaches for Therapeutic Use? Front. Immunol. 2018, 9, 2938. [Google Scholar] [CrossRef] [Green Version]
- Gheisari, M.; Ahmadzadeh, A.; Nobari, N.; Iranmanesh, B.; Mozafari, N. Autologous Fat Grafting in the Treatment of Facial Scleroderma. Dermatol. Res. Pract. 2018, 2018, 6568016. [Google Scholar] [CrossRef] [Green Version]
- Almadori, A.; Griffin, M.; Ryan, C.M.; Hunt, D.F.; Hansen, E.; Kumar, R.; Abraham, D.J.; Denton, C.P.; Butler, P.E.M. Stem cell enriched lipotransfer reverses the effects of fibrosis in systemic sclerosis. PLoS ONE 2019, 14, e0218068. [Google Scholar] [CrossRef]
- Strong, A.L.M.; Adidharma, W.; Brown, O.H.; Cederna, P.S. Fat Grafting Subjectively Improves Facial Skin Elasticity and Hand Function of Scleroderma Patients. Plast. Reconstr. Surg.-Glob. Open 2021, 9, e3373. [Google Scholar] [CrossRef]
- Pignatti, M.; Spinella, A.; Cocchiara, E.; Boscaini, G.; Lusetti, I.L.; Citriniti, G.; Lumetti, F.; Setti, G.; Dominici, M.; Salvarani, C.; et al. Autologous Fat Grafting for the Oral and Digital Complications of Systemic Sclerosis: Results of a Prospective Study. Aesthetic Plast. Surg. 2020, 44, 1820–1832. [Google Scholar] [CrossRef]
- Kølle, S.-F.T.; Fischer-Nielsen, A.; Mathiasen, A.B.; Elberg, J.J.; Oliveri, R.S.; Glovinski, P.V.; Kastrup, J.; Kirchhoff, M.; Rasmussen, B.S.; Talman, M.-L.M.; et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: A randomised placebo-controlled trial. Lancet 2013, 382, 1113–1120. [Google Scholar] [CrossRef]
- Onesti, M.G.; Fioramonti, P.; Carella, S.; Fino, P.; Marchese, C.; Scuderi, N. Improvement of Mouth Functional Disability in Systemic Sclerosis Patients over One Year in a Trial of Fat Transplantation versus Adipose-Derived Stromal Cells. Stem Cells Int. 2016, 2016, 2416192. [Google Scholar] [CrossRef] [Green Version]
- Berl, A.; Shir-Az, O.; Perk, N.; Levy, A.; Levy, Y.; Shalom, A. Total Facial Autologous Fat Grafting for Treating Skin Manifestations in Scleroderma. Life 2022, 12, 1997. [Google Scholar] [CrossRef]
- Modarressi, A. Platlet Rich Plasma (PRP) Improves Fat Grafting Outcomes. World J. Plast. Surg. 2013, 2, 6–13. [Google Scholar]
- Cervelli, V.; Gentile, P.; Scioli, M.G.; Grimaldi, M.; Casciani, C.U.; Spagnoli, L.G.; Orlandi, A. Application of Platelet-Rich Plasma in Plastic Surgery: Clinical and In Vitro Evaluation. Tissue Eng. Part C Methods 2009, 15, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Virzì, F.; Bianca, P.; Giammona, A.; Apuzzo, T.; Di Franco, S.; Mangiapane, L.R.; Colorito, M.L.; Catalano, D.; Scavo, E.; Nicotra, A.; et al. Combined platelet-rich plasma and lipofilling treatment provides great improvement in facial skin-induced lesion regeneration for scleroderma patients. Stem Cell Res. Ther. 2017, 8, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blezien, O.; D’Andrea, F.; Nicoletti, G.F.; Ferraro, G.A. Effects of Fat Grafting Containing Stem Cells in Microstomia and Microcheilia Derived from Systemic Sclerosis. Aesthetic Plast. Surg. 2017, 41, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Abellan Lopez, M.; Philandrianos, C.; Daumas, A.; Velier, M.; Arcani, R.; Jouve, E.; Jaloux, C.; Bertrand, B.; Magalon, J.; Dignat-George, F.; et al. Assessing the effect of PRP addition to facial micro-lipofilling for patients suffering from Scleroderma: A prospective routine care analysis. Ann. Chir. Plast Esthet. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Guillaume-Jugnot, P.; Daumas, A.; Magalon, J.; Jouve, E.; Nguyen, P.-S.; Truillet, R.; Mallet, S.; Casanova, D.; Giraudo, L.; Veran, J.; et al. Autologous adipose-derived stromal vascular fraction in patients with systemic sclerosis: 12-month follow-up. Rheumatology 2016, 55, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Daumas, A.; Magalon, J.; Jouve, E.; Truillet, R.; Casanova, D.; Giraudo, L.; Veran, J.; Benyamine, A.; Dignat-George, F.; Sabatier, F.; et al. Long-term follow-up after autologous adipose-derived stromal vascular fraction injection into fingers in systemic sclerosis patients. Curr. Res. Transl. Med. 2017, 65, 40–43. [Google Scholar] [CrossRef]
- Park, Y.; Lee, Y.; Koh, J.; Lee, J.; Min, H.-K.; Kim, M.; Kim, K.; Lee, S.; Rhie, J.; Kim, W.-U.; et al. Clinical Efficacy and Safety of Injection of Stromal Vascular Fraction Derived from Autologous Adipose Tissues in Systemic Sclerosis Patients with Hand Disability: A Proof-Of-Concept Trial. J. Clin. Med. 2020, 9, 3023. [Google Scholar] [CrossRef]
- Khanna, D.; Caldron, P.; Martin, R.W.; Kafaja, S.; Spiera, R.; Shahouri, S.; Shah, A.; Hsu, V.; Ervin, J.; Simms, R.; et al. Adipose-Derived Regenerative Cell Transplantation for the Treatment of Hand Dysfunction in Systemic Sclerosis: A Randomized Clinical Trial. Arthritis Rheumatol. 2022, 74, 1399–1408. [Google Scholar] [CrossRef]
- Daumas, A.; Magalon, J.; Jouve, E.; Casanova, D.; Philandrianos, C.; Abellan Lopez, M.; Mallet, S.; Veran, J.; Auquit-Auckbur, I.; Farge, D.; et al. Adipose tissue-derived stromal vascular fraction for treating hands of patients with systemic sclerosis: A multicentre randomized trial Autologous AD-SVF versus placebo in systemic sclerosis. Rheumatology 2022, 61, 1936–1947. [Google Scholar] [CrossRef]
- Del Bene, M.; Pozzi, M.R.; Rovati, L.; Mazzola, I.; Erba, G.; Bonomi, S. Autologous Fat Grafting for Scleroderma-Induced Digital Ulcers. An Effective Technique in Patients with Systemic Sclerosis. Handchir. Mikrochir. Plast. Chir. 2014, 46, 242–247. [Google Scholar] [CrossRef]
- Tonnard, P.; Verpaele, A.; Peeters, G.; Hamdi, M.; Cornelissen, M.; Declercq, H. Nanofat Grafting: Basic Research and Clinical Applications. Plast. Reconstr. Surg. 2013, 132, 1017–1026. [Google Scholar] [CrossRef]
- Lv, Q.; Li, X.; Qi, Y.; Gu, Y.; Liu, Z.; Ma, G.-E. Volume Retention After Facial Fat Grafting and Relevant Factors: A Systematic Review and Meta-analysis. Aesthetic Plast. Surg. 2020, 45, 506–520. [Google Scholar] [CrossRef]
- Liao, H.-T.; Marra, K.G.; Rubin, J.P. Application of Platelet-Rich Plasma and Platelet-Rich Fibrin in Fat Grafting: Basic Science and Literature Review. Tissue Eng. Part B Rev. 2014, 20, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Sclafani, A.; Azzi, J. Platelet Preparations for Use in Facial Rejuvenation and Wound Healing: A Critical Review of Current Literature. Aesthetic Plast. Surg. 2015, 39, 495–505. [Google Scholar] [CrossRef]
- Willemsen, J.C.N.; Van Dongen, J.; Spiekman, M.; Vermeulen, K.M.; Harmsen, M.; Van Der Lei, B.; Stevens, H.P.J. The Addition of Platelet-Rich Plasma to Facial Lipofilling: A Double-Blind, Placebo-Controlled, Randomized Trial. Plast. Reconstr. Surg. 2018, 141, 331–343. [Google Scholar] [CrossRef]
- Willemsen, J.C.N.; Spiekman, M.; Stevens, H.P.J.; van der Lei, B.; Harmsen, M.C. Platelet-Rich Plasma Influences Expansion and Paracrine Function of Adipose-Derived Stromal Cells in a Dose-Dependent Fashion. Plast. Reconstr. Surg. 2016, 137, 554e–565e. [Google Scholar] [CrossRef]
- Ghazouane, R.; Bertrand, B.; Philandrianos, C.; Veran, J.; Abellan, M.; Francois, P.; Velier, M.; Orneto, C.; Piccerelle, P.; Magalon, J. What About the Rheological Properties of PRP/Microfat Mixtures in Fat Grafting Procedure? Aesthetic Plast. Surg. 2017, 41, 1217–1221. [Google Scholar] [CrossRef]
- Abellan Lopez, M.; Bertrand, B.; Kober, F.; Boucekine, M.; De Fromont De Bouailles, M.; Vogtensperger, M.; Bernard, M.; Casanova, D.; Magalon, J.; Sabatier, F. The Use of Higher Proportions of Platelet-Rich Plasma to Enrich Microfat Has Negative Effects. Plast. Reconstr. Surg. 2020, 145, 130–140. [Google Scholar] [CrossRef]
- Kumar, P.; Kandoi, S.; Misra, R.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar] [CrossRef]
- Műzes, G.; Sipos, F. Mesenchymal Stem Cell-Derived Secretome: A Potential Therapeutic Option for Autoimmune and Immune-Mediated Inflammatory Diseases. Cells 2022, 11, 2300. [Google Scholar] [CrossRef]
- Dabrowska, S.; Andrzejewska, A.; Janowski, M.; Lukomska, B. Immunomodulatory and Regenerative Effects of Mesenchymal Stem Cells and Extracellular Vesicles: Therapeutic Outlook for Inflammatory and Degenerative Diseases. Front. Immunol. 2021, 11, 591065. [Google Scholar] [CrossRef] [PubMed]
- Maumus, M.; Rozier, P.; Boulestreau, J.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Opportunities and Challenges for Clinical Translation. Front. Bioeng. Biotechnol. 2020, 8, 997. [Google Scholar] [CrossRef] [PubMed]




| Publications | Fat Harvesting and Adipose Tissue Processing | Administration | Patient Characteristics | Follow-Up | Clinical Evaluation of Treatment | Side Effects |
|---|---|---|---|---|---|---|
| ADIPOSE TISSUE | ||||||
| Del Papa et al. [52] 2015 Open-label study | Coleman’s technique (12G cannula followed by a centrifugation at 700 g for 3 min) | Up to atotal amount of 16 mL/patient Injections using a 19G cannula | 20 women with dcSSc | 1 and 3 months | (1) Increase in maximum interincisal distance at 3 months * (2) Gradual increase in mouth perimeter measurement from baseline to 3 months * (3) Durometer measurements in perioral areas: perioral skin hardness decreased * (4) Labial capillaroscopy: increased number of capillaries with a more regular microvascular architecture * (5) Histopathological findings: reduction of dermo-epidermal junction flattening, reconstruction of normal ridge pattern and dermal papillae, and increase in microvascular density (6) Patient satisfaction: 16 claimed to be very satisfied and 4 satisfied | Small areas of ecchymosis |
| Sautereau et al. [49] 2016 Open-label study | Microfat harvesting technique (14G cannula) Filtration of the microfat in a closed-circuit system Puregraft® | Median quantity of fat injected was 17 mL (6.2 to 23 mL) Four entry points: 2 along the nasogenian grooves and 2 along the lower lip Injections using a 21G cannula | 14 women 6 lcSSc 8 dcSSc | 3 and 6 months | (1) MHISS score: mean decrease of 7.3 at 3 months and 10.8 at 6 months * (2) Improvement in perioral modified Rodnan skin score * (3) Mouth opening increased with a decrease in the VAS assessing discomfort related to mouth opening limitation * (4) Oral Sicca Syndrome: Xerostomia Inventory score improved at 6 months. The time to melt a sugar cube on the tongue decreased. VAS focusing on handicaps related to dry mouth decreased at 3 and 6 months * (5) Facial Pain: pain induced by palpation of the face muscles and VAS for facial pain decreased * (6) Cutometer measurements of skin elasticity showed no significant change (7) Patient satisfaction: 9 patients (75%) were very satisfied or satisfied, 2 moderately satisfied, and 1 unsatisfied (8) Global disability: no significant change in SSc-HAQ score was observed | Small areas of bruising and local pain Perioral sensitiv emanifestation (n = 1) Trigeminal neuralgia (n = 1) |
| Gheisari et al. [63] 2018 Open-label study | Liposuction with a 3 mm cannula followed by sedimentation of the adipose tissue for 10 min | 15 to 40 mL of fat injected/ patient Injections using an 18G cannula | 16 women 6 lcSSc 10 dcSSc | 3 months | (1) Increased Mouth opening * (2) MHISS score: mean decrease of 6.12 * (3) Reduction of Face Rodnan score * (4) Cutaneous resonance running time showed no significant change (5) Aesthetic effect: 13 patients showed an improvement (6) Patient satisfaction: 10 patients were very satisfied, 2 somewhat satisfied, and 3 unsatisfied | Bruising at the zone of fat harvest |
| Almadori et al. [64] 2019 Open-label study | Coleman’s technique (3 mm cannula followed by a centrifugation 3000 rpm 3 min) | Mean quantity of fat injected was 10.2 mL Injections using a 14G cannula after small skin incisions | 61 women and 1 man 36 lcSSc 26 dcSSc | Mean follow-up after the last treatment 12.41 months (6–53 months) 29 patients received ≤ 2 treatments 33 ≥ 3 treatments | (1) MHISS score: mean decrease of 6.87 * with a greater improvement for patients that received ≥3 treatments (2) Psychological outcomes: Improvement in all psychological measures: VAS, DAS24 score, HADS-A score, HADS-D score, and BFNE scale * (3) Volumetric augmentation outcome: reduction in perioral wrinkling and ridges, improvement in lip volumes and increased vermillion | Bruising, swelling, and tenderness of donor site Superficial wound infection at the recipient site (n = 1) |
| Strong et al. [65] 2021 Open-label study | Coleman’s technique (3 mm cannula followed by a centrifugation 3000 rpm 3 min) | Mean quantity of fat injected was 26.1 mL (range 7.5–71 mL) after small incisions along the lateral commissures | 10 women | Evaluation at 1, 3, 6 and 12 weeks Mean follow-up = 6.2 months | (1) Volumetric augmentation outcome: reduction perioral rhytids, improvement in soft tissue volume (2) Patient satisfaction: subjective improvement of skin quality | Minor adverse events (AEs): pain, bruising and swelling at the donor site and minor bruising at the recipient site |
| Pignatti et al. [66] 2020 Open-label study | Coleman’s technique (3 mm cannula followed by a centrifugation at 3000 rpm for 3 min) | A total of 16 mL divided in 8 sites around the mouth into subcutaneous and submucosal planes with retrograde technique | In the perioral area: 10/17 received 2 treatments and 7/17 received 3 treatments at 6 months interval | From 12 to 18 months after the first procedure | (1) Patient satisfaction: improvement of perioral skin tension (2) Oral Sicca Syndrome: subjective amelioration of xerostomia (3) No significant change of the mouth opening distance (4) MHISS score: the trend towards improvement | Small ecchymotic areas around the mouth |
| Berl et al. [69] 2022 Open-label study | Liposuction with a 3 or 4 mm cannula followed by sedimentation of the adipose tissue for 10 min Transformation of macrografts to micrografts by transferring fat between syringues via a stopcock valve | Mean volume injected was 72 mL (range 20–180 mL) Injection using ColemanTM micro infiltration cannulas | 17 patients (16 dcSSc,1 lcSSc), 9 had multiple surgeries (MS): 5 had 2MS, 2 had 3MS, 2 had 4MS | 1 week, 1 and 3 months | (1) Mouth opening distance improved at 3 months (mean of 0.85 cm range 0.2–2.5) (2) Overall satisfaction (1–7 scale) was high (mean 5.2) and 88% of patients would be willing to repeat the procedure | Postoperative hematoma (n = 3) and postoperative pain (n = 10) |
| ADIPOSE-DERIVED STROMAL/STEM CELLS | ||||||
| Onesti et al. [68] 2016 Open-label study | Liposuction with a one-hole 3 mm cannula followed by sedimentation of the adipose tissue for 15 min | Two injections of treatment at 3-month intervals ADSCs group: injection in perioral area of 32 × 105 cells in 4 mL of hyaluronic acid with a 30G ½ needle Fat grafting group: injection of 16 mL in the perioral region at sub-cutaneous level with a 2 mm cannula | 10 patients ASCs group: 5 patients Fat grafting group: 5 patients | 1 week, 1 month and 1 year | (1) Increase in maximum interincisal distance but no significant difference between groups (2) MHISS score: improvement but no statistically significant difference between groups (3) VAS scale: improvement but no significant difference between groups (4) Patient satisfaction: in the ASCs group, 20% of the patients were satisfied, 80% very satisfied vs. in the fat grafting group, 80% of patients were satisfied and 20% very satisfied | No information about safety |
| Publications | Fat Harvesting and Adipose Tissue Processing | Administration | Patient Characteristics | Follow-Up | Clinical Evaluation of Treatment | Side Effects |
|---|---|---|---|---|---|---|
| ADIPOSE TISSUE | ||||||
| Bank et al. [54] 2014 Open-label study | Coleman’s technique and sedimentation | Median quantity of fat injected was 26 mL (11 to 30 mL) Injections using an 18G cannula after an incision made with an 18G needle | 13 patients with Raynaud phenomenon including 9 patients with SSc | Average follow-up of 17.9 months | (1) Raynaud phenomenon pain: improvement in pain * (2) Cold attacks: improvement in number, duration and severity * (3) Twelve hands (57%) had at least one DU before treatment compared to only 5 (24%) after treatment * (4) Increased blood flow per imaging noted in 5 of 11 hands tested | Recurrence of ulceration with increased severity of Raynaud’s attacks (n = 1), Cellulitis (n = 1), Transient digital numbness (n = 1) |
| Bene et al. [80] 2014 Open-label study | Coleman’s technique (3 mm cannula followed by a centrifugation 3000 at rpm for 3 min) | 2–3 mL of fat injected/affected fingers, at the border of the larger ulcers with different depths, or at the finger base for the smaller digital ulcers, with blunt cannula | 9 patients for a total of 15 digital ulcers, refractory to pharmacological treatment and wound care. 5 lcSSc 4 dcSSc | From 6 to 24 months Clinical outcomes were evaluated at 3 months | (1) DU Healing: 10/15 DU, 5/9 patients (2) Ulcer reduction >50%: 2 (3) Pain reduction: 7/9 patients | No ulcer recurrence in the same location No adverse reactions at the sites of injection |
| Del Papa et al. [52] 2015 Open-label study | Coleman’s technique (18G cannula followed by centrifugation at 920 g for 3 min) | 0.5 to 1 mL of fat injected at the base of the affected finger under local anesthesia, using an 18G cannula A small skin incision was made at the site of injection | 15 women with only one active DU more than 5 months old prior to enrollment 8 lcSSc 7 dcSSc | 1, 3, and 6 months | (1) Time to healing of cardinal ulcers: mean time was 4.23 weeks (range 2–7), no new DU appeared in any of the treated patients (2) DU pain severity (VAS): reduction in pain intensity * The amounts of analgesic drugs drastically decreased after 1 week and none of the patients were still taking analgesics after 1 month (3) Nailfold Video Capillaroscopy: an increase in the capillary count was observed * | No |
| Del Papa et al. [53] 2019 Monocentric randomized controlled study | Coleman’s technique (18G cannula followed by a centrifugation of 920 g for 3 min) Sham procedure consisting in false liposuction followed by the injection of saline solution at the base of the affected finger. | 0.5 to 1 mL of adipose tissue injected at the base of the finger with the DU, using an 18G cannula Sham procedure consisting of injection of saline solution | 38 patients with only one active ischemic DU lasting for at least 6 weeks 25 patients (23 women, 16 dcSSc) treated with autologous adipose tissue and 13 women (7 dcSSc) received saline solution | 4 and 8 weeks | (1) Prevalence of patients in whom DU healing was observed within 8 weeks: 92.0% of patients actively treated achieved DU healing vs. only 1 of the 13 patients who received saline solution * (2) DU pain severity (VAS): reduction in pain intensity in actively treated patients * (3) Nailfold Video Capillaroscopy: an increase in capillary numbers in the affected finger was observed in the actively treated patients * | No |
| Strong et al. [65] 2021 Monocentric open-label study | Coleman’s technique (3 mm cannula followed by a centrifugation at 3000 rpm for 3 min) | Mean volume of 53.2 (range 30–78 mL) of fat injected into the bilateral hands | 10 women 5 patients received fat grafting to the hands | Evaluation at 1, 3, 6 and 12 weeks Mean follow-up = 6.2 months | (1) Skin elasticity: significant subjective improvement (2) Hand volume: significant subjective improvement (3) Some improvement in mobility | Minor AEs: pain, bruising and swelling at the donor site and minor bruising at the recipient site |
| Pignatti et al. [66] 2020 Monocentric open-label study | Coleman’s technique (12G cannula followed by a centrifugation at 3000 rpm for 3 min) Procedure repeated every 6 months for 2 or 3 times. | 0.5 to 1 mL at the base on each side of each finger, for a total up to 10 mL/hand after skin access created by 19G needle | 12/25 patients received 3 injections in the fingers at 6-month intervals | From 12 to 18 months after the first procedure | (1) Complete healing of DU in 8/9 patients (2) Significant improvement of hand tension (3) Differences in clinometric measures not significant (4) RCS: trend towards improvement (5) mRSS: not significantly improved (6) No significant decrease in pain (SF-MPQ) and affective descriptors | Temporary oedema and paresthesia in the hands of 2 patients |
| ADIPOSE DERIVED–STROMAL VASCULAR FRACTION | ||||||
| Granel et al. [47] 2015 Monocentric open-label study | Coleman’s technique (3 mm cannula) AD-SVF processed with Celution 800/CRS system (Cytori Therapeutics) | Injection with a 25G cannula of 0.5 mL of AD-SVF into each lateral side of each digit, both hands. | 12 patients with a hand disability (CHFS > 20/90) | 6 months | (1) CHFS: 47.4% and 56.0% decrease at M2 and M6 in comparison to baseline was observed (p < 0.001) (2) RCS: improvement * (3) Hand pain (VAS): improvement * (4) SHAQ: improvement * (5) Numbers of DU: decrease in total number of DU (6) Circumference of the fingers, MRSS focused on hands et global MRSS decreased from baseline * (7) Capillaroscopy evaluation: changes observed (8) Hand strength and mobility: improvement | 4 minor AEs potentially related to the procedure: abdominal bruises (n = 2), transient paresthesia (n = 1), and located pain (n = 1) |
| Park et al. [77] 2020 Monocentric open-label study | Suction-assisted Coleman technique AD-SVF processed in a closed system SmartX® kit (DongKoo Bio & Pharma Co., Ltd.) | Injection of 0.6 mL/finger, at the proximal in 3 points from the metacarpophalangeal joint to the tip of the finger. Digital nerve block method was used | 18 patients 8 dcSSc 10 lcSSc | 2, 6, 12 and 24 weeks | (1) mRSS: significant improvement during the follow-up * (2) mRSS applied to hands: significant improvement at 6, 12 and 24 weeks * (3) RCS: slight improvement (4) CHFS: increase tendency (5) Hand oedema: significant decrease in mean circumference of both hands at 24 weeks * (6) DU: 31.6% were healed at 24 weeks (7) Disease-related QOL: improvement (8) Kapandji score, Hand pain (VAS), capillaroscopy evaluation: no change | 5 minor AEs: transient paresthesia (n = 1), dropout due to dizziness after lidocaine injection (n = 1), transient pallor in fingers (n = 3), |
| Daumas et al. [79] 2022 Double-blind, multicentric, phase II randomized controlled study | Coleman’s technique (3 mm cannula) AD-SVF processed with Celution 800/CRS system (Cytori Therapeutics) | Injection with a 25G cannula of 0.5 mL of AD-SVF into each lateral side of each digit, both hands. | 40 patients AD-SVF group: 20 (10 dcSSc and 10 lcSSc) Placebo group: 20 were (5 dcSSc and 15 lcSSc). | 7 days, 1, 3 and 6 months | (1) Improvement of CHFS score at 3 and 6 months in both groups but no difference between groups (2) Improvement of the SHAQ, hand pain severity (VAS), mRSS applied to hands and Raynaud’s phenomenon severity at 3 and 6 months without significance between groups (3) Mean number of healed ulcers/patients was lower for the AD-SVF versus placebo groups | Severe AEs related to the worsening of the underlying SSc (AD-SVF group: n = 7) Severe AE attributed to the surgical procedure (placebo group: n = 1) |
| Khanna et al. [78] 2022 Double-blind, multicentric, phase II randomized controlled study | Liposuction using manual aspiration (no information about the cannula type) AD-SVF processed with Celution 800/CRS system (Cytori Therapeutics) | Placebo: Ringer’s lactate + 0.1–0.2 mL of the patient’s blood Treatment: 0.5 mL containing 4 million viable nucleated cells/finger into each lateral side of each digit, both hands using a 25G needle. | 88 patients AD-SVF group: 48 patients (32 dcSSc and 16 lcSSc) Placebo group: 40 patients (19 dcSSc and 21 lcSSc) | 48 weeks | (1) CHFS: numerically higher for the AD-SVF group vs placebo group (2) HAQ: greater improvement for dcSSc group (3) RCS: greater improvement for dcSSc group | Severe AE in the AD-SVF group: pneumonia (n = 2) AE in the placebo group: anemia, hypotension, joint effusion, angina, upper GI tract hemorrhage (n = 8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coulange Zavarro, A.; Velier, M.; Arcani, R.; Abellan Lopez, M.; Simoncini, S.; Benyamine, A.; Gomes De Pinho, Q.; Coatmeur, R.; Wang, J.; Xia, J.; et al. Adipose Tissue and Adipose-Tissue-Derived Cell Therapies for the Treatment of the Face and Hands of Patients Suffering from Systemic Sclerosis. Biomedicines 2023, 11, 348. https://doi.org/10.3390/biomedicines11020348
Coulange Zavarro A, Velier M, Arcani R, Abellan Lopez M, Simoncini S, Benyamine A, Gomes De Pinho Q, Coatmeur R, Wang J, Xia J, et al. Adipose Tissue and Adipose-Tissue-Derived Cell Therapies for the Treatment of the Face and Hands of Patients Suffering from Systemic Sclerosis. Biomedicines. 2023; 11(2):348. https://doi.org/10.3390/biomedicines11020348
Chicago/Turabian StyleCoulange Zavarro, Anouck, Mélanie Velier, Robin Arcani, Maxime Abellan Lopez, Stéphanie Simoncini, Audrey Benyamine, Quentin Gomes De Pinho, Raphael Coatmeur, Jiucun Wang, Jingjing Xia, and et al. 2023. "Adipose Tissue and Adipose-Tissue-Derived Cell Therapies for the Treatment of the Face and Hands of Patients Suffering from Systemic Sclerosis" Biomedicines 11, no. 2: 348. https://doi.org/10.3390/biomedicines11020348