Nanoparticle Formulations of Antioxidants for the Management of Oxidative Stress in Stroke: A Review
Abstract
:1. Introduction
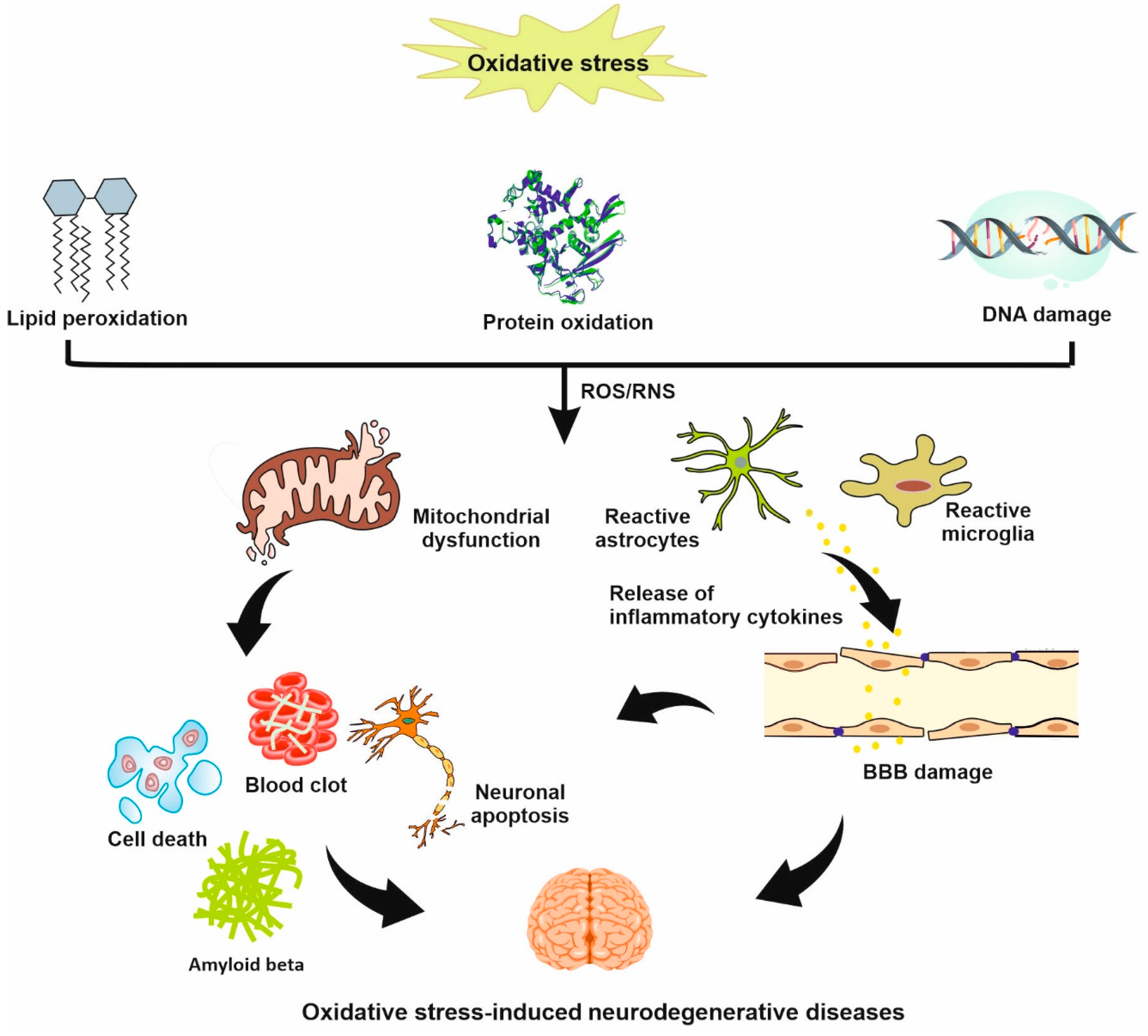
2. Oxidative Stress in Stroke
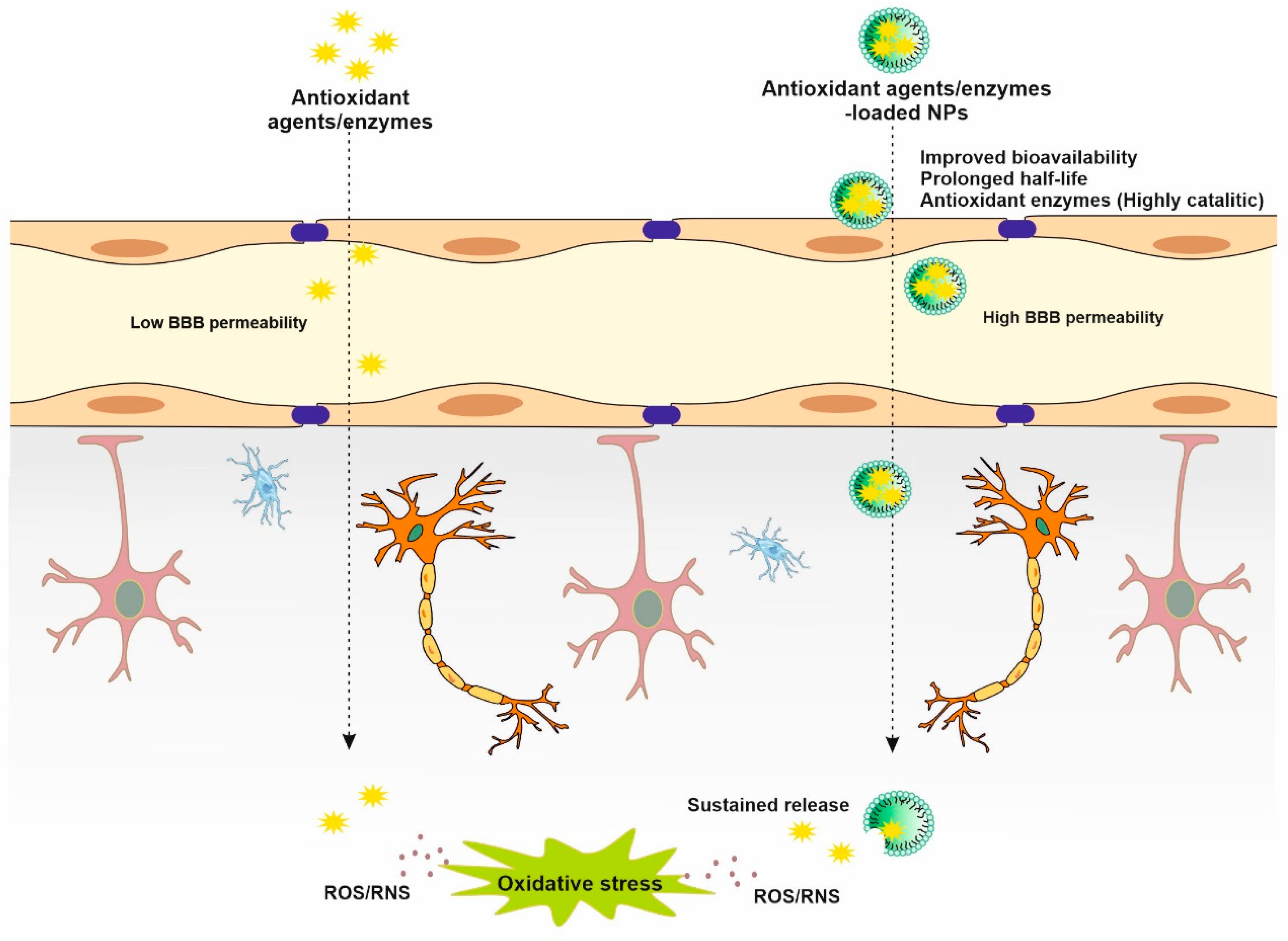
3. Antioxidant NPs in Stroke
3.1. Lipid-Based NPs
3.2. Polymer-Based NPs
3.3. Polysaccharide-Based NPs
3.4. Carbon-Based NPs
3.5. Metal NPs
3.6. Ceramic NPs
3.7. Other NPs
4. Safety Concern of NPs
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Peng, B.; Chen, Z.; Yu, J.; Deng, G.; Bao, Y.; Ma, C.; Du, F.; Sheu, W.C.; Kimberly, W.T.; et al. Brain-targeting, acid-responsive antioxidant nanoparticles for stroke treatment and drug delivery. Bioact. Mater. 2022, 16, 57–65. [Google Scholar] [CrossRef]
- Yu, W.; Xuan, C.; Liu, B.; Zhou, L.; Yin, N.; Gong, E.; Zhang, Z.; Li, Y.; Zhang, K.; Shi, J. Carrier-free programmed spherical nucleic acid for effective ischemic stroke therapy via self-delivery antisense oligonucleotide. Nano Res. 2022, 16, 735–745. [Google Scholar] [CrossRef]
- Deng, G.; Ma, C.; Zhao, H.; Zhang, S.; Liu, J.; Liu, F.; Chen, Z.; Chen, A.T.; Yang, X.; Avery, J.; et al. Anti-edema and antioxidant combination therapy for ischemic stroke via glyburide-loaded betulinic acid nanoparticles. Theranostics 2019, 9, 6991–7002. [Google Scholar] [CrossRef]
- Shahi, S.; Farhoudi, M.; Dizaj, S.M.; Sharifi, S.; Sadigh-Eteghad, S.; Goh, K.W.; Ming, L.C.; Dhaliwal, J.S.; Salatin, S. The Link between Stroke Risk and Orodental Status—A Comprehensive Review. J. Clin. Med. 2022, 11, 5854. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Qi, Z.; Guo, X.; Liu, X.; Shi, W.; Liu, Y.; Du, L. The enhanced protective effects of salvianic acid A: A functionalized nanoparticles against ischemic stroke through increasing the permeability of the blood-brain barrier. Nano Res. 2020, 13, 2791–2802. [Google Scholar] [CrossRef]
- Bao, Q.; Hu, P.; Xu, Y.; Cheng, T.; Wei, C.; Pan, L.; Shi, J. Simultaneous blood–brain barrier crossing and protection for stroke treatment based on edaravone-loaded ceria nanoparticles. ACS Nano 2018, 12, 6794–6805. [Google Scholar] [CrossRef]
- Wu, H.; Peng, B.; Mohammed, F.S.; Gao, X.; Qin, Z.; Sheth, K.N.; Zhou, J.; Jiang, Z. Brain Targeting, Antioxidant Polymeric Nanoparticles for Stroke Drug Delivery and Therapy. Small 2022, 18, e2107126. [Google Scholar] [CrossRef]
- Shi, J.; Yu, W.; Xu, L.; Yin, N.; Liu, W.; Zhang, K.; Liu, J.; Zhang, Z. Bioinspired nanosponge for salvaging ischemic stroke via free radical scavenging and self-adapted oxygen regulating. Nano Lett. 2019, 20, 780–789. [Google Scholar]
- Zhang, C.Y.; Dong, X.; Gao, J.; Lin, W.; Liu, Z.; Wang, Z. Nanoparticle-induced neutrophil apoptosis increases survival in sepsis and alleviates neurological damage in stroke. Sci. Adv. 2019, 5, eaax7964. [Google Scholar] [CrossRef]
- Farhoudi, M.; Sadigh-Eteghad, S.; Mahmoudi, J.; Farjami, A.; Mahmoudian, M.; Salatin, S. The Therapeutic Benefits of Intravenously Administrated Nanoparticles in Stroke and Age-related Neurodegenerative Diseases. Curr. Pharm. Des. 2022, 28, 1985–2000. [Google Scholar] [CrossRef]
- Lv, W.; Liu, Y.; Li, S.; Lv, L.; Lu, H.; Xin, H. Advances of nano drug delivery system for the theranostics of ischemic stroke. J. Nanobiotechnology 2022, 20, 248. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, J.; Huang, S.; Siaw-Debrah, F.; Nyanzu, M.; Zhuge, Q. Polyacrylic acid-coated nanoparticles loaded with recombinant tissue plasminogen activator for the treatment of mice with ischemic stroke. Biochem. Biophys. Res. Commun. 2019, 516, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Essig, F.; Kollikowski, A.M.; Müllges, W.; Stoll, G.; Haeusler, K.G.; Schuhmann, M.K.; Pham, M. Local Cerebral Recombinant Tissue Plasminogen Activator Concentrations During Acute Stroke. JAMA Neurol. 2021, 78, 615–617. [Google Scholar] [CrossRef]
- Gainey, J.; Brecthtel, L.; Blum, B.; Keels, A.; Madeline, L.; Lowther, E.; Nathaniel, T. Functional Outcome Measures of Recombinant Tissue Plasminogen Activator–Treated Stroke Patients in the Telestroke Technology. J. Exp. Neurosci. 2018, 12, 1179069518793412. [Google Scholar] [CrossRef]
- Maghsoodi, M.; Rahmani, M.; Ghavimi, H.; Montazam, S.H.; Soltani, S.; Alami, M.; Salatin, S.; Jelvehgari, M. Fast dissolving sublingual films containing sumatriptan alone and combined with methoclopramide: Evaluation in vitro drug release and mucosal permeation. Pharm Sci. 2016, 22, 153–163. [Google Scholar] [CrossRef]
- Chittora, R.; Jain, S. Application of Nanotechnology in Stroke Recovery. In Regenerative Therapies in Ischemic Stroke Recovery; Raza, S.S., Ed.; Springer Nature: Singapore, 2022; pp. 31–51. [Google Scholar] [CrossRef]
- Farhoudi, M.; Sadigh-Eteghad, S.; Farjami, A.; Salatin, S. Nanoparticle and Stem Cell Combination Therapy for the Management of Stroke. Curr. Pharm. Des. 2022, 29, 15–29. [Google Scholar]
- Diez-Iriepa, D.; Knez, D.; Gobec, S.; Iriepa, I.; de Los Ríos, C.; Bravo, I.; López-Muñoz, F.; Marco-Contelles, J.; Hadjipavlou-Litina, D. Polyfunctionalized α-Phenyl-tert-butyl (benzyl) nitrones: Multifunctional Antioxidants for Stroke Treatment. Antioxidants 2022, 11, 1735. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Cheng, C.; Li, G.; Xie, J.; Shen, M.; Chen, Q.; Li, W.; He, W.; Qiu, P. Design, synthesis and biological evaluation of chalcone analogues with novel dual antioxidant mechanisms as potential anti-ischemic stroke agents. Acta Pharm. Sin. B 2019, 9, 335–350. [Google Scholar] [CrossRef]
- Khaksar, S.; Bigdeli, M.; Samiee, A.; Shirazi-Zand, Z. Antioxidant and anti-apoptotic effects of cannabidiol in model of ischemic stroke in rats. Brain Res. Bull. 2022, 180, 118–130. [Google Scholar] [CrossRef]
- Elsayed, W.M.; Abdel-Gawad, E.-H.A.; Mesallam, D.I.A.; El-Serafy, T.S. The relationship between oxidative stress and acute ischemic stroke severity and functional outcome. Egypt. J. Neurol. Psychiatry Neurosurg. 2020, 56, 74. [Google Scholar] [CrossRef]
- Manzanero, S.; Santro, T.; Arumugam, T.V. Neuronal oxidative stress in acute ischemic stroke: Sources and contribution to cell injury. Neurochem. Int. 2013, 62, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Gerhke, S.A.; Shibli, J.A.; Salles, M.B. Potential of the use of an antioxidant compound to promote peripheral nerve regeneration after injury. Neural Regen. Res. 2015, 10, 1063. [Google Scholar]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003. [Google Scholar]
- Ashok, A.; Andrabi, S.S.; Mansoor, S.; Kuang, Y.; Kwon, B.K.; Labhasetwar, V. Antioxidant Therapy in Oxidative Stress-Induced Neurodegenerative Diseases: Role of Nanoparticle-Based Drug Delivery Systems in Clinical Translation. Antioxidants 2022, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Dogan, O.; Kisa, U.; Erdemoglu, A.K.; Kacmaz, M.; Caglayan, O.; Kurku, H. Oxidative and nitrosative stress in patients with ischemic stroke. J. Lab. Med. 2018, 42, 195–200. [Google Scholar] [CrossRef]
- Hu, W.; Qiang, T.; Chai, L.; Liang, T.; Ren, L.; Cheng, F.; Li, C.; James, T.D. Simultaneous tracking of autophagy and oxidative stress during stroke with an ICT-TBET integrated ratiometric two-photon platform. Chem. Sci. 2022, 13, 5363–5373. [Google Scholar] [CrossRef] [PubMed]
- Chioua, M.; Martínez-Alonso, E.; Gonzalo-Gobernado, R.; Ayuso, M.I.; Escobar-Peso, A.; Infantes, L.; Hadjipavlou-Litina, D.; Montoya, J.J.; Montaner, J.; Alcázar, A.; et al. New Quinolylnitrones for Stroke Therapy: Antioxidant and Neuroprotective (Z)-N-tert-Butyl-1-(2-chloro-6-methoxyquinolin-3-yl)methanimine Oxide as a New Lead-Compound for Ischemic Stroke Treatment. J. Med. Chem. 2019, 62, 2184–2201. [Google Scholar] [CrossRef] [PubMed]
- Fabian, R.H.; Derry, P.J.; Rea, H.C.; Dalmeida, W.V.; Nilewski, L.G.; Sikkema, W.K.; Mandava, P.; Tsai, A.-L.; Mendoza, K.; Berka, V. Efficacy of novel carbon nanoparticle antioxidant therapy in a severe model of reversible middle cerebral artery stroke in acutely hyperglycemic rats. Front. Neurol. 2018, 9, 199. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, S.; Fan, X. Role of Polyphenols as Antioxidant Supplementation in Ischemic Stroke. Oxidative Med. Cell. Longev. 2021, 2021, 5471347. [Google Scholar] [CrossRef]
- Jelinek, M.; Jurajda, M.; Duris, K. Oxidative Stress in the Brain: Basic Concepts and Treatment Strategies in Stroke. Antioxidants 2021, 10, 1886. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, Y.; Wang, L.; Li, G.; Gao, J.; Wang, Y. Development of L-carnosine functionalized iron oxide nanoparticles loaded with dexamethasone for simultaneous therapeutic potential of blood brain barrier crossing and ischemic stroke treatment. Drug Deliv. 2021, 28, 380–389. [Google Scholar] [CrossRef]
- Salatin, S.; Lotfipour, F.; Jelvehgari, M. A brief overview on nano-sized materials used in the topical treatment of skin and soft tissue bacterial infections. Expert Opin. Drug Deliv. 2019, 16, 1313–1331. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Sharifi, S.; Samani, A.; Ahmadian, E.; Eftekhari, A.; Derakhshankhah, H.; Jafari, S.; Mokhtarpour, M.; Salatin, S. Oral delivery of proteins and peptides by mucoadhesive nanoparticles. Biointerface Res. Appl. Chem. 2019, 9, 3849–3852. [Google Scholar]
- Rabanel, J.M.; Piec, P.A.; Landri, S.; Patten, S.A.; Ramassamy, C. Transport of PEGylated-PLA nanoparticles across a blood brain barrier model, entry into neuronal cells and in vivo brain bioavailability. J. Control. Release Off. J. Control. Release Soc. 2020, 328, 679–695. [Google Scholar] [CrossRef]
- Kang, L.; Jiang, D.; Dalong, N.; Ehlerding, E.; Wang, R.; Cai, W. Antioxidative neuroprotective role of iron-gallic acid coordination nanoparticles and PET/MR usage in an ischemia stroke model. Soc. Nuclear Med. 2019, 60, 113. [Google Scholar]
- Silveira, P.C.L.; Rodrigues, M.S.; Gelain, D.P.; de Oliveira, J. Gold nanoparticles application to the treatment of brain dysfunctions related to metabolic diseases: Evidence from experimental studies. Metab. Brain Dis. 2023, 38, 123–135. [Google Scholar] [CrossRef]
- Alami-Milani, M.; Zakeri-Milani, P.; Valizadeh, H.; Fathi, M.; Salatin, S.; Salehi, R.; Jelvehgari, M. PLA–PCL–PEG–PCL–PLA based micelles for improving the ocular permeability of dexamethasone: Development, characterization, and in vitro evaluation. Pharm. Dev. Technol. 2020, 25, 704–719. [Google Scholar] [CrossRef]
- Sharma, H.S.; Muresanu, D.F.; Ozkizilcik, A.; Sahib, S.; Tian, Z.R.; Lafuente, J.V.; Castellani, R.J.; Nozari, A.; Feng, L.; Buzoianu, A.D. Superior antioxidant and anti-ischemic neuroprotective effects of cerebrolysin in heat stroke following intoxication of engineered metal Ag and Cu nanoparticles: A comparative biochemical and physiological study with other stroke therapies. Prog. Brain Res. 2021, 266, 301–348. [Google Scholar]
- Muresanu, D.F.; Sharma, A.; Ryan Tian, Z.; Smith, M.A.; Shanker Sharma, H. Nanowired drug delivery of antioxidant compound H-290/51 enhances neuroprotection in hyperthermia-induced neurotoxicity. CNS Neurol. Disord.-Drug Targets Former. Curr. Drug Targets-CNS Neurol. Disord. 2012, 11, 50–64. [Google Scholar]
- Komsiiska, D. Oxidative stress and stroke: A review of upstream and downstream antioxidant therapeutic options. Comp. Clin. Pathol. 2019, 28, 915–926. [Google Scholar] [CrossRef]
- Salatin, S.; Jelvehgari, M. Desirability function approach for development of a thermosensitive and bioadhesive nanotransfersome–hydrogel hybrid system for enhanced skin bioavailability and antibacterial activity of cephalexin. Drug Dev. Ind. Pharm. 2020, 46, 1318–1333. [Google Scholar] [CrossRef]
- Salatin, S.; Lotfipour, F.; Jelvehgari, M. Preparation and characterization of a novel thermosensitive and bioadhesive cephalexin nanohydrogel: A promising platform for topical antibacterial delivery. Expert Opin. Drug Deliv. 2020, 17, 881–893. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, W.; Jin, X.; Shang, X.; Wu, X.; Wen, L.; Li, S.; Hong, Y.; Ke, J.; Xu, Y. Enhanced brain delivery of hypoxia-sensitive liposomes by hydroxyurea for rescue therapy of hyperacute ischemic stroke. Nanoscale 2023, 15, 11625–11646. [Google Scholar] [CrossRef]
- Salatin, S. Nanoparticles as potential tools for improved antioxidant enzyme delivery. J. Adv. Chem. Pharm. Mater. JACPM 2018, 1, 65–66. [Google Scholar]
- Kikuchi, T.; Fukuta, T.; Agato, Y.; Yanagida, Y.; Ishii, T.; Koide, H.; Shimizu, K.; Oku, N.; Asai, T. Suppression of Cerebral Ischemia/Reperfusion Injury by Efficient Release of Encapsulated Ifenprodil From Liposomes Under Weakly Acidic pH Conditions. J. Pharm. Sci. 2019, 108, 3823–3830. [Google Scholar] [CrossRef]
- Zhao, Y.; Xin, Z.; Li, N.; Chang, S.; Chen, Y.; Geng, L.; Chang, H.; Shi, H.; Chang, Y.-Z. Nano-liposomes of lycopene reduces ischemic brain damage in rodents by regulating iron metabolism. Free Radic. Biol. Med. 2018, 124, 1–11. [Google Scholar] [CrossRef]
- Sinha, J.; Das, N.; Basu, M.K. Liposomal antioxidants in combating ischemia-reperfusion injury in rat brain. Biomed. Pharmacother. 2001, 55, 264–271. [Google Scholar] [CrossRef]
- Li, N.; Feng, L.; Tan, Y.; Xiang, Y.; Zhang, R.; Yang, M. Preparation, characterization, pharmacokinetics and biodistribution of baicalin-loaded liposome on cerebral ischemia-reperfusion after iv administration in rats. Molecules 2018, 23, 1747. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Wan, J.; Yang, Q.; Xiang, Y.; Ni, L.; Long, Y.; Cui, M.; Ci, Z.; Tang, D.; et al. Preparation, Characterization and in vivo Study of Borneol-Baicalin-Liposomes for Treatment of Cerebral Ischemia-Reperfusion Injury. Int. J. Nanomed. 2020, 15, 5977–5989. [Google Scholar] [CrossRef]
- Yun, X.; Maximov, V.D.; Yu, J.; Vertegel, A.A.; Kindy, M.S. Nanoparticles for targeted delivery of antioxidant enzymes to the brain after cerebral ischemia and reperfusion injury. J. Cereb. Blood Flow Metab. 2013, 33, 583–592. [Google Scholar] [CrossRef]
- Ashafaq, M.; Intakhab Alam, M.; Khan, A.; Islam, F.; Khuwaja, G.; Hussain, S.; Ali, R.; Alshahrani, S.; Antar Makeen, H.; Alhazmi, H.A.; et al. Nanoparticles of resveratrol attenuates oxidative stress and inflammation after ischemic stroke in rats. Int. Immunopharmacol. 2021, 94, 107494. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Rad, A.A.; Safaei, N.; Salatin, S.; Ahmadian, E.; Sharifi, S.; Vahed, S.Z.; Lotfipour, F.; Shahi, S. The application of nanomaterials in cardiovascular diseases: A review on drugs and devices. J. Pharm. Pharm. Sci. 2019, 22, 501–515. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Sarkar, S.; Mandal, A.K.; Das, N. Neuroprotective role of nanoencapsulated quercetin in combating ischemia-reperfusion induced neuronal damage in young and aged rats. PLoS ONE 2013, 8, e57735. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, E.E.; Waters, E.S.; Yang, X.; Fagan, M.M.; Scheulin, K.M.; Sneed, S.E.; Cheek, S.R.; Jeon, J.H.; Shin, S.K.; Kinder, H.A.; et al. Tanshinone IIA-Loaded Nanoparticle and Neural Stem Cell Therapy Enhances Recovery in a Pig Ischemic Stroke Model. Stem Cells Transl. Med. 2022, 11, 1061–1071. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Ma, C.; Li, T.; Yang, L. Preparation of baicalin-loaded ligand-modified nanoparticles for nose-to-brain delivery for neuroprotection in cerebral ischemia. Drug Deliv. 2022, 29, 1282–1298. [Google Scholar] [CrossRef]
- Waters, E.S.; Kaiser, E.E.; Yang, X.; Fagan, M.M.; Scheulin, K.M.; Jeon, J.H.; Shin, S.K.; Kinder, H.A.; Kumar, A.; Platt, S.R.; et al. Intracisternal administration of tanshinone IIA-loaded nanoparticles leads to reduced tissue injury and functional deficits in a porcine model of ischemic stroke. IBRO Neurosci. Rep. 2021, 10, 18–30. [Google Scholar] [CrossRef]
- Andrabi, S.S.; Yang, J.; Gao, Y.; Kuang, Y.; Labhasetwar, V. Nanoparticles with antioxidant enzymes protect injured spinal cord from neuronal cell apoptosis by attenuating mitochondrial dysfunction. J. Control. Release Off. J. Control. Release Soc. 2020, 317, 300–311. [Google Scholar] [CrossRef]
- Reddy, M.K.; Labhasetwar, V. Nanoparticle-mediated delivery of superoxide dismutase to the brain: An effective strategy to reduce ischemia-reperfusion injury. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 1384–1395. [Google Scholar] [CrossRef]
- Petro, M.; Jaffer, H.; Yang, J.; Kabu, S.; Morris, V.B.; Labhasetwar, V. Tissue plasminogen activator followed by antioxidant-loaded nanoparticle delivery promotes activation/mobilization of progenitor cells in infarcted rat brain. Biomaterials 2016, 81, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Morris, V.B.; Labhasetwar, V.; Ghorpade, A. Nanoparticle-mediated catalase delivery protects human neurons from oxidative stress. Cell Death Dis. 2013, 4, e903. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Li, S.-Y. Nano-curcumin simultaneously protects the blood–brain barrier and reduces M1 microglial activation during cerebral ischemia–reperfusion injury. ACS Appl. Mater. Interfaces 2019, 11, 3763–3770. [Google Scholar] [CrossRef] [PubMed]
- Mei, T.; Kim, A.; Vong, L.B.; Marushima, A.; Puentes, S.; Matsumaru, Y.; Matsumura, A.; Nagasaki, Y. Encapsulation of tissue plasminogen activator in pH-sensitive self-assembled antioxidant nanoparticles for ischemic stroke treatment—Synergistic effect of thrombolysis and antioxidant. Biomaterials 2019, 215, 119209. [Google Scholar] [CrossRef]
- Rajkovic, O.; Gourmel, C.; d’Arcy, R.; Wong, R.; Rajkovic, I.; Tirelli, N.; Pinteaux, E. Reactive Oxygen Species-Responsive Nanoparticles for the Treatment of Ischemic Stroke. Adv. Ther. 2019, 2, 1900038. [Google Scholar] [CrossRef]
- Lee, D.; Bae, S.; Hong, D.; Lim, H.; Yoon, J.H.; Hwang, O.; Park, S.; Ke, Q.; Khang, G.; Kang, P.M. H2O2-responsive molecularly engineered polymer nanoparticles as ischemia/reperfusion-targeted nanotherapeutic agents. Sci. Rep. 2013, 3, 2233. [Google Scholar] [CrossRef]
- Yoshitomi, T.; Nagasaki, Y. Nitroxyl radical-containing nanoparticles for novel nanomedicine against oxidative stress injury. Nanomedicine 2011, 6, 509–518. [Google Scholar] [CrossRef]
- Wilson, B.; Mohamed Alobaid, B.N.; Geetha, K.M.; Jenita, J.L. Chitosan nanoparticles to enhance nasal absorption and brain targeting of sitagliptin to treat Alzheimer’s disease. J. Drug Deliv. Sci. Technol. 2021, 61, 102176. [Google Scholar] [CrossRef]
- Ding, Y.; Qiao, Y.; Wang, M.; Zhang, H.; Li, L.; Zhang, Y.; Ge, J.; Song, Y.; Li, Y.; Wen, A. Enhanced Neuroprotection of Acetyl-11-Keto-β-Boswellic Acid (AKBA)-Loaded O-Carboxymethyl Chitosan Nanoparticles Through Antioxidant and Anti-Inflammatory Pathways. Mol. Neurobiol. 2016, 53, 3842–3853. [Google Scholar] [CrossRef]
- Yuan, J.; Li, L.; Yang, Q.; Ran, H.; Wang, J.; Hu, K.; Pu, W.; Huang, J.; Wen, L.; Zhou, L.; et al. Targeted Treatment of Ischemic Stroke by Bioactive Nanoparticle-Derived Reactive Oxygen Species Responsive and Inflammation-Resolving Nanotherapies. ACS Nano 2021, 15, 16076–16094. [Google Scholar] [CrossRef]
- Halawani, R.F.; AbdElgawad, H.; Aloufi, F.A.; Balkhyour, M.A.; Zrig, A.; Hassan, A.H.A. Synergistic effect of carbon nanoparticles with mild salinity for improving chemical composition and antioxidant activities of radish sprouts. Front. Plant Sci. 2023, 14, 1158031. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Tsai, S.K.; Chih, C.L.; Chiang, L.Y.; Hsieh, H.M.; Teng, C.M.; Tsai, M.C. Neuroprotective effect of hexasulfobutylated C60 on rats subjected to focal cerebral ischemia. Free Radic. Biol. Med. 2001, 30, 643–649. [Google Scholar] [CrossRef]
- Vani, J.R.; Mohammadi, M.T.; Foroshani, M.S.; Jafari, M. Polyhydroxylated fullerene nanoparticles attenuate brain infarction and oxidative stress in rat model of ischemic stroke. EXCLI J. 2016, 15, 378–390. [Google Scholar] [CrossRef] [PubMed]
- TaghiMohammadi, M.; Sobhani, Z.S.; Darabi, S. Fullerenol nanoparticles decrease brain infarction through potentiation of superoxide dismutase activity during cerebral ischemia-reperfusion injury. Razavi Int. J. Med. 2016, 4, 31–36. [Google Scholar]
- Dal Bosco, L.; Weber, G.E.; Parfitt, G.M.; Cordeiro, A.P.; Sahoo, S.K.; Fantini, C.; Klosterhoff, M.C.; Romano, L.A.; Furtado, C.A.; Santos, A.P.; et al. Biopersistence of PEGylated Carbon Nanotubes Promotes a Delayed Antioxidant Response after Infusion into the Rat Hippocampus. PLoS ONE 2015, 10, e0129156. [Google Scholar] [CrossRef]
- Derry, P.J.; Nilewski, L.G.; Sikkema, W.K.; Mendoza, K.; Jalilov, A.; Berka, V.; McHugh, E.A.; Tsai, A.-L.; Tour, J.M.; Kent, T.A. Catalytic oxidation and reduction reactions of hydrophilic carbon clusters with NADH and cytochrome C: Features of an electron transport nanozyme. Nanoscale 2019, 11, 10791–10807. [Google Scholar] [CrossRef]
- Samuel, E.L.; Marcano, D.C.; Berka, V.; Bitner, B.R.; Wu, G.; Potter, A.; Fabian, R.H.; Pautler, R.G.; Kent, T.A.; Tsai, A.-L. Highly efficient conversion of superoxide to oxygen using hydrophilic carbon clusters. Proc. Natl. Acad. Sci. USA 2015, 112, 2343–2348. [Google Scholar] [CrossRef]
- Dharmalingam, P.; Talakatta, G.; Mitra, J.; Wang, H.; Derry, P.J.; Nilewski, L.G.; McHugh, E.A.; Fabian, R.H.; Mendoza, K.; Vasquez, V.; et al. Pervasive Genomic Damage in Experimental Intracerebral Hemorrhage: Therapeutic Potential of a Mechanistic-Based Carbon Nanoparticle. ACS Nano 2020, 14, 2827–2846. [Google Scholar] [CrossRef]
- Manuja, A.; Kumar, B.; Kumar, R.; Chhabra, D.; Ghosh, M.; Manuja, M.; Brar, B.; Pal, Y.; Tripathi, B.N.; Prasad, M. Metal/metal oxide nanoparticles: Toxicity concerns associated with their physical state and remediation for biomedical applications. Toxicol. Rep. 2021, 8, 1970–1978. [Google Scholar] [CrossRef]
- Grimaud, A.; Diaz-Morales, O.; Han, B.; Hong, W.T.; Lee, Y.-L.; Giordano, L.; Stoerzinger, K.A.; Koper, M.; Shao-Horn, Y. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 2017, 9, 457–465. [Google Scholar] [CrossRef]
- Chen, W.; Li, S.; Wang, J.; Sun, K.; Si, Y. Metal and metal-oxide nanozymes: Bioenzymatic characteristics, catalytic mechanism, and eco-environmental applications. Nanoscale 2019, 11, 15783–15793. [Google Scholar] [CrossRef] [PubMed]
- Van der Zande, M.; Vandebriel, R.J.; Van Doren, E.; Kramer, E.; Herrera Rivera, Z.; Serrano-Rojero, C.S.; Gremmer, E.R.; Mast, J.; Peters, R.J.; Hollman, P.C. Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano 2012, 6, 7427–7442. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.; Geertsma, R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef]
- Daniel, S.K.; Tharmaraj, V.; Sironmani, T.A.; Pitchumani, K. Toxicity and immunological activity of silver nanoparticles. Appl. Clay Sci. 2010, 48, 547–551. [Google Scholar] [CrossRef]
- Wen, R.; Yang, X.; Hu, L.; Sun, C.; Zhou, Q.; Jiang, G. Brain-targeted distribution and high retention of silver by chronic intranasal instillation of silver nanoparticles and ions in Sprague–Dawley rats. J. Appl. Toxicol. 2016, 36, 445–453. [Google Scholar] [CrossRef]
- Yoon, Y.; Truong, P.L.; Lee, D.; Ko, S.H. Metal-oxide nanomaterials synthesis and applications in flexible and wearable sensors. ACS Nanosci. Au 2021, 2, 64–92. [Google Scholar] [CrossRef]
- Takamiya, M.; Miyamoto, Y.; Yamashita, T.; Deguchi, K.; Ohta, Y.; Ikeda, Y.; Matsuura, T.; Abe, K. Neurological and pathological improvements of cerebral infarction in mice with platinum nanoparticles. J. Neurosci. Res. 2011, 89, 1125–1133. [Google Scholar] [CrossRef]
- Takamiya, M.; Miyamoto, Y.; Yamashita, T.; Deguchi, K.; Ohta, Y.; Abe, K. Strong neuroprotection with a novel platinum nanoparticle against ischemic stroke-and tissue plasminogen activator-related brain damages in mice. Neuroscience 2012, 221, 47–55. [Google Scholar] [CrossRef]
- Rao, S.; Lin, Y.; Lin, R.; Liu, J.; Wang, H.; Hu, W.; Chen, B.; Chen, T. Traditional Chinese medicine active ingredients-based selenium nanoparticles regulate antioxidant selenoproteins for spinal cord injury treatment. J. Nanobiotechnol. 2022, 20, 278. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Basu, A.; Biswas, J.; Sen, T.; Bhattacharya, S. Chemoprotective and chemosensitizing properties of selenium nanoparticle (Nano-Se) during adjuvant therapy with cyclophosphamide in tumor-bearing mice. Mol. Cell. Biochem. 2017, 424, 13–33. [Google Scholar] [CrossRef]
- Im, G.B.; Kim, Y.G.; Yoo, T.Y.; Kim, Y.H.; Kim, K.; Hyun, J.; Soh, M.; Hyeon, T.; Bhang, S.H. Ceria Nanoparticles as Copper Chaperones that Activate SOD1 for Synergistic Antioxidant Therapy to Treat Ischemic Vascular Diseases. Adv. Mater. 2023, 35, 2208989. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Zhao, M.; Chen, H.; Lenahan, C.; Zhou, X.; Ou, Y.; He, Y. The role of nanomaterials in stroke treatment: Targeting oxidative stress. Oxidative Med. Cell. Longev. 2021, 2021, 8857486. [Google Scholar] [CrossRef] [PubMed]
- Hirst, S.M.; Karakoti, A.; Singh, S.; Self, W.; Tyler, R.; Seal, S.; Reilly, C.M. Bio-distribution and in vivo antioxidant effects of cerium oxide nanoparticles in mice. Environ. Toxicol. 2013, 28, 107–118. [Google Scholar] [CrossRef]
- Jeong, H.-G.; Cha, B.G.; Kang, D.-W.; Kim, D.Y.; Ki, S.K.; Kim, S.I.; Han, J.H.; Yang, W.; Kim, C.K.; Kim, J. Ceria nanoparticles synthesized with aminocaproic acid for the treatment of subarachnoid hemorrhage. Stroke 2018, 49, 3030–3038. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shen, Y.; Wu, Y.; Yang, Y.; Wu, J.; Zhou, P.; Lu, X.; Guo, Z. An intrinsic therapy of gold nanoparticles in focal cerebral ischemia-reperfusion injury in rats. J. Biomed. Nanotechnol. 2013, 9, 1017–1028. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, Y.; Liu, Y.; Guo, Z.; Bai, T.; Zhou, P.; Wu, J.; Yang, Q.; Liu, Z.; Lu, X. Intrinsic Effects of Gold Nanoparticles on Oxygen-Glucose Deprivation/Reperfusion Injury in Rat Cortical Neurons. Neurochem. Res. 2019, 44, 1549–1566. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Li, X.; Qiao, S.; Huang, G.; Hermann, D.M.; Doeppner, T.R.; Zeng, M.; Liu, W.; Xu, G.; et al. A Co-Doped Fe3O4 Nanozyme Shows Enhanced Reactive Oxygen and Nitrogen Species Scavenging Activity and Ameliorates the Deleterious Effects of Ischemic Stroke. ACS Appl. Mater. Interfaces 2021, 13, 46213–46224. [Google Scholar] [CrossRef]
- Guan, D.; Li, Y.; Peng, X.; Zhao, H.; Mao, Y.; Cui, Y. Thymoquinone protects against cerebral small vessel disease: Role of antioxidant and anti-inflammatory activities. J. Biol. Regul. Homeost. Agents 2018, 32, 225–231. [Google Scholar]
- Ahmadi, M.; Agah, E.; Nafissi, S.; Jaafari, M.R.; Harirchian, M.H.; Sarraf, P.; Faghihi-Kashani, S.; Hosseini, S.J.; Ghoreishi, A.; Aghamollaii, V. Safety and efficacy of nanocurcumin as add-on therapy to riluzole in patients with amyotrophic lateral sclerosis: A pilot randomized clinical trial. Neurotherapeutics 2018, 15, 430–438. [Google Scholar] [CrossRef]
- Dolati, S.; Babaloo, Z.; Ayromlou, H.; Ahmadi, M.; Rikhtegar, R.; Rostamzadeh, D.; Roshangar, L.; Nouri, M.; Mehdizadeh, A.; Younesi, V. Nanocurcumin improves regulatory T-cell frequency and function in patients with multiple sclerosis. J. Neuroimmunol. 2019, 327, 15–21. [Google Scholar] [CrossRef]
- Saghari, Y.; Movahedi, M.; Tebianian, M.; Entezari, M. Investigating the Effect of Curcumin and Betanin Nanoparticles on Antioxidant and Inflammatory Response in Rats Suffering from Brain Ischemia/Reperfusion. J. Shahid Sadoughi Univ. Med. Sci. 2023, 31, 6526–6536. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Ji, X.; Askhatova, D.; Du, R.; Lu, L.; Shi, J. Comprehensive Insights into the Multi-Antioxidative Mechanisms of Melanin Nanoparticles and Their Application To Protect Brain from Injury in Ischemic Stroke. J. Am. Chem. Soc. 2017, 139, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Dou, C.; Xia, Y.; Li, B.; Zhao, M.; Yu, P.; Zheng, Y.; El-Toni, A.M.; Atta, N.F.; Galal, A. Neutrophil-like cell-membrane-coated nanozyme therapy for ischemic brain damage and long-term neurological functional recovery. Acs Nano 2021, 15, 2263–2280. [Google Scholar] [CrossRef] [PubMed]
- Wingard, C.J.; Walters, D.M.; Cathey, B.L.; Hilderbrand, S.C.; Katwa, P.; Lin, S.; Ke, P.C.; Podila, R.; Rao, A.; Lust, R.M. Mast cells contribute to altered vascular reactivity and ischemia-reperfusion injury following cerium oxide nanoparticle instillation. Nanotoxicology 2011, 5, 531–545. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salatin, S.; Farhoudi, M.; Farjami, A.; Maleki Dizaj, S.; Sharifi, S.; Shahi, S. Nanoparticle Formulations of Antioxidants for the Management of Oxidative Stress in Stroke: A Review. Biomedicines 2023, 11, 3010. https://doi.org/10.3390/biomedicines11113010
Salatin S, Farhoudi M, Farjami A, Maleki Dizaj S, Sharifi S, Shahi S. Nanoparticle Formulations of Antioxidants for the Management of Oxidative Stress in Stroke: A Review. Biomedicines. 2023; 11(11):3010. https://doi.org/10.3390/biomedicines11113010
Chicago/Turabian StyleSalatin, Sara, Mehdi Farhoudi, Afsaneh Farjami, Solmaz Maleki Dizaj, Simin Sharifi, and Shahriar Shahi. 2023. "Nanoparticle Formulations of Antioxidants for the Management of Oxidative Stress in Stroke: A Review" Biomedicines 11, no. 11: 3010. https://doi.org/10.3390/biomedicines11113010





