Granulocyte Colony-Stimulating Factor Ameliorates Endothelial Activation and Thrombotic Diathesis Biomarkers in a Murine Model of Hind Limb Ischemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Limb Ischemia
2.3. Laser Doppler Perfusion Imaging
2.4. Enzyme-Linked Immunosorbent Assay
2.5. Study Ethics
2.6. Statistical Analysis
3. Results
3.1. Granulocyte Colony-Stimulating Factor Administration Reduces Endothelial Activation
3.2. Granulocyte Colony-Stimulating Factor Administration Attenuates Thrombotic Diathesis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herrington, W.; Lacey, B.; Sherliker, P.; Armitage, J.; Lewington, S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ. Res. 2016, 118, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Gomar, F.; Perez-Quilis, C.; Leischik, R.; Lucia, A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann. Transl. Med. 2016, 4, 256. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Matsushita, K.; Aboyans, V.; Hess, C.N.; Hicks, C.W.; Kwan, T.W.; McDermott, M.M.; Misra, S.; Ujueta, F.; on behalf of the American Heart Association Council on Epidemiology and Prevention; et al. Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e171–e191. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef]
- Leng, G.C.; Lee, A.J.; Fowkes, F.G.; Lowe, G.D.; Housley, E. The relationship between cigarette smoking and cardiovascular risk factors in peripheral arterial disease compared with ischaemic heart disease. The Edinburgh Artery Study. Eur. Heart J. 1995, 16, 1542–1548. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Gogalniceanu, P.; Lancaster, R.T.; Patel, V.I. Clinical Assessment of Peripheral Arterial Disease of the Lower Limbs. N. Engl. J. Med. 2018, 378, e24. [Google Scholar] [CrossRef]
- Criqui, M.H.; Langer, R.D.; Fronek, A.; Feigelson, H.S.; Klauber, M.R.; McCann, T.J.; Browner, D. Mortality over a period of 10 years in patients with peripheral arterial disease. N. Engl. J. Med. 1992, 326, 381–386. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Bjorck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries Endorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e726–e779. [Google Scholar] [CrossRef]
- Arai, M.; Misao, Y.; Nagai, H.; Kawasaki, M.; Nagashima, K.; Suzuki, K.; Tsuchiya, K.; Otsuka, S.; Uno, Y.; Takemura, G.; et al. Granulocyte colony-stimulating factor: A noninvasive regeneration therapy for treating atherosclerotic peripheral artery disease. Circ. J. 2006, 70, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Fujita, Y.; Katayama, M.; Baba, R.; Shibakawa, M.; Yoshikawa, K.; Katakami, N.; Furukawa, Y.; Tsukie, T.; Nagano, T.; et al. Long-term clinical outcome after intramuscular transplantation of granulocyte colony stimulating factor-mobilized CD34 positive cells in patients with critical limb ischemia. Atherosclerosis 2012, 224, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Mavromatis, K.; Ko, Y.A.; Rogers, S.C.; Dhindsa, D.S.; Goodwin, C.; Patel, R.; Martini, M.A.; Prasad, M.; Mokhtari, A.; et al. Rationale and design of the granulocyte-macrophage colony stimulating factor in peripheral arterial disease (GPAD-3) study. Contemp. Clin. Trials 2020, 91, 105975. [Google Scholar] [CrossRef]
- Zhang, Y.; Adachi, Y.; Iwasaki, M.; Minamino, K.; Suzuki, Y.; Nakano, K.; Koike, Y.; Mukaide, H.; Shigematsu, A.; Kiriyama, N.; et al. G-CSF and/or M-CSF accelerate differentiation of bone marrow cells into endothelial progenitor cells in vitro. Oncol. Rep. 2006, 15, 1523–1527. [Google Scholar] [CrossRef]
- Briasoulis, A.; Tousoulis, D.; Antoniades, C.; Papageorgiou, N.; Stefanadis, C. The role of endothelial progenitor cells in vascular repair after arterial injury and atherosclerotic plaque development. Cardiovasc. Ther. 2011, 29, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Brunner, S.; Huber, B.C.; Fischer, R.; Groebner, M.; Hacker, M.; David, R.; Zaruba, M.M.; Vallaster, M.; Rischpler, C.; Wilke, A.; et al. G-CSF treatment after myocardial infarction: Impact on bone marrow-derived vs cardiac progenitor cells. Exp. Hematol. 2008, 36, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Sun, C.; Xu, X.; Zhou, J.; Wu, Y.; Tian, Y.; Ma, A.; Liu, Z. Early use of granulocyte colony stimulating factor improves survival in a rabbit model of chronic myocardial ischemia. J. Cardiol. 2013, 61, 87–94. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Yagita, Y.; Oyama, N.; Terasaki, Y.; Omura-Matsuoka, E.; Sasaki, T.; Kitagawa, K. Granulocyte colony-stimulating factor enhances arteriogenesis and ameliorates cerebral damage in a mouse model of ischemic stroke. Stroke 2011, 42, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Duelsner, A.; Gatzke, N.; Glaser, J.; Hillmeister, P.; Li, M.; Lee, E.J.; Lehmann, K.; Urban, D.; Meyborg, H.; Stawowy, P.; et al. Granulocyte colony-stimulating factor improves cerebrovascular reserve capacity by enhancing collateral growth in the circle of Willis. Cerebrovasc. Dis. 2012, 33, 419–429. [Google Scholar] [CrossRef]
- Li, M.; Wang, Z.; Xia, H.; Yu, L.; Hu, Z. Vildagliptin and G-CSF Improved Angiogenesis and Survival after Acute Myocardial Infarction. Arch. Med. Res. 2019, 50, 133–141. [Google Scholar] [CrossRef]
- Ren, P.; Zhang, M.; Dai, S. Therapeutic effects of coronary granulocyte colony-stimulating factor on rats with chronic ischemic heart disease. Open Life Sci. 2020, 15, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Traupe, T.; Stoller, M.; Gloekler, S.; Meier, P.; Seiler, C. The effect of pegylated granulocyte colony-stimulating factor on collateral function and myocardial ischaemia in chronic coronary artery disease: A randomized controlled trial. Eur. J. Clin. Investig. 2019, 49, e13035. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Yamazaki, S.; Hanada, S.; Kobayashi, S.; Tsukamoto, T.; Haruna, T.; Sakaguchi, K.; Sakai, K.; Obara, H.; Morishita, K.; et al. Outcome from a Randomized Controlled Clinical Trial- Improvement of Peripheral Arterial Disease by Granulocyte Colony-Stimulating Factor-Mobilized Autologous Peripheral-Blood-Mononuclear Cell Transplantation (IMPACT). Circ. J. 2018, 82, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Zafarghandi, M.R.; Ravari, H.; Aghdami, N.; Namiri, M.; Moazzami, K.; Taghiabadi, E.; Fazel, A.; Pournasr, B.; Farrokhi, A.; Sharifian, R.A.; et al. Safety and efficacy of granulocyte-colony-stimulating factor administration following autologous intramuscular implantation of bone marrow mononuclear cells: A randomized controlled trial in patients with advanced lower limb ischemia. Cytotherapy 2010, 12, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, A.; Katayama, M.; Handa, N.; Kinoshita, M.; Takano, H.; Horii, M.; Sadamoto, K.; Yokoyama, A.; Yamanaka, T.; Onodera, R.; et al. Intramuscular transplantation of G-CSF-mobilized CD34(+) cells in patients with critical limb ischemia: A phase I/IIa, multicenter, single-blinded, dose-escalation clinical trial. Stem Cells 2009, 27, 2857–2864. [Google Scholar] [CrossRef]
- Ohtake, T.; Mochida, Y.; Ishioka, K.; Oka, M.; Maesato, K.; Moriya, H.; Hidaka, S.; Higashide, S.; Ioji, T.; Fujita, Y.; et al. Autologous Granulocyte Colony-Stimulating Factor-Mobilized Peripheral Blood CD34 Positive Cell Transplantation for Hemodialysis Patients with Critical Limb Ischemia: A Prospective Phase II Clinical Trial. Stem Cells Transl. Med. 2018, 7, 774–782. [Google Scholar] [CrossRef]
- Eton, D.; Zhou, G.; He, T.C.; Bartholomew, A.; Patil, R. Filgrastim, fibrinolysis, and neovascularization. J. Tissue Eng. Regen. Med. 2022, 16, 496–510. [Google Scholar] [CrossRef]
- Kawamura, I.; Takemura, G.; Tsujimoto, A.; Watanabe, T.; Kanamori, H.; Esaki, M.; Kobayashi, H.; Takeyama, T.; Kawaguchi, T.; Goto, K.; et al. Treatment of leg ischemia with biodegradable gelatin hydrogel microspheres incorporating granulocyte colony-stimulating factor. J. Cardiovasc. Pharmacol. 2011, 57, 416–423. [Google Scholar] [CrossRef]
- Barnes, J.A.; Eid, M.A.; Creager, M.A.; Goodney, P.P. Epidemiology and Risk of Amputation in Patients with Diabetes Mellitus and Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1808–1817. [Google Scholar] [CrossRef]
- Nakayama, M.; Asari, Y. Angiogenesis achieved by granulocyte colony-stimulating factor in combination with bypass surgery in 2 cases of critical limb ischemia. Circ. J. 2008, 72, 1385–1387. [Google Scholar] [CrossRef] [Green Version]
- Sacramento, C.B.; Cantagalli, V.D.; Grings, M.; Carvalho, L.P.; Baptista-Silva, J.C.; Beutel, A.; Bergamaschi, C.T.; de Campos Junior, R.R.; de Moraes, J.Z.; Takiya, C.M.; et al. Granulocyte-macrophage colony-stimulating factor gene based therapy for acute limb ischemia in a mouse model. J. Gene Med. 2009, 11, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Liaudet, L.; Vassalli, G.; Pacher, P. Role of peroxynitrite in the redox regulation of cell signal transduction pathways. Front. Biosci. (Landmark Ed.) 2009, 14, 4809–4814. [Google Scholar] [CrossRef] [PubMed]
- Diers, A.R.; Broniowska, K.A.; Hogg, N. Nitrosative stress and redox-cycling agents synergize to cause mitochondrial dysfunction and cell death in endothelial cells. Redox Biol. 2013, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Antonopoulos, A.S.; Oikonomou, E.; Tsioufis, C.; Tousoulis, D. Inflammatory Mediators of Platelet Activation: Focus on Atherosclerosis and COVID-19. Int. J. Mol. Sci. 2021, 22, 11170. [Google Scholar] [CrossRef]
- Oikonomou, E.; Leopoulou, M.; Theofilis, P.; Antonopoulos, A.S.; Siasos, G.; Latsios, G.; Mystakidi, V.C.; Antoniades, C.; Tousoulis, D. A link between inflammation and thrombosis in atherosclerotic cardiovascular diseases: Clinical and therapeutic implications. Atherosclerosis 2020, 309, 16–26. [Google Scholar] [CrossRef]
- Hwang, S.J.; Ballantyne, C.M.; Sharrett, A.R.; Smith, L.C.; Davis, C.E.; Gotto, A.M., Jr.; Boerwinkle, E. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 1997, 96, 4219–4225. [Google Scholar] [CrossRef]
- Blann, A.D.; Seigneur, M.; Steiner, M.; Miller, J.P.; McCollum, C.N. Circulating ICAM-1 and VCAM-1 in peripheral artery disease and hypercholesterolaemia: Relationship to the location of atherosclerotic disease, smoking, and in the prediction of adverse events. Thromb. Haemost. 1998, 79, 1080–1085. [Google Scholar]
- Santos, J.C.D.; Cruz, M.S.; Bortolin, R.H.; Oliveira, K.M.; Araujo, J.N.G.; Duarte, V.H.R.; Silva, A.; Santos, I.; Dantas, J.M.O.; Paiva, M.; et al. Relationship between circulating VCAM-1, ICAM-1, E-selectin and MMP9 and the extent of coronary lesions. Clinics 2018, 73, e203. [Google Scholar] [CrossRef]
- Edlinger, C.; Lichtenauer, M.; Wernly, B.; Pistulli, R.; Paar, V.; Prodinger, C.; Krizanic, F.; Thieme, M.; Kammler, J.; Jung, C.; et al. Disease-specific characteristics of vascular cell adhesion molecule-1 levels in patients with peripheral artery disease. Heart Vessel. 2019, 34, 976–983. [Google Scholar] [CrossRef]
- Cooke, J.P.; Meng, S. Vascular Regeneration in Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Papadimitriou, C.; Vamvakou, G.; Katsichti, P.; Venetsanou, K.; Stamatelopoulos, K.; Papamichael, C.; Dimopoulos, A.M.; Lekakis, J. Treatment with granulocyte colony stimulating factor is associated with improvement in endothelial function. Growth Factors 2008, 26, 117–124. [Google Scholar] [CrossRef]
- Iwata, Y.; Fujimoto, Y.; Morino, T.; Sugimoto, K.; Ohkubo, K.; Kadohira, T.; Fukushima, K.; Kitahara, H.; Komuro, I.; Kobayashi, Y. Effects of stem cell mobilization by granulocyte colony-stimulating factor on endothelial function after sirolimus-eluting stent implantation: A double-blind, randomized, placebo-controlled clinical trial. Am. Heart J. 2013, 165, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Arslan, O.; Akan, H.; Arat, M.; Dalva, K.; Ozcan, M.; Gurman, G.; Ilhan, O.; Konuk, N.; Beksac, M.; Uysal, A.; et al. Soluble adhesion molecules (sICAM-1, sL-Selectin, sE-Selectin, sCD44) in healthy allogenic peripheral stem-cell donors primed with recombinant G-CSF. Cytotherapy 2000, 2, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Sudhoff, T.; Sohngen, D. Circulating endothelial adhesion molecules (sE-selectin, sVCAM-1 and sICAM-1) during rHuG-CSF-stimulated stem cell mobilization. J. Hematother. Stem Cell Res. 2002, 11, 147–151. [Google Scholar] [CrossRef]
- Fuste, B.; Mazzara, R.; Escolar, G.; Merino, A.; Ordinas, A.; Diaz-Ricart, M. Granulocyte colony-stimulating factor increases expression of adhesion receptors on endothelial cells through activation of p38 MAPK. Haematologica 2004, 89, 578–585. [Google Scholar]
- Juhan-Vague, I.; Collen, D. On the role of coagulation and fibrinolysis in atherosclerosis. Ann. Epidemiol. 1992, 2, 427–438. [Google Scholar] [CrossRef]
- Declerck, P.J.; Gils, A. Three decades of research on plasminogen activator inhibitor-1: A multifaceted serpin. Semin. Thromb. Hemost. 2013, 39, 356–364. [Google Scholar] [CrossRef]
- Vaughan, D.E. PAI-1 and atherothrombosis. J. Thromb. Haemost. 2005, 3, 1879–1883. [Google Scholar] [CrossRef]
- Smith, F.B.; Lee, A.J.; Rumley, A.; Fowkes, F.G.; Lowe, G.D. Tissue-plasminogen activator, plasminogen activator inhibitor and risk of peripheral arterial disease. Atherosclerosis 1995, 115, 35–43. [Google Scholar] [CrossRef]
- Bjorck, M.; Lepkowska Eriksson, M.; Bylock, A.; Steuer, J.; Wanhainen, A.; Carlsson, B.C.; Bock, D.; Kragsterman, B. Plasminogen activator inhibitor-1 levels and activity decrease after intervention in patients with critical limb ischaemia. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Khoukaz, H.B.; Ji, Y.; Braet, D.J.; Vadali, M.; Abdelhamid, A.A.; Emal, C.D.; Lawrence, D.A.; Fay, W.P. Drug Targeting of Plasminogen Activator Inhibitor-1 Inhibits Metabolic Dysfunction and Atherosclerosis in a Murine Model of Metabolic Syndrome. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Bilir, C.; Engin, H.; Temi, Y.B.; Toka, B.; Karabag, T. Acute myocardial infarction caused by filgrastim: A case report. Case Rep. Oncol. Med. 2012, 2012, 784128. [Google Scholar] [CrossRef]
- Kawachi, Y.; Watanabe, A.; Uchida, T.; Yoshizawa, K.; Kurooka, N.; Setsu, K. Acute arterial thrombosis due to platelet aggregation in a patient receiving granulocyte colony-stimulating factor. Br. J. Haematol. 1996, 94, 413–416. [Google Scholar] [CrossRef]
- Steppich, B.A.; Demetz, G.; Schulz, S.; von Wedel, J.; Pogatsa-Murray, G.; Braun, S.L.; Stein, A.; Kastrati, A.; Schomig, A.; Ott, I. Effects of G-CSF on systemic inflammation, coagulation and platelet activation in patients with acute myocardial infarction. Thromb. Res. 2011, 127, 119–121. [Google Scholar] [CrossRef] [PubMed]

| Control | p | GCSF | p | |
|---|---|---|---|---|
| sE-Selectin (Day 1), ng/mL | 4.24 ± 0.88 | 0.34 | 4.63 ± 0.95 | 0.04 |
| sE-Selectin (Day 28), ng/mL | 2.69 ± 1.01 | 3.03 ± 0.46 | ||
| sICAM-1 (Day 1), ng/mL | 1.92 ± 0.16 | 0.14 | 2.02 ± 0.20 | 0.01 |
| sICAM-1 (Day 28), ng/mL | 1.41 ± 0.19 | 1.37 ± 0.18 | ||
| sVCAM-1 (Day 1), MFI | 23,834 ± 84 | 0.23 | 23,746 ± 101 | 0.04 |
| sVCAM-1 (Day 28), MFI | 23,575 ± 151 | 23,075 ± 592 | ||
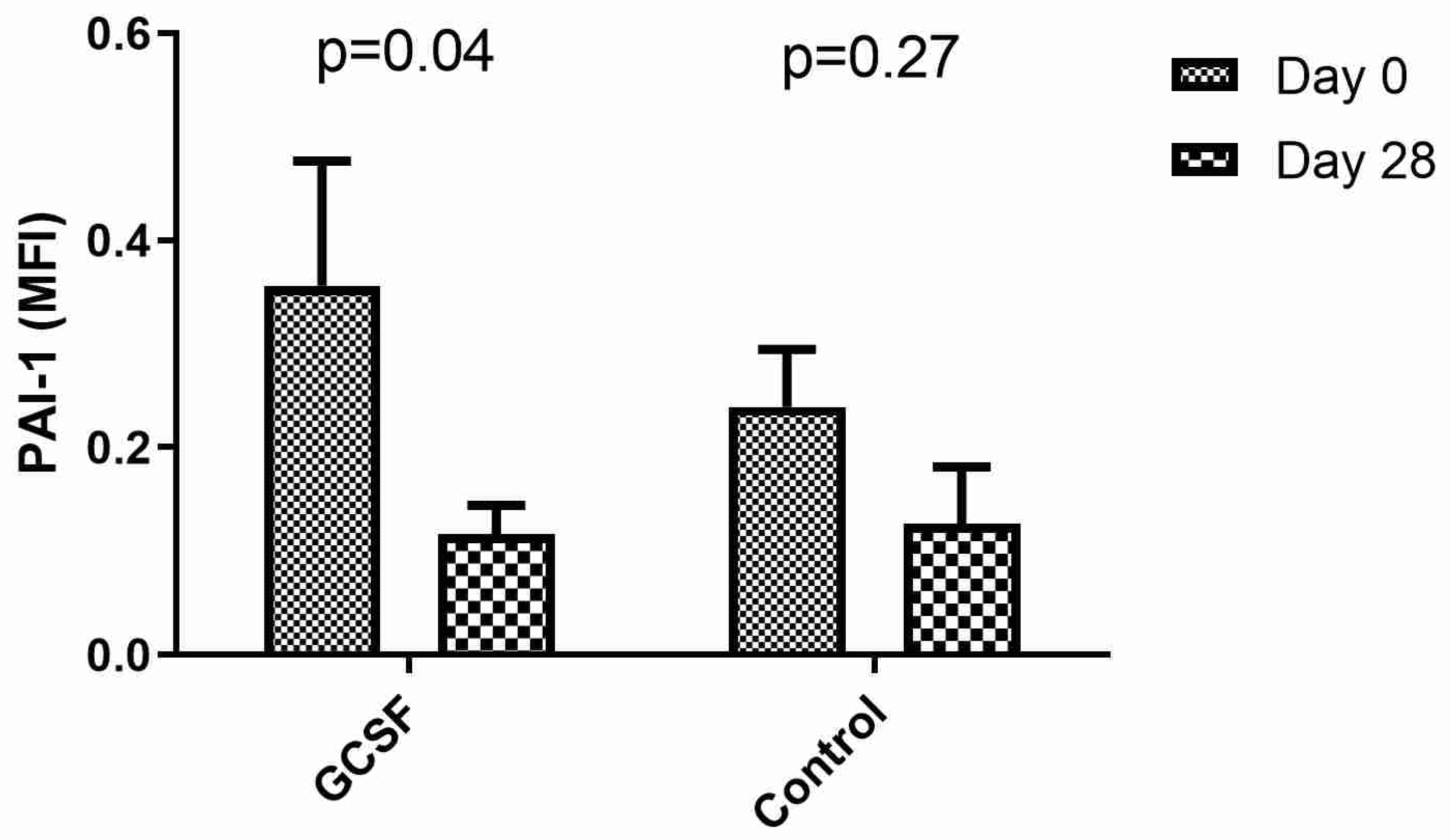
| PAI-1 (Day 1), MFI | 0.239 ± 0.055 | 0.27 | 0.356 ± 0.121 | 0.04 |
| PAI-1 (Day 28), MFI | 0.126 ± 0.055 | 0.122 ± 0.026 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valatsou, A.; Theofilis, P.; Simantiris, S.; Vogiatzi, G.; Briasoulis, A.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Pantopoulou, A.; Nasiri-Ansari, N.; et al. Granulocyte Colony-Stimulating Factor Ameliorates Endothelial Activation and Thrombotic Diathesis Biomarkers in a Murine Model of Hind Limb Ischemia. Biomedicines 2022, 10, 2303. https://doi.org/10.3390/biomedicines10092303
Valatsou A, Theofilis P, Simantiris S, Vogiatzi G, Briasoulis A, Sagris M, Oikonomou E, Antonopoulos AS, Pantopoulou A, Nasiri-Ansari N, et al. Granulocyte Colony-Stimulating Factor Ameliorates Endothelial Activation and Thrombotic Diathesis Biomarkers in a Murine Model of Hind Limb Ischemia. Biomedicines. 2022; 10(9):2303. https://doi.org/10.3390/biomedicines10092303
Chicago/Turabian StyleValatsou, Angeliki, Panagiotis Theofilis, Spyridon Simantiris, Georgia Vogiatzi, Alexandros Briasoulis, Marios Sagris, Evangelos Oikonomou, Alexios S. Antonopoulos, Alkistis Pantopoulou, Narjes Nasiri-Ansari, and et al. 2022. "Granulocyte Colony-Stimulating Factor Ameliorates Endothelial Activation and Thrombotic Diathesis Biomarkers in a Murine Model of Hind Limb Ischemia" Biomedicines 10, no. 9: 2303. https://doi.org/10.3390/biomedicines10092303





