UV-B Filter Octylmethoxycinnamate Is a Modulator of the Serotonin and Histamine Receptors in Human Umbilical Arteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Preparation of HUA-Rings
2.3. Vascular Reactivity Experiments
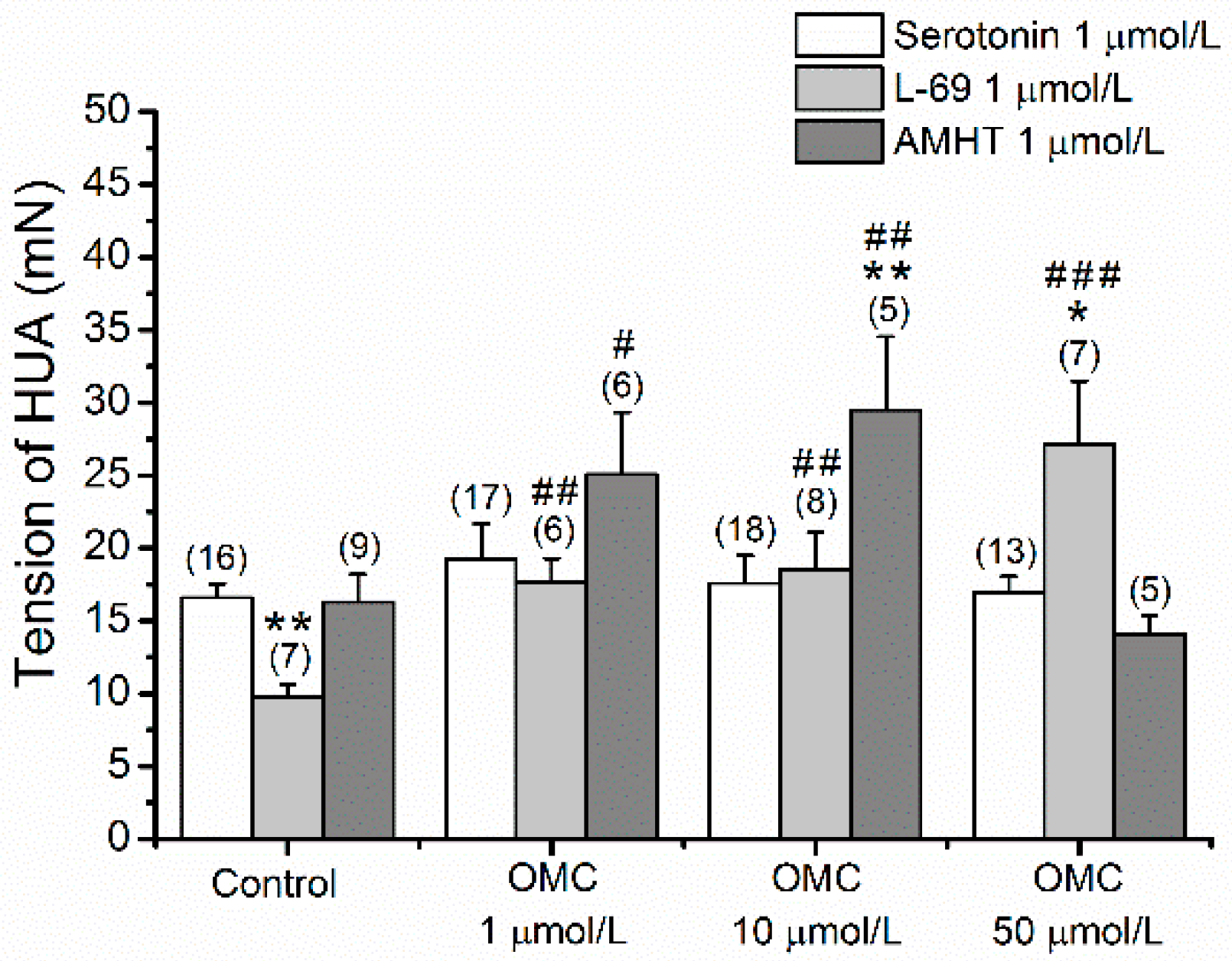
- The HUA rings were contracted using an agonist of 5-HT2A receptors, alpha-methyl-5-hydroxytryptamine (AMHT; 1 µmol/L), and after reaching a stable contraction, 1 µmol/L of AS19, an agonist of 5-HT7 receptors, was added to each ring, and the % of relaxation was measured.
- The HUA rings were contracted using the 5-HT1B and 5-HT1D receptor agonist, L-694247 (L69; 1 µmol/L). In responsive HUA-rings, after reaching a stable contraction, 1 µmol/L of AS19, an agonist of 5-HT7 receptors, was added to each ring and the % of relaxation was measured.
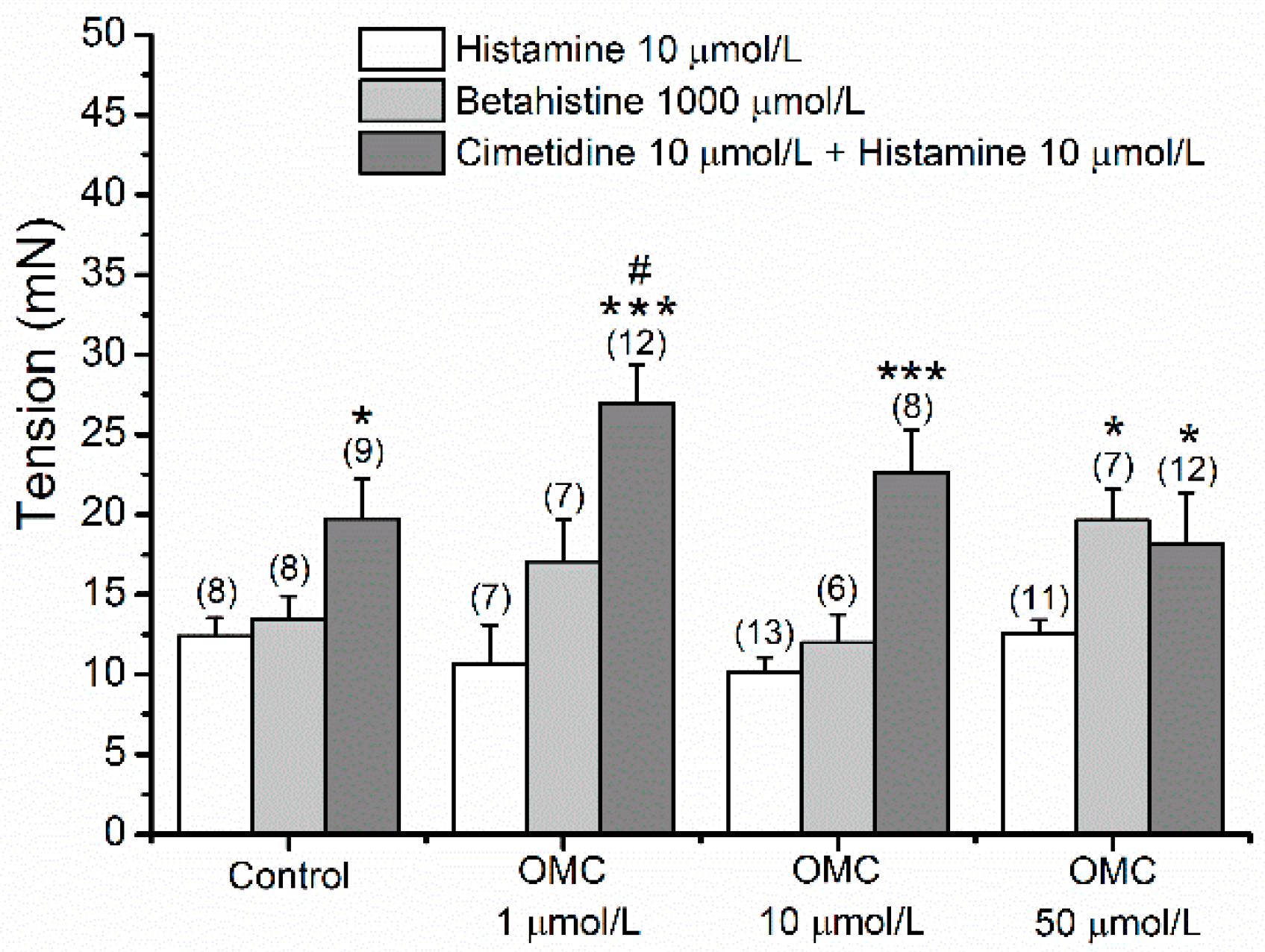
- The HUA rings were contracted using an agonist of H1 receptors, betahistine (BHI, 1000 µmol/L), and after reaching a stable contraction (~10 min), 100 µmol/L of dimaprit, an agonist of H2 receptors, was added to each ring and the % of relaxation was measured.
- The HUA rings were exposed to an antagonist of the H2 receptor, cimetidine (10 µmol/L), and after ~15 min, 10 µmol/L of Hist, an unspecific agonist of Hist receptors, was added to each ring and the % contraction was measured.
2.4. Smooth Muscle Cells Dissociation and Culture
2.5. Real-Time Quantitative Polymerase Chain Reaction (qPCR)
2.6. Drugs and Chemicals
2.7. Statistical Analysis
3. Results
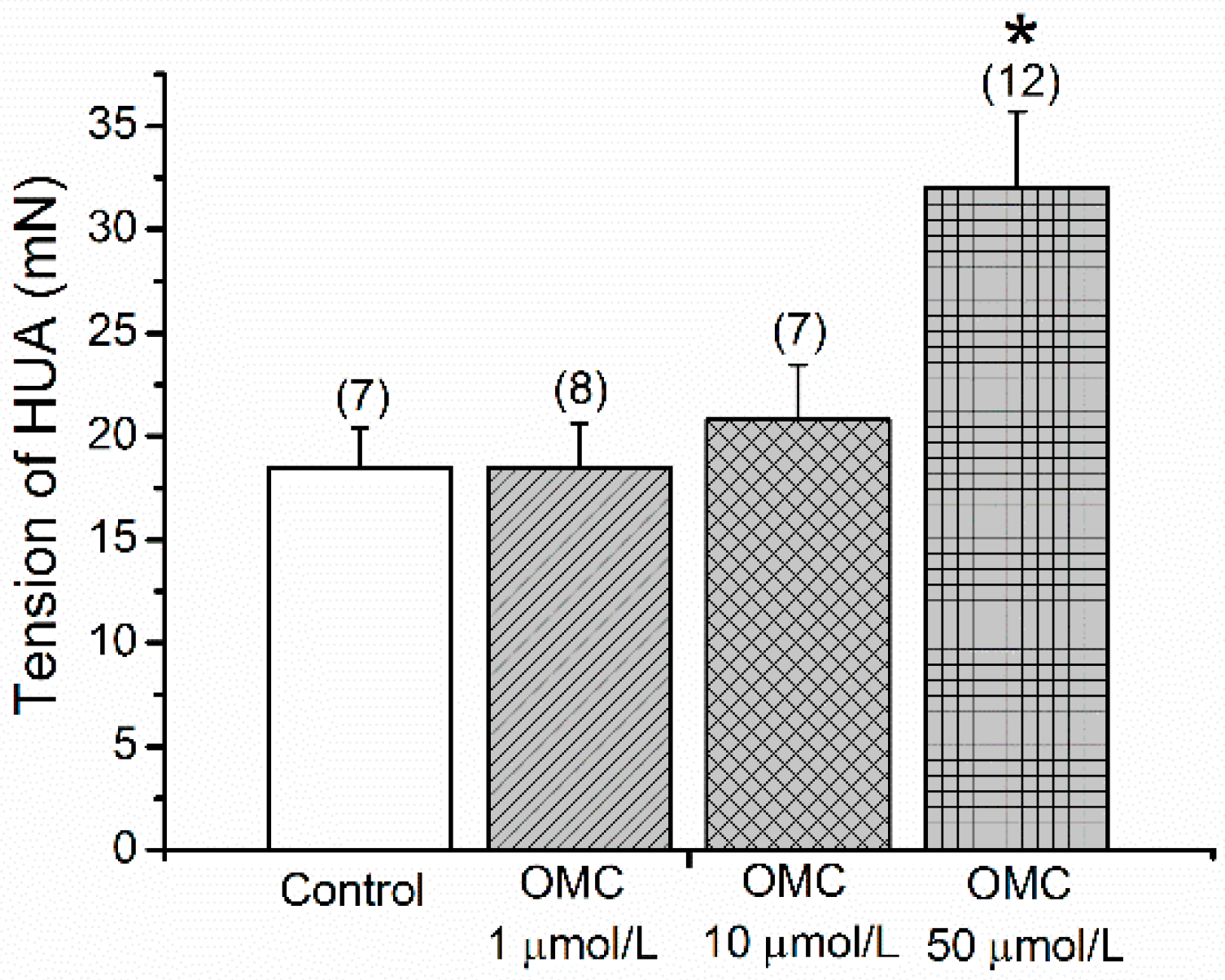
3.1. Direct Effects of OMC on Basal Tension of the Human Vasculature
3.2. Long-Term Effects of OMC on Human Arteries Contracted with Serotonin
3.3. Long-Term Effects of OMC on Vascular Responses of Human Arteries to Agonists and Antagonists of Different 5-HT Receptors
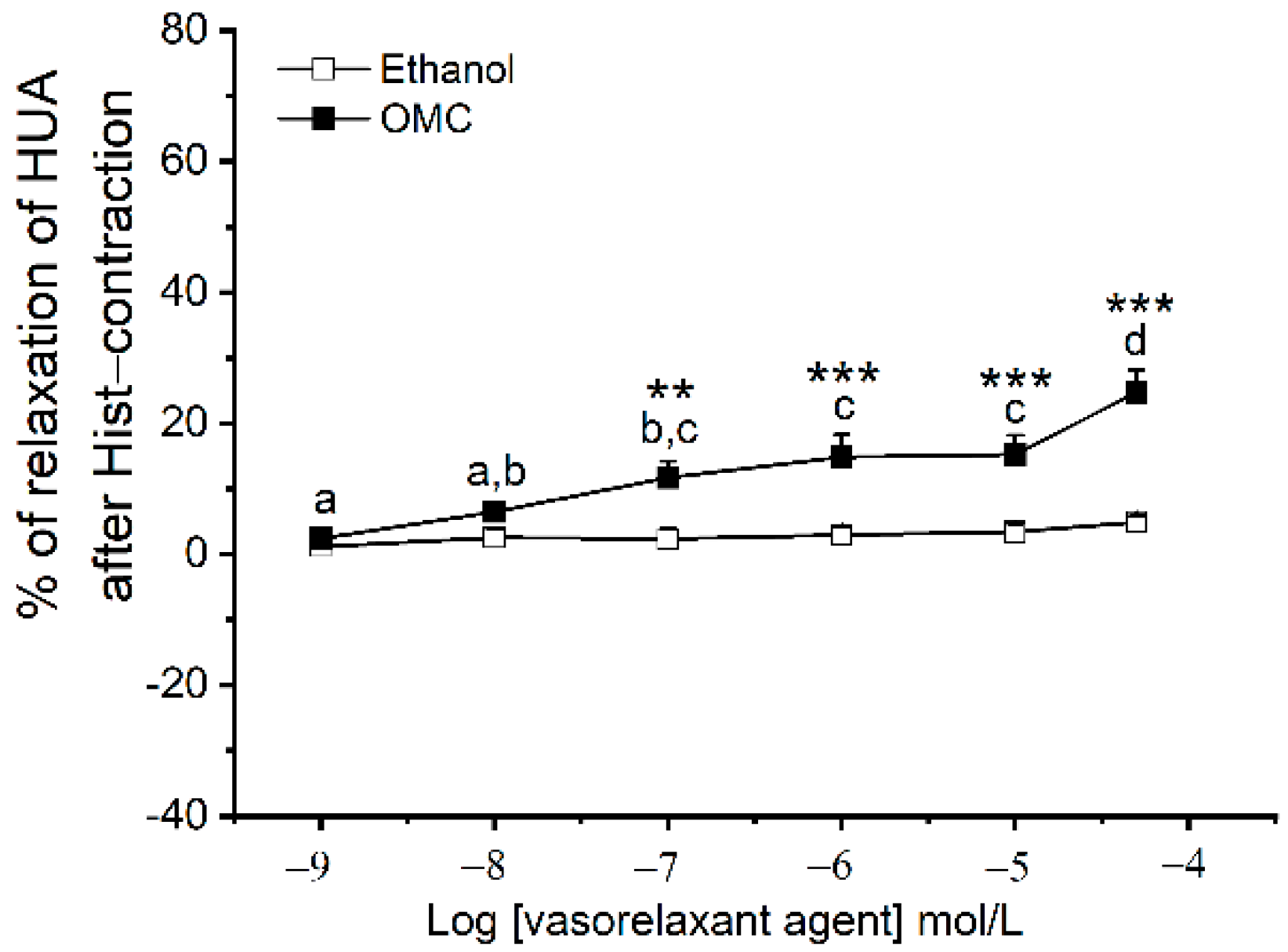
3.4. Long-Term Effects of OMC on Human Arteries Contracted with Histamine
3.5. Long-Term Effects of OMC on Vascular Responses of Human Arteries to Agonists and Antagonists of Different Hist Receptors
3.6. Effects of OMC on the Expression of 5-HT and Hist Receptors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, S.L.; Lim, H.W. Review of environmental effects of oxybenzone and other sunscreen active ingredients. J. Am. Acad. Dermatol. 2019, 80, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Lorigo, M.; Cairrao, E. Antioxidants as stabilizers of UV filters: An example for the UV-B filter octylmethoxycinnamate. Biomed. Dermatol. 2019, 3. [Google Scholar] [CrossRef]
- Maipas, S.; Nicolopoulou-Stamati, P. Sun lotion chemicals as endocrine disruptors. Horm.-Int. J. Endocrinol. Metab. 2015, 14, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Mancebo, S.E.; Hu, J.Y.; Wang, S.Q. Sunscreens A Review of Health Benefits, Regulations, and Controversies. Dermatol. Clin. 2014, 32, 427. [Google Scholar] [CrossRef] [PubMed]
- Ruszkiewicz, J.A.; Pinkas, A.; Ferrer, B.; Peres, T.V.; Tsatsakis, A.; Aschner, M. Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol. Rep. 2017, 4, 245–259. [Google Scholar] [CrossRef]
- Paul, S.P. Ensuring the Safety of Sunscreens, and Their Efficacy in Preventing Skin Cancers: Challenges and Controversies for Clinicians, Formulators, and Regulators. Front. Med. 2019, 6, 195. [Google Scholar] [CrossRef] [Green Version]
- Lorigo, M.; Mariana, M.; Cairrao, E. Photoprotection of ultraviolet-B filters: Updated review of endocrine disrupting properties. Steroids 2018, 131, 46–58. [Google Scholar] [CrossRef]
- Siller, A.; Blaszak, S.C.; Lazar, M.; Olasz Harken, E. Update About the Effects of the Sunscreen Ingredients Oxybenzone and Octinoxate on Humans and the Environment. Plast. Surg. Nurs. Off. J. Am. Soc. Plast. Reconstr. Surg. Nurses 2019, 39, 157–160. [Google Scholar] [CrossRef]
- Suh, S.; Pham, C.; Smith, J.; Mesinkovska, N.A. The banned sunscreen ingredients and their impact on human health: A systematic review. Int. J. Dermatol. 2020, 59, 1033–1042. [Google Scholar] [CrossRef]
- Ouchene, L.; Litvinov, I.V.; Netchiporouk, E. Hawaii and Other Jurisdictions Ban Oxybenzone or Octinoxate Sunscreens Based on the Confirmed Adverse Environmental Effects of Sunscreen Ingredients on Aquatic Environments. J. Cutan. Med. Surg. 2019, 23, 648–649. [Google Scholar] [CrossRef]
- Schreurs, R.H.; Sonneveld, E.; Jansen, J.H.; Seinen, W.; van der Burg, B. Interaction of polycyclic musks and UV filters with the estrogen receptor (ER), androgen receptor (AR), and progesterone receptor (PR) in reporter gene bioassays. Toxicol. Sci. Off. J. Soc. Toxicol. 2005, 83, 264–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, R.; Cotton, B.; Lichtensteiger, W.; Schlumpf, M. UV filters with antagonistic action at androgen receptors in the MDA-kb2 cell transcriptional-activation assay. Toxicol. Sci. Off. J. Soc. Toxicol. 2003, 74, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.H. Evidence of the Possible Harm of Endocrine-Disrupting Chemicals in Humans: Ongoing Debates and Key Issues. Endocrinol. Metab. 2018, 33, 44–52. [Google Scholar] [CrossRef]
- Padula, A.M.; Monk, C.; Brennan, P.A.; Borders, A.; Barrett, E.S.; McEvoy, C.T.; Foss, S.; Desai, P.; Alshawabkeh, A.; Wurth, R.; et al. A review of maternal prenatal exposures to environmental chemicals and psychosocial stressors-implications for research on perinatal outcomes in the ECHO program. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2020, 40, 10–24. [Google Scholar] [CrossRef]
- Rager, J.E.; Bangma, J.; Carberry, C.; Chao, A.; Grossman, J.; Lu, K.; Manuck, T.A.; Sobus, J.R.; Szilagyi, J.; Fry, R.C. Review of the environmental prenatal exposome and its relationship to maternal and fetal health. Reprod. Toxicol. 2020, 98, 1–12. [Google Scholar] [CrossRef]
- Kelley, A.S.; Banker, M.; Goodrich, J.M.; Dolinoy, D.C.; Burant, C.; Domino, S.E.; Smith, Y.R.; Song, P.X.K.; Padmanabhan, V. Early pregnancy exposure to endocrine disrupting chemical mixtures are associated with inflammatory changes in maternal and neonatal circulation. Sci. Rep. 2019, 9, 5422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorigo, M.; Mariana, M.; Feiteiro, J.; Cairrao, E. How is the human umbilical artery regulated? J. Obstet. Gynaecol. Res. 2018, 44, 1193–1201. [Google Scholar] [CrossRef] [Green Version]
- Lorigo, M.; Cairrao, E. Fetoplacental vasculature as a model to study human cardiovascular endocrine disruption. Mol. Asp. Med. 2021, 101054. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Domínguez-Pérez, M.; Mercado, I.; Villarreal-Molina, M.T.; Jacobo-Albavera, L. Use of Human Umbilical Vein Endothelial Cells (HUVEC) as a Model to Study Cardiovascular Disease: A Review. Appl. Sci. 2020, 10, 938. [Google Scholar] [CrossRef] [Green Version]
- Velarde, F.; Castaneda, V.; Morales, E.; Ortega, M.; Ocana, E.; Alvarez-Barreto, J.; Grunauer, M.; Eguiguren, L.; Caicedo, A. Use of Human Umbilical Cord and Its Byproducts in Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 117. [Google Scholar] [CrossRef]
- Lorigo, M.; Quintaneiro, C.; Lemos, M.C.; Martinez-de-Oliveira, J.; Breitenfeld, L.; Cairrao, E. UV-B Filter Octylmethoxycinnamate Induces Vasorelaxation by Ca(2+) Channel Inhibition and Guanylyl Cyclase Activation in Human Umbilical Arteries. Int. J. Mol. Sci. 2019, 20, 1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorigo, M.; Quintaneiro, C.; Maia, C.J.; Breitenfeld, L.; Cairrao, E. UV-B filter octylmethoxycinnamate impaired the main vasorelaxant mechanism of human umbilical artery. Chemosphere 2021, 277, 130302. [Google Scholar] [CrossRef] [PubMed]
- Provitera, L.; Cavallaro, G.; Griggio, A.; Raffaeli, G.; Amodeo, I.; Gulden, S.; Lattuada, D.; Ercoli, G.; Lonati, C.; Tomaselli, A.; et al. Cyclic nucleotide-dependent relaxation in human umbilical vessels. J. Physiol. Pharmacol. 2019, 70, 619–630. [Google Scholar] [CrossRef]
- Weiner, C.P.; Gelfan, R.; Socol, M.L. Intrapartum treatment of preeclamptic hypertension by ketanserin—A serotonin receptor antagonist. Am. J. Obstet. Gynecol. 1984, 149, 576–578. [Google Scholar] [CrossRef]
- Weiner, C.P.; Socol, M.L.; Vaisrub, N. Control of preeclamptic hypertension by ketanserin, a new serotonin receptor antagonist. Am. J. Obstet. Gynecol. 1984, 149, 496–500. [Google Scholar] [CrossRef]
- Bahado-Singh, R.; Poon, L.C.; Yilmaz, A.; Syngelaki, A.; Turkoglu, O.; Kumar, P.; Kirma, J.; Allos, M.; Accurti, V.; Li, J.; et al. Integrated Proteomic and Metabolomic prediction of Term Preeclampsia. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Bolte, A.C.; van Geijn, H.P.; Dekker, G.A. Pathophysiology of preeclampsia and the role of serotonin. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 95, 12–21. [Google Scholar] [CrossRef]
- Brew, O.; Sullivan, M.H. The links between maternal histamine levels and complications of human pregnancy. J. Reprod. Immunol. 2006, 72, 94–107. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Gloria, S.; Marques, J.; Feiteiro, J.; Marcelino, H.; Verde, I.; Cairrao, E. Tributyltin role on the serotonin and histamine receptors in human umbilical artery. Toxicol. In Vitro 2018, 50, 210–216. [Google Scholar] [CrossRef]
- Schlumpf, M.; Cotton, B.; Conscience, M.; Haller, V.; Steinmann, B.; Lichtensteiger, W. In vitro and in vivo estrogenicity of UV screens. Environ. Health Perspect. 2001, 109, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Vela-Soria, F.; Gallardo-Torres, M.E.; Ballesteros, O.; Diaz, C.; Perez, J.; Navalon, A.; Fernandez, M.F.; Olea, N. Assessment of parabens and ultraviolet filters in human placenta tissue by ultrasound-assisted extraction and ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2017, 1487, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Janjua, N.R.; Kongshoj, B.; Andersson, A.M.; Wulf, H.C. Sunscreens in human plasma and urine after repeated whole-body topical application. J. Eur. Acad. Derm. Venereol. 2008, 22, 456–461. [Google Scholar] [CrossRef]
- Tang, Z.-R.; Xu, X.-L.; Deng, S.-L.; Lian, Z.-X.; Yu, K. Oestrogenic Endocrine Disruptors in the Placenta and the Fetus. Int. J. Mol. Sci. 2020, 21, 1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, A.; Leung, S.W.; Lao, T.T.; Man, R.Y. 5-hydroxytryptamine and thromboxane A2 as physiologic mediators of human umbilical artery closure. J. Soc. Gynecol. Investig. 2003, 10, 490–495. [Google Scholar] [CrossRef]
- Lovren, F.; Li, X.F.; Lytton, J.; Triggle, C. Functional characterization and m-RNA expression of 5-HT receptors mediating contraction in human umbilical artery. Br. J. Pharmacol. 1999, 127, 1247–1255. [Google Scholar] [CrossRef] [Green Version]
- Gokina, N.I.; Bevan, J.A. Histamine-induced depolarization: Ionic mechanisms and role in sustained contraction of rabbit cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2094–H2104. [Google Scholar] [CrossRef]
- Afifi, Y.; Churchill, D. Pharmacological treatment of hypertension in pregnancy. Curr. Pharm. Des. 2003, 9, 1745–1753. [Google Scholar] [CrossRef]
- Silva, R.E.R.; Alencar Silva, A.; Pereira-de-Morais, L.; de Sousa Almeida, N.; Iriti, M.; Kerntopf, M.R.; Menezes, I.R.A.; Coutinho, H.D.M.; Barbosa, R. Relaxant Effect of Monoterpene (-)-Carveol on Isolated Human Umbilical Cord Arteries and the Involvement of Ion Channels. Molecules 2020, 25, 2681. [Google Scholar] [CrossRef]
- Gupta, S.; Hanff, L.M.; Visser, W.; Steegers, E.A.; Saxena, P.R.; Vulto, A.G.; MaassenVanDenBrink, A. Functional reactivity of 5-HT receptors in human umbilical cord and maternal subcutaneous fat arteries after normotensive or pre-eclamptic pregnancy. J. Hypertens. 2006, 24, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, C.; Bodelsson, G.; Bodelsson, M.; Stjernquist, M. Characterization of 5-hydroxytryptamine receptors mediating circular smooth muscle contraction in the human umbilical artery. Gynecol. Obstet. Investig. 1999, 47, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Sabolovic Rudman, S.; Mustapic, M.; Kosec, V.; Pivac, N.; Rudman, F.; Muck-Seler, D. Serotonin risk factors for the development of hypertension in pregnancy. Arch. Gynecol. Obs. 2015, 291, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Irge, E.; Halici, Z.; Yilmaz, M.; Cadirci, E.; Karakus, E. Evaluation of 5-HT7 receptor expression in the placentae of normal and pre-eclamptic women. Clin. Exp. Hypertens. 2016, 38, 189–193. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J.; Mattson, M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010, 13, 1763–1811. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Mattson, M.P. How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech. Dis. 2017, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Arslan Azizoglu, G.; Tuncay Tanriverdi, S.; Aydin Kose, F.; Ballar Kirmizibayrak, P.; Ozer, O. Dual-Prevention for UV-Induced Skin Damage: Incorporation of Melatonin-Loaded Elastic Niosomes into Octyl Methoxycinnamate Pickering Emulsions. AAPS PharmSciTech 2017, 18, 2987–2998. [Google Scholar] [CrossRef]
- Trovato Salinaro, A.; Pennisi, M.; Di Paola, R.; Scuto, M.; Crupi, R.; Cambria, M.T.; Ontario, M.L.; Tomasello, M.; Uva, M.; Maiolino, L.; et al. Neuroinflammation and neurohormesis in the pathogenesis of Alzheimer’s disease and Alzheimer-linked pathologies: Modulation by nutritional mushrooms. Immun. Ageing I A 2018, 15, 8. [Google Scholar] [CrossRef] [Green Version]
- Miquel, S.; Champ, C.; Day, J.; Aarts, E.; Bahr, B.A.; Bakker, M.; Bánáti, D.; Calabrese, V.; Cederholm, T.; Cryan, J.; et al. Poor cognitive ageing: Vulnerabilities, mechanisms and the impact of nutritional interventions. Ageing Res. Rev. 2018, 42, 40–55. [Google Scholar] [CrossRef] [Green Version]
- Arnanz, A.; Garcia-Velasco, J.A.; Neyro, J.L. Calcifediol (25OHD) Deficiency and Its Treatment in Women’s Health and Fertility. Nutrients 2022, 14, 1820. [Google Scholar] [CrossRef]
- Neale, R.E.; Khan, S.R.; Lucas, R.M.; Waterhouse, M.; Whiteman, D.C.; Olsen, C.M. The effect of sunscreen on vitamin D: A review. Br. J. Dermatol. 2019, 181, 907–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar-Cordero, M.J.; Lasserrot-Cuadrado, A.; Mur-Villar, N.; León-Ríos, X.A.; Rivero-Blanco, T.; Pérez-Castillo, I.M. Vitamin D, preeclampsia and prematurity: A systematic review and meta-analysis of observational and interventional studies. Midwifery 2020, 87, 102707. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Fang, R.; Yu, R.; Chen, D.; Zhao, J.; Xiao, J. Maternal Vitamin D Status in the Late Second Trimester and the Risk of Severe Preeclampsia in Southeastern China. Nutrients 2017, 9, 138. [Google Scholar] [CrossRef] [Green Version]
- Fogacci, S.; Fogacci, F.; Banach, M.; Michos, E.D.; Hernandez, A.V.; Lip, G.Y.H.; Blaha, M.J.; Toth, P.P.; Borghi, C.; Cicero, A.F.G. Vitamin D supplementation and incident preeclampsia: A systematic review and meta-analysis of randomized clinical trials. Clin. Nutr. 2020, 39, 1742–1752. [Google Scholar] [CrossRef]
- Serrano-Díaz, N.C.; Gamboa-Delgado, E.M.; Domínguez-Urrego, C.L.; Vesga-Varela, A.L.; Serrano-Gómez, S.E.; Quintero-Lesmes, D.C. Vitamin D and risk of preeclampsia: A systematic review and meta-analysis. Biomed. Rev. Inst. Nac. Salud 2018, 38 (Suppl. S1), 43–53. [Google Scholar] [CrossRef]
- Shi, D.-D.; Wang, Y.; Guo, J.-J.; Zhou, L.; Wang, N. Vitamin D Enhances Efficacy of Oral Nifedipine in Treating Preeclampsia with Severe Features: A Double Blinded, Placebo-Controlled and Randomized Clinical Trial. Front. Pharmacol. 2017, 8, 865. [Google Scholar] [CrossRef] [Green Version]




| Gene | GenBank Accession No. | Primer (5′-3′) | Annealing Temperature (°C) |
|---|---|---|---|
| β-actin | NM_001101.5 | Forward: 5-CAT CCT CAC CCT GAA GTA CCC-3 Reverse: 5-AGC CTG GAT AGC AAC GTA CAT G -3 | 60 |
| 5-HT2A | NM_001165947.5 | Forward: 5-TCT TTC AGC TTC CTC CCT CA-3 Reverse: 5-TGC AGG ACT CTT TGC AGA TG -3 | 60 |
| H1 | NM_001098212.2 | Forward: 5-CAC ACT GAA CCC CCT CAT CT-3 Reverse: 5-GGC CTT CGT CCT CTA TTT CC-3 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorigo, M.; Quintaneiro, C.; Breitenfeld, L.; Cairrao, E. UV-B Filter Octylmethoxycinnamate Is a Modulator of the Serotonin and Histamine Receptors in Human Umbilical Arteries. Biomedicines 2022, 10, 1054. https://doi.org/10.3390/biomedicines10051054
Lorigo M, Quintaneiro C, Breitenfeld L, Cairrao E. UV-B Filter Octylmethoxycinnamate Is a Modulator of the Serotonin and Histamine Receptors in Human Umbilical Arteries. Biomedicines. 2022; 10(5):1054. https://doi.org/10.3390/biomedicines10051054
Chicago/Turabian StyleLorigo, Margarida, Carla Quintaneiro, Luiza Breitenfeld, and Elisa Cairrao. 2022. "UV-B Filter Octylmethoxycinnamate Is a Modulator of the Serotonin and Histamine Receptors in Human Umbilical Arteries" Biomedicines 10, no. 5: 1054. https://doi.org/10.3390/biomedicines10051054









