Recombinant Klotho Protein Ameliorates Myocardial Ischemia/Reperfusion Injury by Attenuating Sterile Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Experimental Animals
2.2. Experimental Rat Model of Myocardial I/R Injury
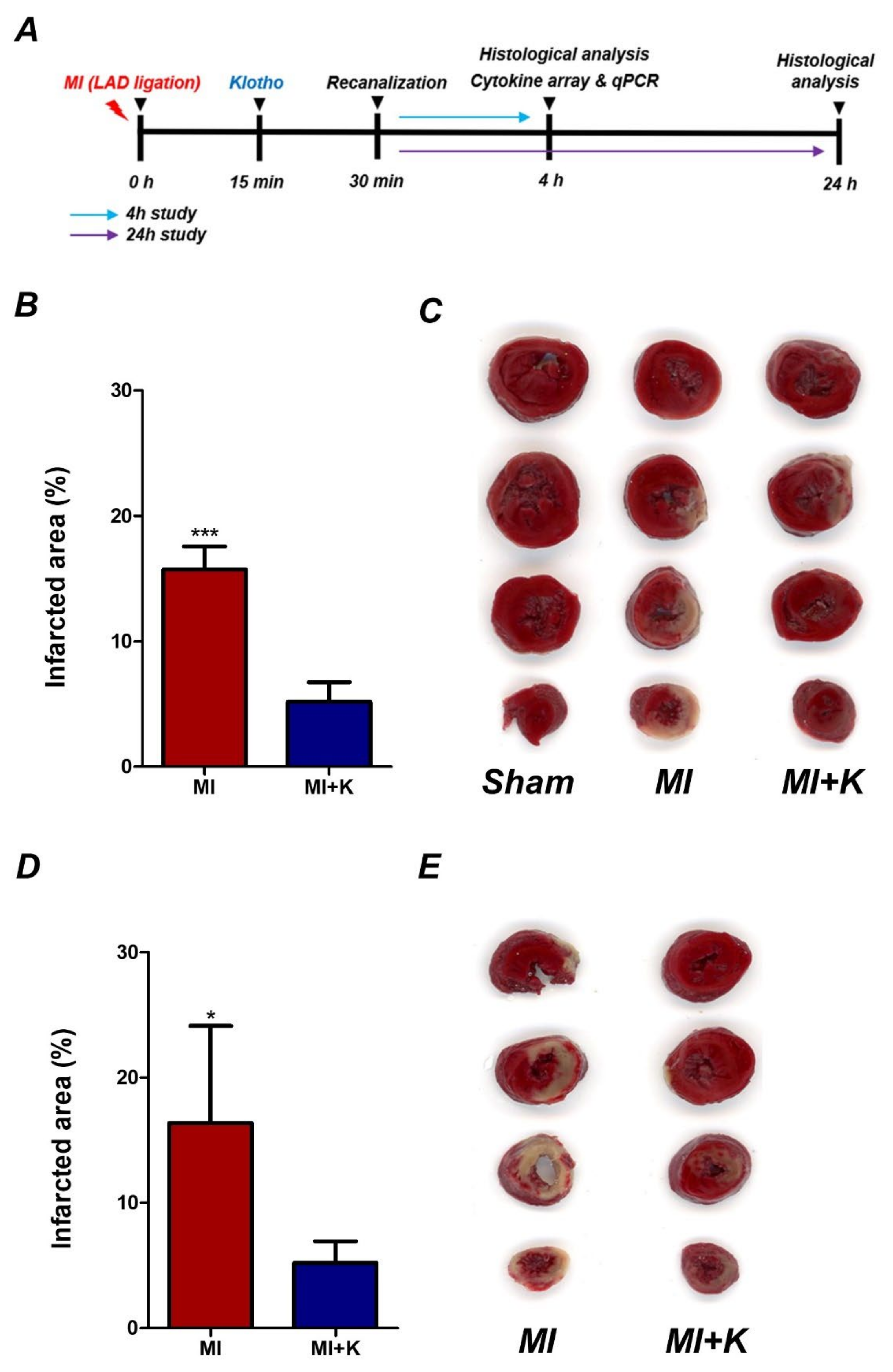
2.3. Experiment Protocol
2.4. Assessment of Infarct Volume
2.5. Immunohistochemistry Analysis
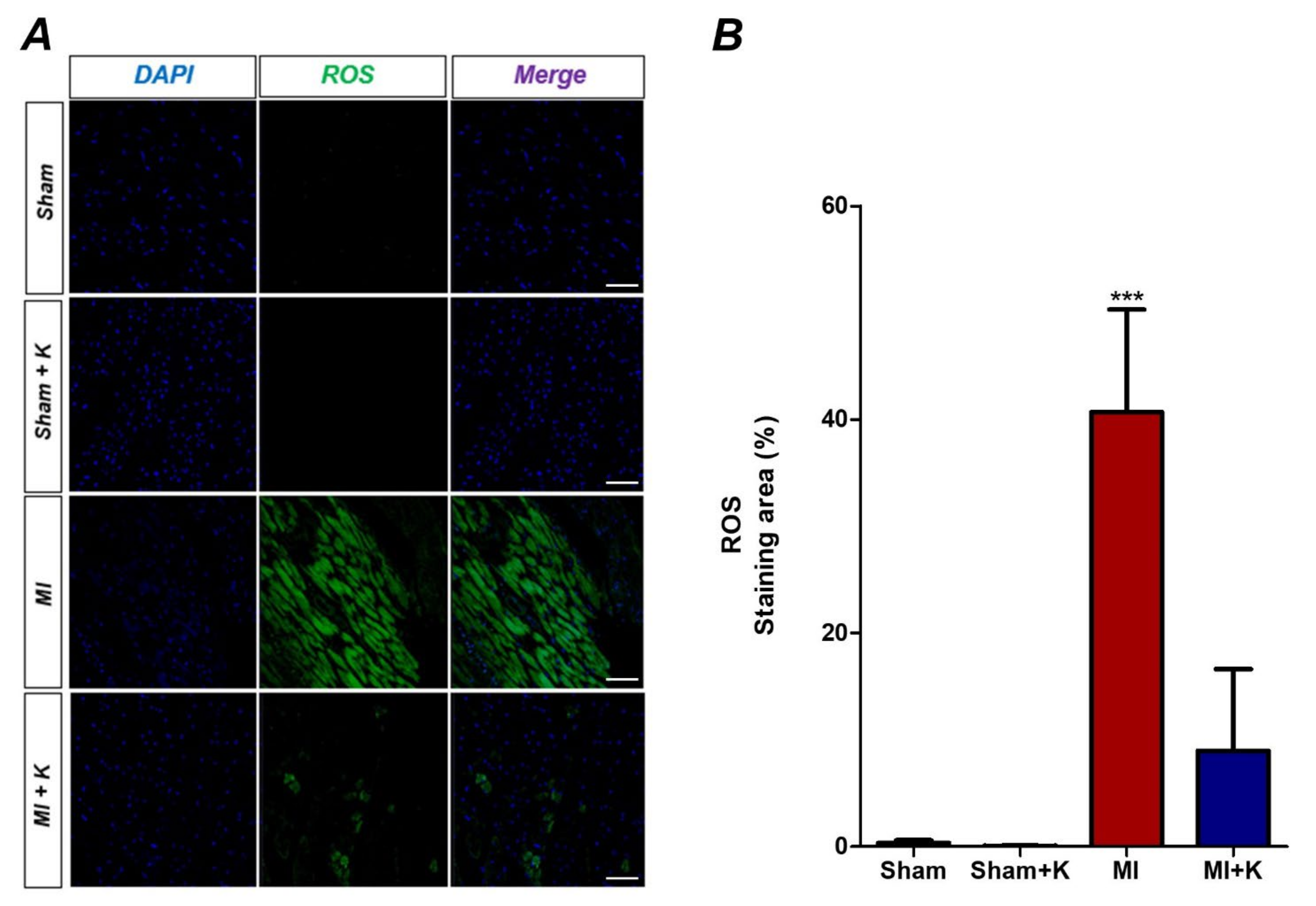
2.6. Detection of Intracellular Levels of Reactive Oxygen Species (ROS)
2.7. Enzyme-Linked Immunosorbent Assay (ELISA) for Detection of Cardiac Troponin T (cTnT) and HMGB1
2.8. Real-Time Polymerase Chain Reaction (RT-PCR)
2.9. Terminal Deoxynucleotidyl Transferase (TdT)-Mediated dUTP Nick End Labeling (TUNEL) Assay
2.10. Cytokine Array
2.11. Statistical Analysis
3. Results
3.1. Optimal rKL Dose for Myocardial Protection
3.2. Administration of rKL Protein Reduces Infarct Volume in Myocardial I/R Injury
3.3. Administration of rKL Protein Reduces Intracellular Levels of Reactive Oxygen Species (ROS)
3.4. Administration of rKL Protein Attenuates the Extracellular Release of HMGB1 from Peri-infarct Tissue after Myocardial I/R Injury and Effects of the Administration of rKL Protein on HMGB1 Levels in the Plasma
3.5. Effects of the Administration of rKL Protein on cTnT
3.6. Administration of rKL Protein Inhibited Expression of Pro-Inflammatory Cytokines from Peri-Infarct Regions
3.7. rKL Protein Attenuates Apoptosis in the Myocardium After Myocardial I/R Injury (TUNEL Assay)
3.8. Administration of rKL Protein Modulated Cytokine Production in the Myocardial I/R Injury
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAR | Area at risk |
| AMI | Acute myocardial infarction |
| ANOVA | One-way analysis of variance |
| CINC-1 | Cytokine-induced neutrophil chemoatractant 1 |
| cTnT | Cardiac troponin T |
| CXCL5 | C-X-C motif chemokine 5 |
| DCF | 2’,7’-dichlorofluorescein |
| ELISA | Enzyme-linked immunosorbent assay |
| FGF | Fibrous growth factors |
| H2DCFDA | 2’,7’-dichlorodihydrofluorescein diacetate |
| HMGB1 | High mobility group box-1 |
| I/R | Ischemic/reperfusion |
| IGF-1 | Insulin/insulin-like growth factor-1 |
| IL | Interleukin |
| IRA | Infarct-related artery |
| LAD | Left anterior descending coronary artery |
| PCI | Primary percutaneous coronary intervention |
| rKL | Recombinant klotho |
| ROS | Reactive oxygen species |
| RT-PCR | Real-time polymerase chain reaction |
| sICAM-1/CD54 | Intracellular adhesion molecule-1 |
| STEMI | ST elevation myocardial infarction |
| TBS | Tris-buffered saline |
| TLR-4 | Toll-like receptor 4 |
| TNF | Tumor necrosis factor |
| TTC | 2,3,5-triphenyltetrazolium chloride |
| TUNEL | Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling |
References
- Menees, D.S.; Peterson, E.D.; Wang, Y.; Curtis, J.P.; Messenger, J.C.; Rumsfeld, J.S.; Gurm, H.S. Door-to-balloon time and mortality among patients undergoing primary pci. N. Engl. J. Med. 2013, 369, 901–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boersma, E. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients. Eur. Heart J. 2006, 27, 779–788. [Google Scholar] [CrossRef] [Green Version]
- Greulich, S.; Mayr, A.; Gloekler, S.; Seitz, A.; Birkmeier, S.; Schäufele, T.; Bekeredjian, R.; Zuern, C.S.; Seizer, P.; Geisler, T.; et al. Time-dependent myocardial necrosis in patients with st-segment-elevation myocardial infarction without angiographic collateral flow visualized by cardiac magnetic resonance imaging: Results from the multicenter stemi-scar project. J. Am. Heart Assoc. 2019, 8, e012429. [Google Scholar] [CrossRef]
- Seiler, C.; Kirkeeide, R.L.; Gould, K.L. Measurement from arteriograms of regional myocardial bed size distal to any point in the coronary vascular tree for assessing anatomic area at risk. J. Am. Coll. Cardiol. 1993, 21, 783–797. [Google Scholar] [CrossRef] [Green Version]
- Kushner, F.G.; Hand, M.; Smith, S.C., Jr.; King, S.B., 3rd; Anderson, J.L.; Antman, E.M.; Bailey, S.R.; Bates, E.R.; Blankenship, J.C.; Casey, D.E., Jr.; et al. 2009 focused updates: Acc/aha guidelines for the management of patients with st-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and acc/aha/scai guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation 2009, 120, 2271–2306. [Google Scholar] [PubMed]
- Heusch, G.; Gersh, B.J. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: A continual challenge. Eur. Heart J. 2017, 38, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Riezzo, I.; Pascale, N.; Pomara, C.; Turillazzi, E. Ischemia/Reperfusion Injury following Acute Myocardial Infarction: A Critical Issue for Clinicians and Forensic Pathologists. Mediat. Inflamm. 2017, 2017, 7018393. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Targeting myocardial reperfusion injury--the search continues. N. Engl. J. Med. 2015, 373, 1073–1075. [Google Scholar] [CrossRef]
- Fröhlich, G.M.; Meier, P.; White, S.K.; Yellon, D.M.; Hausenloy, D.J. Myocardial reperfusion injury: Looking beyond primary pci. Eur. Heart J. 2013, 34, 1714–1722. [Google Scholar] [CrossRef] [Green Version]
- Beom, J.H.; Kim, J.H.; Seo, J.; Lee, J.H.; Chung, Y.E.; Chung, H.S.; Chung, S.P.; Kim, C.H.; You, J.S. Targeted temperature management at 33 °C or 36 °C induces equivalent myocardial protection by inhibiting hmgb1 release in myocardial ischemia/reperfusion injury. PLoS ONE 2021, 16, e0246066. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, E.J.; Seo, J.; Kavoussi, A.; Chung, Y.E.; Chung, S.P.; Park, I.; Kim, C.H.; You, J.S. Hypothermia inhibits the propagation of acute ischemic injury by inhibiting hmgb1. Mol. Brain 2016, 9, 81. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Guo, Z.; Ding, Z.; Mehta, J.L. Inflammation, autophagy, and apoptosis after myocardial infarction. J. Am. Heart Assoc. 2018, 7, e008024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, H.S.; Yang, J.; Chen, P.; Yang, J.; Bo, S.Q.; Ding, J.W.; Yu, Q.Q. The hmgb1-tlr4 axis contributes to myocardial ischemia/reperfusion injury via regulation of cardiomyocyte apoptosis. Gene 2013, 527, 389–393. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, L.; Li, W.; Fang, J. Role of high-mobility group box-1 in myocardial ischemia/reperfusion injury and the effect of ethyl pyruvate. Exp. Ther. Med. 2015, 9, 1537–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, U.; Tracey, K.J. Hmgb1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 2011, 29, 139–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, C.L.; Zhang, M.Q.; Zhang, Y.; Xu, H.X.; Wang, J.M.; An, G.P.; Wang, Y.Y.; Li, L. Glycyrrhizin protects rat heart against ischemia-reperfusion injury through blockade of hmgb1-dependent phospho-jnk/bax pathway. Acta Pharmacol. Sin. 2012, 33, 1477–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, M.V.; Pedersen, S.; Møgelvang, R.; Skov-Jensen, J.; Flyvbjerg, A. Plasma high-mobility group box 1 levels predict mortality after st-segment elevation myocardial infarction. JACC Cardiovasc. Interv. 2011, 4, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Olejnik, A.; Franczak, A.; Krzywonos-Zawadzka, A.; Kałużna-Oleksy, M.; Bil-Lula, I. The biological role of klotho protein in the development of cardiovascular diseases. Biomed. Res. Int. 2018, 2018, 5171945. [Google Scholar] [CrossRef] [Green Version]
- Manya, H.; Akasaka-Manya, K.; Endo, T. Klotho protein deficiency and aging. Geriatr. Gerontol. Int. 2010, 10 (Suppl. 1), S80–S87. [Google Scholar] [CrossRef]
- Shiraki-Iida, T.; Aizawa, H.; Matsumura, Y.; Sekine, S.; Iida, A.; Anazawa, H.; Nagai, R.; Kuro-o, M.; Nabeshima, Y. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998, 424, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.C.; Shiizaki, K.; Kuro-o, M.; Moe, O.W. Fibroblast growth factor 23 and klotho: Physiology and pathophysiology of an endocrine network of mineral metabolism. Annu. Rev. Physiol. 2013, 75, 503–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, H.J.; Oh, H.; Nam, B.Y.; You, J.S.; Ryu, D.R.; Kang, S.W.; Chung, Y.E. The protective effect of klotho against contrast-associated acute kidney injury via the antioxidative effect. American journal of physiology. Ren. Physiol. 2019, 317, F881–F889. [Google Scholar] [CrossRef]
- Shen, Y.; Yan, Y.; Lu, L.; Qian, Y.; Guan, X.; Zhang, L.; Qi, Y.; Gu, L.; Ding, F. Klotho ameliorates hydrogen peroxide-induced oxidative injury in TCMK-1 cells. Int. Urol. Nephrol. 2018, 50, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hwang, K.H.; Park, K.S.; Kong, I.D.; Cha, S.K. Biological role of anti-aging protein klotho. J. Lifestyle Med. 2015, 5, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Zheng, S.; Feng, X.; Cai, C.; Ye, X.; Liu, P. Klotho gene improves oxidative stress injury after myocardial infarction. Exp. Ther. Med. 2021, 21, 52. [Google Scholar] [CrossRef]
- Sahu, A.; Mamiya, H.; Shinde, S.N.; Cheikhi, A.; Winter, L.L.; Vo, N.V.; Stolz, D.; Roginskaya, V.; Tang, W.Y.; St Croix, C.; et al. Age-related declines in α-klotho drive progenitor cell mitochondrial dysfunction and impaired muscle regeneration. Nat. Commun. 2018, 9, 4859. [Google Scholar] [CrossRef]
- Amaro-Gahete, F.J.; Jurado-Fasoli, L.; Sanchez-Delgado, G.; García-Lario, J.V.; Castillo, M.J.; Ruiz, J.R. Relationship between plasma s-klotho and cardiometabolic risk in sedentary adults. Aging 2020, 12, 2698–2710. [Google Scholar] [CrossRef]
- Memmos, E.; Sarafidis, P.; Pateinakis, P.; Tsiantoulas, A.; Faitatzidou, D.; Giamalis, P.; Vasilikos, V.; Papagianni, A. Soluble klotho is associated with mortality and cardiovascular events in hemodialysis. BMC Nephrol. 2019, 20, 217. [Google Scholar] [CrossRef] [Green Version]
- Semba, R.D.; Cappola, A.R.; Sun, K.; Bandinelli, S.; Dalal, M.; Crasto, C.; Guralnik, J.M.; Ferrucci, L. Plasma klotho and cardiovascular disease in adults. J. Am. Geriatr. Soc. 2011, 59, 1596–1601. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.E.; Yang, D.; Li, L.; Wang, W.; Peng, Y.; Chen, C.; Chen, P.; Xia, X.; Wang, H.; Jiang, J.; et al. Prolyl hydroxylase domain protein 2 silencing enhances the survival and paracrine function of transplanted adipose-derived stem cells in infarcted myocardium. Circ. Res. 2013, 113, 288–300. [Google Scholar] [CrossRef]
- Li, L.; Yu, Q.; Liang, W. Use of 2,3,5-triphenyltetrazolium chloride-stained brain tissues for immunofluorescence analyses after focal cerebral ischemia in rats. Pathol. Res. Pract. 2018, 214, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Reid, E.; Graham, D.; Lopez-Gonzalez, M.R.; Holmes, W.M.; Macrae, I.M.; McCabe, C. Penumbra detection using pwi/dwi mismatch mri in a rat stroke model with and without comorbidity: Comparison of methods. J. Cereb. Blood Flow Metab. 2012, 32, 1765–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loukili, N.; Rosenblatt-Velin, N.; Li, J.; Clerc, S.; Pacher, P.; Feihl, F.; Waeber, B.; Liaudet, L. Peroxynitrite induces hmgb1 release by cardiac cells in vitro and hmgb1 upregulation in the infarcted myocardium in vivo. Cardiovasc. Res. 2011, 89, 586–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrassy, M.; Volz, H.C.; Igwe, J.C.; Funke, B.; Eichberger, S.N.; Kaya, Z.; Buss, S.; Autschbach, F.; Pleger, S.T.; Lukic, I.K.; et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation 2008, 117, 3216–3226. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Jin, Y.; Shin, J.H.; Kim, I.D.; Lee, H.K.; Park, S.; Han, P.L.; Lee, J.K. Glycyrrhizic acid affords robust neuroprotection in the postischemic brain via anti-inflammatory effect by inhibiting hmgb1 phosphorylation and secretion. Neurobiol. Dis. 2012, 46, 147–156. [Google Scholar] [CrossRef]
- Gong, G.; Xiang, L.; Yuan, L.; Hu, L.; Wu, W.; Cai, L.; Yin, L.; Dong, H. Protective effect of glycyrrhizin, a direct hmgb1 inhibitor, on focal cerebral ischemia/reperfusion-induced inflammation, oxidative stress, and apoptosis in rats. PLoS ONE 2014, 9, e89450. [Google Scholar] [CrossRef] [Green Version]
- Mollica, L.; De Marchis, F.; Spitaleri, A.; Dallacosta, C.; Pennacchini, D.; Zamai, M.; Agresti, A.; Trisciuoglio, L.; Musco, G.; Bianchi, M.E. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem. Biol. 2007, 14, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Kuribayashi, T. Elimination half-lives of interleukin-6 and cytokine-induced neutrophil chemoattractant-1 synthesized in response to inflammatory stimulation in rats. Lab. Anim. Res. 2018, 34, 80–83. [Google Scholar] [CrossRef]
- Ivetic, A. Signals regulating l-selectin-dependent leucocyte adhesion and transmigration. Int. J. Biochem. Cell Biol. 2013, 45, 550–555. [Google Scholar] [CrossRef]
- Rothlein, R.; Dustin, M.L.; Marlin, S.D.; Springer, T.A. A human intercellular adhesion molecule (icam-1) distinct from lfa-1. J. Immunol. 1986, 137, 1270–1274. [Google Scholar]
- Chang, M.S.; McNinch, J.; Basu, R.; Simonet, S. Cloning and characterization of the human neutrophil-activating peptide (ena-78) gene. J. Biol. Chem. 1994, 269, 25277–25282. [Google Scholar] [CrossRef]
- Choi, H.A.; Badjatia, N.; Mayer, S.A. Hypothermia for acute brain injury--mechanisms and practical aspects. Nature reviews. Neurology 2012, 8, 214–222. [Google Scholar] [PubMed]
- Hong, J.M.; Lee, J.S.; Song, H.J.; Jeong, H.S.; Choi, H.A.; Lee, K. Therapeutic hypothermia after recanalization in patients with acute ischemic stroke. Stroke 2014, 45, 134–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, N.; Wetterslev, J.; Cronberg, T.; Erlinge, D.; Gasche, Y.; Hassager, C.; Horn, J.; Hovdenes, J.; Kjaergaard, J.; Kuiper, M.; et al. Targeted temperature management at 33 °C versus 36 °C after cardiac arrest. N. Engl. J. Med. 2013, 369, 2197–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myung, J.; Beom, J.-H.; Kim, J.-H.; Woo, J.-S.; Park, I.; Chung, S.-P.; Chung, Y.-E.; You, J.-S. Recombinant Klotho Protein Ameliorates Myocardial Ischemia/Reperfusion Injury by Attenuating Sterile Inflammation. Biomedicines 2022, 10, 894. https://doi.org/10.3390/biomedicines10040894
Myung J, Beom J-H, Kim J-H, Woo J-S, Park I, Chung S-P, Chung Y-E, You J-S. Recombinant Klotho Protein Ameliorates Myocardial Ischemia/Reperfusion Injury by Attenuating Sterile Inflammation. Biomedicines. 2022; 10(4):894. https://doi.org/10.3390/biomedicines10040894
Chicago/Turabian StyleMyung, Jinwoo, Jin-Ho Beom, Ju-Hee Kim, Ji-Sun Woo, Incheol Park, Sung-Phil Chung, Yong-Eun Chung, and Je-Sung You. 2022. "Recombinant Klotho Protein Ameliorates Myocardial Ischemia/Reperfusion Injury by Attenuating Sterile Inflammation" Biomedicines 10, no. 4: 894. https://doi.org/10.3390/biomedicines10040894





