Cytokine Profiles Differentiate Symptomatic from Asymptomatic PTSD in Service Members and Veterans with Chronic Traumatic Brain Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Traumatic Brain Injury Characterization
2.3. Participant Grouping Based on PCL-C
2.4. Cytokine Concentration Measurement
2.5. Self-Report Behavioral Symptom Measures
2.6. Statistical Methods
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Behavioral Symptoms
3.3. Protein Biomarkers
3.4. Correlation Analysis between Plasma Proteins and Behavioral Symptoms
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lindquist, L.K.; Love, H.C.; Elbogen, E.B. Traumatic Brain Injury in Iraq and Afghanistan Veterans: New Results From a National Random Sample Study. J. Neuropsychiatry 2017, 29, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Hoencamp, R.; Vermetten, E.; Tan, E.C.; Putter, H.; Leenen, L.P.; Hamming, J.F. Systematic review of the prevalence and characteristics of battle casualties from NATO coalition forces in Iraq and Afghanistan. Injury 2014, 45, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Department of Defense. Numbers for Traumatic Brain Injury Worldwide; Department of Defense: Washington, DC, USA, 2022. [Google Scholar]
- Awan, N.; DiSanto, D.; Juengst, S.B.; Kumar, R.G.; Bertisch, H.; Niemeier, J.; Fann, J.R.; Kesinger, M.R.; Sperry, J.; Wagner, A.K. Evaluating the Cross-Sectional and Longitudinal Relationships Predicting Suicidal Ideation Following Traumatic Brain Injury. J. Head Trauma Rehabil. 2020, 36, E18–E29. [Google Scholar] [CrossRef]
- Greer, N.; Ackland, P.; Sayer, N.; Spoont, M.; Taylor, B.; MacDonald, R.; McKenzie, L.; Rosebush, C.; Wilt, T.J. Relationship of Deployment-Related Mild Traumatic Brain Injury to Posttraumatic Stress Disorder, Depressive Disorders, Substance Use Disorders, Suicidal Ideation, and Anxiety Disorders: A Systematic Review; Department of Veterans Affairs: Washington, DC, USA, 2019. [Google Scholar]
- McIntire, K.L.; Crawford, K.M.; Perrin, P.B.; Sestak, J.L.; Aman, K.; Walter, L.A.; Page, D.B.; Wen, H.; Randolph, B.O.; Brunner, R.C.; et al. Factors Increasing Risk of Suicide after Traumatic Brain Injury: A State-of-the-Science Review of Military and Civilian Studies. Brain Inj. 2021, 35, 151–163. [Google Scholar] [CrossRef]
- De Berardis, D.; Vellante, F.; Fornaro, M.; Anastasia, A.; Olivieri, L.; Rapini, G.; Serroni, N.; Orsolini, L.; Valchera, A.; Carano, A.; et al. Alexithymia, suicide ideation, affective temperaments and homocysteine levels in drug naïve patients with post-traumatic stress disorder: An exploratory study in the everyday ‘real world’ clinical practice. Int. J. Psychiatry Clin. Pract. 2020, 24, 83–87. [Google Scholar] [CrossRef] [PubMed]
- De Berardis, D.; Marini, S.; Serroni, N.; Iasevoli, F.; Tomasetti, C.; de Bartolomeis, A.; Mazza, M.; Tempesta, D.; Valchera, A.; Fornaro, M.; et al. Targeting the Noradrenergic System in Posttraumatic Stress Disorder: A Systematic Review and Meta-Analysis of Prazosin Trials. Curr. Drug Targets 2015, 16, 1094–1106. [Google Scholar] [CrossRef]
- Yurgil, K.A.; Barkauskas, D.A.; Vasterling, J.J.; Nievergelt, C.M.; Larson, G.E.; Schork, N.J.; Litz, B.T.; Nash, W.P.; Baker, D.G.; Marine Resiliency Study Team. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty Marines. JAMA Psychiatry 2014, 71, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Stein, M.B.; Kessler, R.C.; Heeringa, S.G.; Jain, S.; Campbell-Sills, L.; Colpe, L.J.; Fullerton, C.S.; Nock, M.K.; Sampson, N.A.; Schoenbaum, M.; et al. Prospective longitudinal evaluation of the effect of deployment-acquired traumatic brain injury on posttraumatic stress and related disorders: Results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). Am. J. Psychiatry 2015, 172, 1101–1111. [Google Scholar] [CrossRef] [Green Version]
- Stein, M.B.; Jain, S.; Giacino, J.T.; Levin, H.; Dikmen, S.; Nelson, L.D.; Vassar, M.J.; Okonkwo, D.O.; Diaz-Arrastia, R.; Robertson, C.S.; et al. Risk of Posttraumatic Stress Disorder and Major Depression in Civilian Patients After Mild Traumatic Brain Injury: A TRACK-TBI Study. JAMA Psychiatry 2019, 76, 249–258. [Google Scholar] [CrossRef]
- Warren, A.M.; Boals, A.; Elliott, T.R.; Reynolds, M.; Weddle, R.J.; Holtz, P.; Trost, Z.; Foreman, M.L. Mild traumatic brain injury increases risk for the development of posttraumatic stress disorder. J. Trauma Acute Care Surg. 2015, 79, 1062–1066. [Google Scholar] [CrossRef]
- Whelan-Goodinson, R.; Ponsford, J.; Johnston, L.; Grant, F. Psychiatric disorders following traumatic brain injury: Their nature and frequency. J. Head Trauma Rehabil. 2009, 24, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Risbrough, V.B.; Vaughn, M.N.; Friend, S.F. Role of Inflammation in Traumatic Brain Injury-Associated Risk for Neuropsychiatric Disorders: State of the Evidence and Where Do We Go From Here. Biol. Psychiatry 2022, 91, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Rodney, T.; Taylor, P.; Dunbar, K.; Perrin, N.; Lai, C.; Roy, M.; Gill, J. High IL-6 in military personnel relates to multiple traumatic brain injuries and post-traumatic stress disorder. Behav. Brain Res. 2020, 392, 112715. [Google Scholar] [CrossRef] [PubMed]
- Kanefsky, R.; Motamedi, V.; Mithani, S.; Mysliwiec, V.; Gill, J.M.; Pattinson, C.L. Mild traumatic brain injuries with loss of consciousness are associated with increased inflammation and pain in military personnel. Psychiatry Res. 2019, 279, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Pierce, M.E.; Hayes, J.; Huber, B.R.; Jeromin, A.; Fortier, C.B.; Fonda, J.R.; Lasseter, H.; Chaby, L.; McGlinchey, R.; Milberg, W. Plasma biomarkers associated with deployment trauma and its consequences in post-9/11 era veterans: Initial findings from the TRACTS longitudinal cohort. Transl. Psychiatry 2022, 12, 80. [Google Scholar] [CrossRef]
- Kruse, J.L.; Olmstead, R.; Hellemann, G.; Breen, E.C.; Tye, S.J.; Brooks, J.O., 3rd; Wade, B.; Congdon, E.; Espinoza, R.; Narr, K.L.; et al. Interleukin-8 and lower severity of depression in females, but not males, with treatment-resistant depression. J. Psychiatr. Res. 2021, 140, 350–356. [Google Scholar] [CrossRef]
- Juengst, S.B.; Kumar, R.G.; Failla, M.D.; Goyal, A.; Wagner, A.K. Acute Inflammatory Biomarker Profiles Predict Depression Risk Following Moderate to Severe Traumatic Brain Injury. J. Head Trauma Rehabil. 2015, 30, 207–218. [Google Scholar] [CrossRef]
- Ruggiero, K.J.; Del Ben, K.; Scotti, J.; Rabalais, A.E. Psychometric properties of the PTSD checklist—Civilian version. J. Trauma. Stress 2003, 16, 495–502. [Google Scholar] [CrossRef]
- Keane, T.M.; Fairbank, J.A.; Caddell, J.M.; Zimering, R.T.; Taylor, K.L.; Mora, C.A. Clinical evaluation of a measure to assess combat exposure. Psychol. Assess. J. Consult. Clin. Psychol. 1989, 1, 53–55. [Google Scholar] [CrossRef]
- Diener, E.; Emmons, R.A.; Larsen, R.J.; Griffin, S. The Satisfaction With Life Scale. J. Personal. Assess. 1985, 49, 71–75. [Google Scholar] [CrossRef]
- Johns, M.W. Reliability and Factor Analysis of the Epworth Sleepiness Scale. Sleep 1992, 15, 376–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.A. Review of the Neurobehavioral Symptom Inventory. Rehabil. Psychol. 2021, 66, 170–182. [Google Scholar] [CrossRef]
- Fann, J.R.; Bombardier, C.H.; Dikmen, S.; Esselman, P.; Warms, C.A.; Pelzer, E.; Rau, H.; Temkin, N. Validity of the Patient Health Questionnaire-9 in assessing depression following traumatic brain injury. J. Head Trauma Rehabil. 2005, 20, 501–511. [Google Scholar] [CrossRef]
- Ware, J.E., Jr. SF-36 health survey update. Spine 2000, 25, 3130–3139. [Google Scholar] [CrossRef] [PubMed]
- Kossmann, T.; Stahel, P.F.; Lenzlinger, P.M.; Redl, H.; Dubs, R.W.; Trentz, O.; Schlag, G.; Morganti-Kossmann, C. Interleukin-8 Released into the Cerebrospinal Fluid after Brain Injury is Associated with Blood–Brain Barrier Dysfunction and Nerve Growth Factor Production. J. Cereb. Blood Flow Metab. 1997, 17, 280–289. [Google Scholar] [CrossRef]
- Woodcock, T.; Morganti-Kossmann, M.C. The Role of Markers of Inflammation in Traumatic Brain Injury. Front. Neurol. 2013, 4, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aloisi, F.; Care, A.; Borsellino, G.; Gallo, P.; Cabibbo, A.; Testa, U.; Levi, G.; Peschle, C. Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1β, and tumor necrosis factor-α. J. Immunol. 1992, 149, 2358–2366. [Google Scholar]
- Kasahara, T.; Mukaida, N.; Yamashita, K.; Yagisawa, H.; Akahoshi, T.; Matsushima, K. IL-1 and TNF-alpha induction of IL-8 and monocyte chemotactic and activating factor (MCAF) mRNA expression in a human astrocytoma cell line. Immunology 1991, 74, 60–67. [Google Scholar]
- Polat, Ö.; Uçkun, Ö.M.; Tuncer, C.; Belen, A.D. Is IL-8 level an indicator of clinical and radiological status of traumatic brain injury? Turk. J. Trauma Emerg. Surg. 2019, 25, 193–197. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, D.; Guan, Z.; Wang, X. Disturbance of Serum Interleukin-2 and Interleukin-8 Levels in Posttraumatic and Non-Posttraumatic Stress Disorder Earthquake Survivors in Northern China. Neuroimmunomodulation 2007, 14, 248–254. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, X.-Z.; Li, X.; Chen, Z.; Benedek, D.M.; Fullerton, C.S.; Wynn, G.; Naifeh, J.A.; Wu, H.; Benfer, N.; et al. Potential chemokine biomarkers associated with PTSD onset, risk and resilience as well as stress responses in US military service members. Transl. Psychiatry 2020, 10, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieter, J.N.; Engel, S.D. Traumatic Brain Injury and Posttraumatic Stress Disorder: Comorbid Consequences of War. Neurosci. Insights 2019, 14, 1179069519892933. [Google Scholar] [CrossRef] [PubMed]
- Reisman, M. PTSD Treatment for Veterans: What’s Working, What’s New, and What’s Next. Pharm. Ther. 2016, 41, 623–634. [Google Scholar]
- Zou, W.; Feng, R.; Yang, Y. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naïve patients with major depression. PLoS ONE 2018, 13, e0197267. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Suchankova, P.; Ekman, A.; Erhardt, S.; Sellgren, C.; Samuelsson, M.; Westrin, A.; Minthon, L.; Hansson, O.; Träskman-Bendz, L.; et al. Low IL-8 is associated with anxiety in suicidal patients: Genetic variation and decreased protein levels. Acta Psychiatr. Scand. 2015, 131, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Chaban, V.; Clarke, G.J.; Skandsen, T.; Islam, R.; Einarsen, C.E.; Vik, A.; Damås, J.K.; Mollnes, T.E.; Håberg, A.K.; Pischke, S.E. Systemic Inflammation Persists the First Year after Mild Traumatic Brain Injury: Results from the Prospective Trondheim Mild Traumatic Brain Injury Study. J. Neurotrauma 2020, 37, 2120–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sass, D.; Guedes, V.A.; Smith, E.G.; Vorn, R.; Devoto, C.; Edwards, K.A.; Mithani, S.; Hentig, J.; Lai, C.; Wagner, C.; et al. Sex Differences in Behavioral Symptoms and the Levels of Circulating GFAP, Tau, and NfL in Patients With Traumatic Brain Injury. Front. Pharmacol. 2021, 12, 746491. [Google Scholar] [CrossRef]
- Edwards, K.A.; Gill, J.M.; Pattinson, C.L.; Lai, C.; Brière, M.; Rogers, N.J.; Milhorn, D.; Elliot, J.; Carr, W. Interleukin-6 is associated with acute concussion in military combat personnel. BMC Neurol. 2020, 20, 209. [Google Scholar] [CrossRef]
- Lima, B.B.; Hammadah, M.; Wilmot, K.; Pearce, B.D.; Shah, A.; Levantsevych, O.; Kaseer, B.; Obideen, M.; Gafeer, M.M.; Kim, J.H.; et al. Posttraumatic stress disorder is associated with enhanced interleukin-6 response to mental stress in subjects with a recent myocardial infarction. Brain Behav. Immun. 2019, 75, 26–33. [Google Scholar] [CrossRef]
- Hussein, S.; Dalton, B.; Willmund, G.D.; Ibrahim, M.A.A.; Himmerich, H. A Systematic Review of Tumor Necrosis Factor-α in Post-Traumatic Stress Disorder: Evidence from Human and Animal Studies. Psychiatr. Danub. 2017, 29, 407–420. [Google Scholar] [CrossRef]
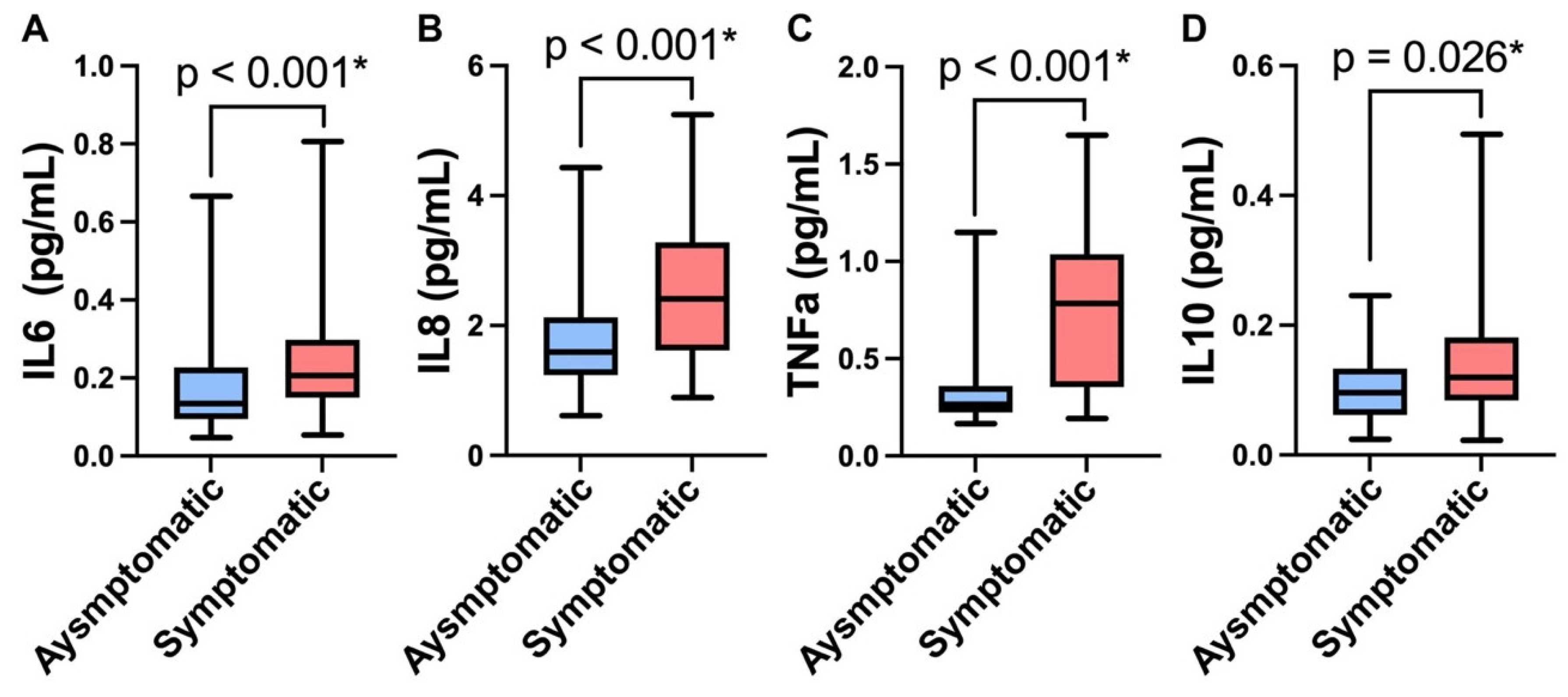
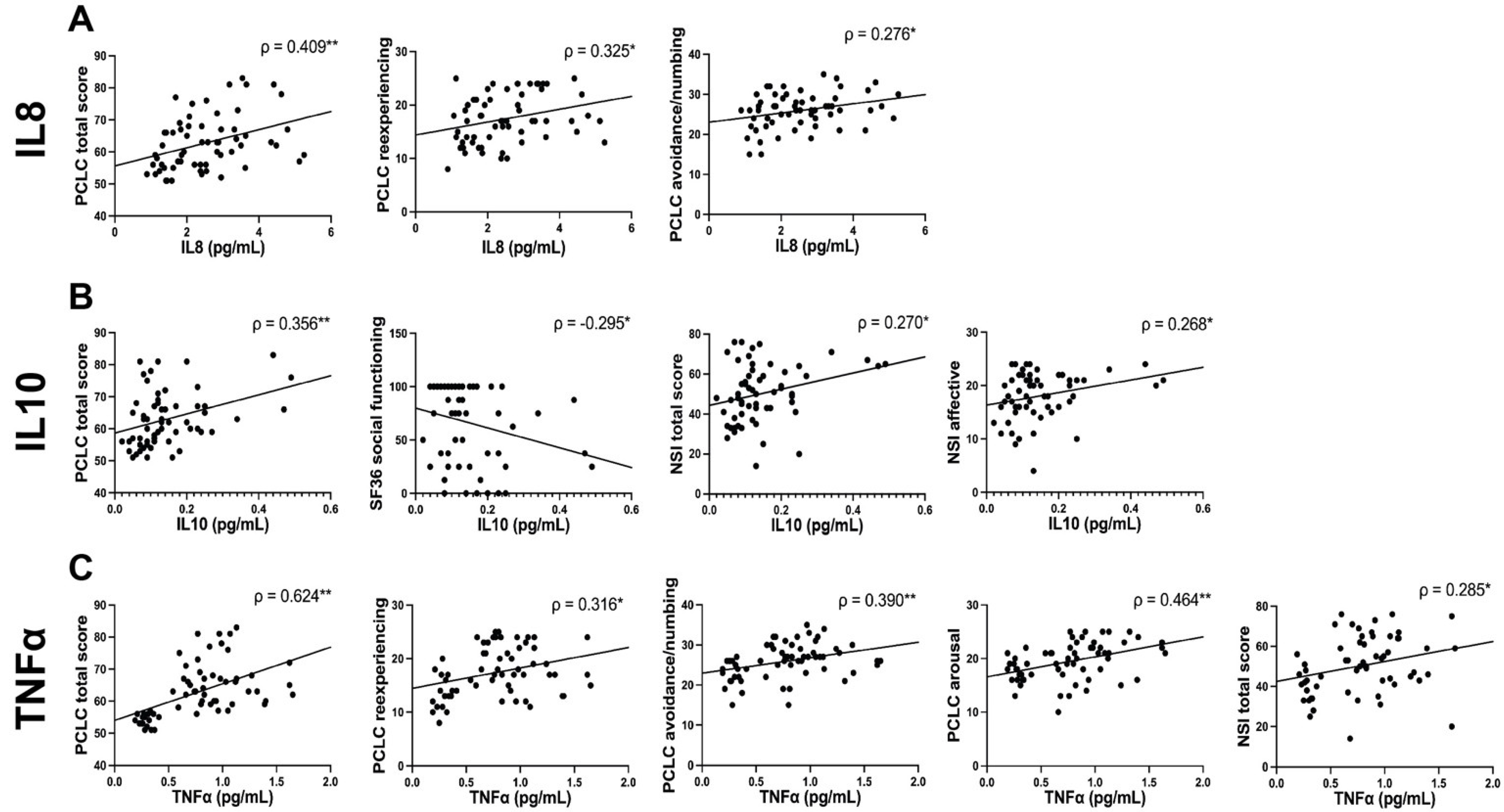
| Characteristic | PTSD High, N = 62 1 | PTSD Low, N = 61 1 | p-Value 2 | Cohen’s d | 95% Confidence Interval |
|---|---|---|---|---|---|
| Age | 37.00 (31.00, 45.00) | 36.00 (29.50, 43.50) | 0.474 | −0.16 | (−0.51, 0.20) |
| Body Mass Index (kg/m2) | 28.75 (26.30, 32.13) | 27.30 (24.65, 29.85) | 0.031 * | −0.39 | (−0.75, −0.04) |
| Sex | 0.165 | ||||
| Male | 47 (75.8%) | 53 (86.9%) | |||
| Female | 15 (24.2%) | 8 (13.1%) | |||
| Race | 0.529 | ||||
| White | 44 (71%) | 50 (82%) | |||
| Black or African-American | 13 (21%) | 7 (11.5%) | |||
| Asian | 3 (4.8%) | 3 (4.9%) | |||
| American Indian or Alaska Native/Inuit | 1 (1.6%) | 1 (1.6%) | |||
| Native Hawaiian or Other Pacific Islander | 1 (1.6%) | 0 (0%) | |||
| Ethnicity | 0.603 | ||||
| Not Hispanic or Latino | 52 (83.9%) | 54 (88.5%) | |||
| Hispanic or Latino | 10 (16.1%) | 7 (11.5%) | |||
| Military Status | 0.108 | ||||
| Active duty military | 42 (67.7%) | 51 (83.6%) | |||
| Retired from military | 10 (16.1%) | 5 (8.2%) | |||
| Veteran | 7 (11.3%) | 1 (1.6%) | |||
| National Guard | 1 (1.6%) | 3 (4.9%) | |||
| Reserve component | 1 (1.6%) | 1 (1.6%) | |||
| Inactive reserve | 1 (1.6%) | 0 (0%) | |||
| Most recent TBI severity | 0.365 | ||||
| mTBI | 57 (91.93%) | 54 (88.52%) | |||
| moTBI | 4 (6.45%) | 3 (4.91%) | |||
| sTBI | 1 (1.61%) | 4 (6.55%) | |||
| Highest TBI severity | 0.034 * | ||||
| mTBI | 49 (79.03%) | 52 (85.24%) | |||
| moTBI | 12 (19.35%) | 4 (6.55%) | |||
| sTBI | 1 (1.61%) | 5 (8.19%) | |||
| Number of TBIs | 4.00 (2.00, 6.00) | 2.00 (1.00, 4.00) | <0.001 * | −0.67 | (−1.03, −0.31) |
| TSI (years) | 4.27 (1.54, 8.70) | 8.26 (3.31, 14.13) | 0.002 * | 0.61 | (0.25, 0.97) |
| CES | 18.00 (8.25, 25.25) | 4.00 (0.00, 17.50) | <0.001 * | −0.71 | (−1.07, −0.34) |
| SWLS | 24.00 (18.00, 29.25) | 22.5 (15.25, 28.75) | 0.347 | −0.18 | (−0.54, 0.17) |
| ESS | 8.50 (5.00, 13.25) | 10.00 (6.00, 14.00) | 0.282 | 0.19 | (−0.17, 0.54) |
| PHQ9 | 4.00 (1.00, 12.50) | 12.00 (2.00, 18.00) | 0.066 | 0.42 | (0.06, 0.77) |
| NSI total | 50.00 (41.00, 61.50) | 7.00 (3.00, 13.00) | <0.001 * | −3.62 | (−4.19, −3.03) |
| NSI vestibular | 4.00 (2.00, 7.00) | 0.00 (0.00, 1.00) | <0.001 * | −1.61 | (−2.02, −1.20) |
| NSI somatosensory | 12.50 (8.75, 17.00) | 1.00 (0.00, 4.00) | <0.001 * | −2.20 | (−2.64, −1.74) |
| NSI cognitive | 12.00 (9.00, 14.00) | 2.00 (0.00, 3.00) | <0.001 * | −2.79 | (−3.29, −2.29) |
| NSI affective | 19.00 (16.00, 21.00) | 2.00 (0.50, 3.50) | <0.001 * | −4.48 | (−5.15, −3.82) |
| PCL-C total | 62.00 (56.00, 67.00) | 20.00 (17.00, 22.00) | <0.001 * | −6.85 | (−7.78, −5.91) |
| PCL-C reexperiencing | 17.00 (13.75, 21.25) | 5.00 (5.00, 6.00) | <0.001 * | −3.54 | (−4.10, −2.96) |
| PCL-C avoidance numbing | 26.00 (23.00, 29.00) | 8.00 (7.00, 9.00) | <0.001 * | −5.59 | (−6.37, −4.80) |
| PCL-C arousal | 20.00 (17.00, 22.00) | 6.00 (5.00, 7.00) | <0.001 * | −4.89 | (−5.60, −4.18) |
| SF-36 subscales | |||||
| Physical Functioning | 95.00 (78.75, 100.00) | 80.00 (50.00, 100.00) | 0.007 * | −0.49 | (−0.85, −0.13) |
| Role Limitations Due To Physical Problems | 50.00 (0.00, 100.00) | 50.00 (0.00, 100.00) | 0.829 | −0.04 | (−0.39, 0.32) |
| Role Limitations Due to Emotional Problems | 66.67 (0.00, 100.00) | 33.33 (0.00, 100.00) | 0.061 | −0.35 | (−0.70, 0.01) |
| Vitality | 47.50 (25.00, 65.00) | 30.00 (12.50, 65.00) | 0.063 | −0.31 | (−0.66, 0.05) |
| Emotional Well-Being | 78.00 (40.00, 92.00) | 60.00 (32.00, 92.00) | 0.191 | −0.30 | (−0.66, 0.05) |
| Social Functioning | 75.00 (37.50, 100.00) | 37.50 (12.50, 100.00) | 0.013 * | −0.49 | (−0.85, −0.13) |
| Pain | 62.50 (31.88, 90.00) | 45.00 (22.50, 80.00) | 0.162 | −0.27 | (−0.62, 0.09) |
| General Health | 70.00 (40.00, 85.00) | 55.00 (40.00, 80.00) | 0.338 | −0.17 | (−0.52, 0.18) |
| Predictors | Exp(B) | p-Value | |
|---|---|---|---|
| Plasma IL6 | |||
| BMI | 1.040 | 0.451 | |
| Number of TBIs | 1.129 | 0.228 | |
| Time since injury | 0.928 | 0.024 * | |
| CES total | 1.056 | 0.006 * | |
| Plasma IL6 | 2.565 | 0.002 * | |
| Plasma IL8 | |||
| BMI | 1.081 | 0.139 | |
| Number of TBIs | 1.298 | 0.014 * | |
| Time since injury | 0.951 | 0.115 | |
| CES total | 1.037 | 0.069 | |
| Plasma IL8 | 4.623 | <0.001 * | |
| Plasma IL10 | |||
| BMI | 1.110 | 0.046 * | |
| Number of TBIs | 1.271 | 0.020 * | |
| Time since injury | 0.931 | 0.031 * | |
| CES total | 1.052 | 0.014 * | |
| Plasma IL10 | 2.666 | 0.001 * | |
| Plasma TNFα | |||
| BMI | 1.065 | 0.289 | |
| Number of TBIs | 1.248 | 0.059 | |
| Time since injury | 0.937 | 0.065 | |
| CES total | 1.048 | 0.033 * | |
| Plasma TNFα | 5.850 | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, E.G.; Hentig, J.; Martin, C.; Wagner, C.; Guedes, V.A.; Edwards, K.A.; Devoto, C.; Dunbar, K.; Roy, M.J.; Gill, J.M. Cytokine Profiles Differentiate Symptomatic from Asymptomatic PTSD in Service Members and Veterans with Chronic Traumatic Brain Injury. Biomedicines 2022, 10, 3289. https://doi.org/10.3390/biomedicines10123289
Smith EG, Hentig J, Martin C, Wagner C, Guedes VA, Edwards KA, Devoto C, Dunbar K, Roy MJ, Gill JM. Cytokine Profiles Differentiate Symptomatic from Asymptomatic PTSD in Service Members and Veterans with Chronic Traumatic Brain Injury. Biomedicines. 2022; 10(12):3289. https://doi.org/10.3390/biomedicines10123289
Chicago/Turabian StyleSmith, Ethan G., James Hentig, Carina Martin, Chelsea Wagner, Vivian A. Guedes, Katie A. Edwards, Christina Devoto, Kerri Dunbar, Michael J. Roy, and Jessica M. Gill. 2022. "Cytokine Profiles Differentiate Symptomatic from Asymptomatic PTSD in Service Members and Veterans with Chronic Traumatic Brain Injury" Biomedicines 10, no. 12: 3289. https://doi.org/10.3390/biomedicines10123289





