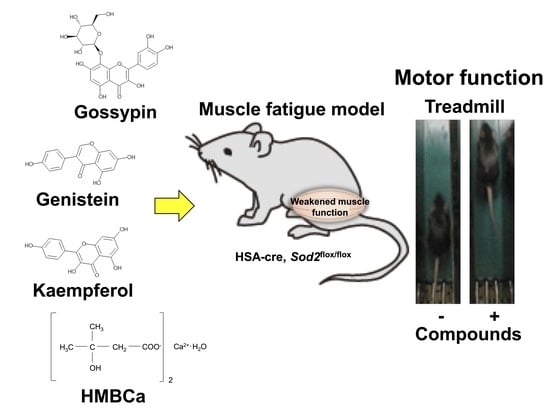
Natural Compounds That Enhance Motor Function in a Mouse Model of Muscle Fatigue
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Genotyping
2.2. Substances
2.3. Administration
2.4. Treadmill Protocol
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HMB | β-hydroxy-β-methylbutyrate |
| I. P. | intraperitoneal |
| muscle-Sod2-/- | muscle-specific SOD2 deficient |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
References
- Hamilton, M.L.; Van Remmen, H.; Drake, J.A.; Yang, H.; Guo, Z.M.; Kewitt, K.; Walter, C.A.; Richardson, A. Does oxidative damage to DNA increase with age? Proc. Natl. Acad. Sci. USA 2001, 98, 10469–10474. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Bigelow, M.L.; Kahl, J.; Singh, R.; Coenen-Schimke, J.; Raghavakaimal, S.; Nair, K.S. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. USA 2005, 102, 5618–5623. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G.; Wehling-Henricks, M. The role of free radicals in the pathophysiology of muscular dystrophy. J. Appl. Physiol. 2007, 102, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, H.; Horie, T.; Ishikawa, S.; Tsuda, C.; Kawakami, S.; Noda, Y.; Kaneko, T.; Tahara, S.; Tachibana, T.; Okabe, M.; et al. Oxidative stress in skeletal muscle causes severe disturbance of exercise activity without muscle atrophy. Free Radic. Biol. Med. 2010, 48, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Nojiri, H.; Kawakami, S.; Uchiyama, S.; Shirasawa, T. Model mice for tissue-specific deletion of the manganese superoxide dismutase gene. Geriatr. Gerontol. Int. 2010, 10 (Suppl. 1), S70–S79. [Google Scholar] [CrossRef]
- Kobayashi, K.; Nojiri, H.; Saita, Y.; Morikawa, D.; Ozawa, Y.; Watanabe, K.; Koike, M.; Asou, Y.; Shirasawa, T.; Yokote, K.; et al. Mitochondrial superoxide in osteocytes perturbs canalicular networks in the setting of age-related osteoporosis. Sci. Rep. 2015, 5, 9148. [Google Scholar] [CrossRef]
- Koike, M.; Nojiri, H.; Ozawa, Y.; Watanabe, K.; Muramatsu, Y.; Kaneko, H.; Morikawa, D.; Kobayashi, K.; Saita, Y.; Sasho, T.; et al. Mechanical overloading causes mitochondrial superoxide and SOD2 imbalance in chondrocytes resulting in cartilage degeneration. Sci. Rep. 2015, 5, 11722. [Google Scholar] [CrossRef]
- Hermes, T.A.; Mizobuti, D.S.; da Rocha, G.L.; da Silva, H.N.M.; Covatti, C.; Pereira, E.C.L.; Ferretti, R.; Minatel, E. Tempol improves redox status in mdx dystrophic diaphragm muscle. Int. J. Exp. Pathol. 2020, 101, 289–297. [Google Scholar] [CrossRef]
- Ganapaty, S.; Chandrashekhar, V.M.; Chitme, H.R.; Narsu, M.L. Free radical scavenging activity of gossypin and nevadensin: An in-vitro evaluation. Ind. J. Pharmacol. 2007, 39, 281–283. [Google Scholar] [CrossRef]
- Kunnumakkara, B.K.; Neir, A.S.; Ahn, K.S.; Pandey, M.K.; Yi, Z.; Liu, M.; Aggarwal, B.B. Gossypin, a pentahydroxy glucosyl flavone, inhibits the transforming growth factor beta-activated kinase-1-mediated NF-κB activation pathway, leading to potentiation of apoptosis, suppression of invasion, and abrogation of osteoclastogenesis. Blood 2007, 109, 5112–5121. [Google Scholar] [CrossRef]
- Wang, L.; Tu, Y.C.; Lian, T.W.; Hung, J.T.; Yen, J.H.; Wu, M.J. Distinctive antioxidant and antiinflammatory effects of flavonols. J. Agric. Food Chem. 2006, 54, 9798–9804. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M. The role of oxidative stress in carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 239–267. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, C.G.; Haenen, G.R.; van Acker, F.A.; van der Vijgh, W.J.; Bast, A. Flavonoids as peroxynitrite scavengers: The role of the hydroxyl groups. Toxicol. In Vitro 2001, 15, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, L.; Rosso, A.; Folador, P.; Figueiredo, M.S.; Pizzolatti, M.G.; Leite, L.D.; Silva, F.R. Insulinomimetic effect of kaempferol 3-neohesperidoside on the rat soleus muscle. J. Nat. Prod. 2008, 71, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Vukovich, M.D.; Slater, G.; Macchi, M.B.; Turner, M.J.; Fallon, K.; Boston, T.; Rathmacher, J. β-hydroxy-β-methylbutyrate (HMB) kinetics and the influence of glucose ingestion in humans. J. Nutr. Biochem. 2001, 12, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.D.; Kang, H.E.; Yang, S.H.; Lee, M.G.; Shin, W.G. Pharmacokinetics and first-pass metabolism of astaxanthin in rats. Br. J. Nutr. 2011, 105, 220–227. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, X.; Cai, Q.; Zhuang, J.; Tan, X.; He, W.; Zhao, M. Protective effect of taxifolin on H2O2-induced H9C2 cell pyroptosis. J. Cent. South Univ. Med. Sci. 2017, 42, 1367–1374. [Google Scholar]
- Haraguchi, H.; Mochida, Y.; Sakai, S.; Masuda, H.; Tamura, Y.; Mizutani, K.; Tanaka, O.; Chou, W.H. Protection against oxidative damage by dihydroflavonols in Engelhardtia chrysolepis. Biosci. Biotechnol. Biochem. 1996, 60, 945–948. [Google Scholar] [CrossRef]
- Roy, A.; Sharma, A.K.; Nellore, K.; Narkar, V.A.; Kumar, A. TAK1 preserves skeletal muscle mass and mitochondrial function through redox homeostasis. FASEB BioAdvances 2020, 2, 538–553. [Google Scholar] [CrossRef]
- Oshida, Y.; Kako, M.; Nakai, N.; Shimomura, Y.; Li, L.; Sato, J.; Ohsawa, I.; Sato, Y. Troglitazone improves insulin-stimulated glucose utilization associated with an increased muscle glycogen content in obese Zucker rats. Endocr. J. 1999, 46, 723–730. [Google Scholar] [CrossRef][Green Version]
- Wilson, J.M.; Fitschen, P.J.; Campbell, B.; Wilson, G.J.; Zanchi, N.; Taylor, L.; Wilborn, C.; Kalman, D.S.; Stout, J.R.; Hoffman, J.R.; et al. International Society of Sports Nutrition Position Stand: Beta-hydroxy-beta-methylbutyrate (HMB). J. Int. Soc. Sports Nutr. 2013, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, N.E.; Gerlinger-Romero, F.; Guimaraes-Ferreira, L.; de Siqueira Filho, M.A.; Felitti, V.; Lira, F.S.; Seelaender, M.; Lancha, A.H., Jr. HMB supplementation: Clinical and athletic performance-related effects and mechanisms of action. Amino Acids 2011, 40, 1015–1025. [Google Scholar] [CrossRef] [PubMed]




| Compounds | Supplier | Dosage (mg/kg) | Administration | Relative Change | t-Test | n |
|---|---|---|---|---|---|---|
| Positive effects | ||||||
| Acacetin | Merck | 50 | I. P. | 1.26 | 0.07 | 5 |
| Aesculin | LKT Laboratories | 100 | I. P. | 1.41 | 0.36 | 5 |
| AICAR | Merck | 500 | I. P. | 1.01 | 0.65 | 4 |
| Alliin | Merck | 10 | I. P. | 1.29 | 0.33 | 5 |
| Allopurinol | Merck | 100 | I. P. | 1.08 | 0.26 | 4 |
| Apigenin | Merck | 50 | I. P. | 1.03 | 0.63 | 5 |
| Apocynin | Merck | 300 | I. P. | 1.27 | 0.31 | 5 |
| Ascorbic acid | Merck | 500 | I. P. | 1.26 | 0.29 | 10 |
| Asparagine | Merck | 500 | I. P. | 1.90 | 0.21 | 4 |
| Astaxanthin | Merck | 500 | Oral | 1.24 | 0.04 | 4 |
| ATP | Merck | 2000 | Oral | 1.07 | 0.96 | 4 |
| Auraptene | Kyoto Univ. | 30 | I. P. | 1.24 | 0.12 | 4 |
| Baicalein | Merck | 500 | I. P. | 1.44 | 0.17 | 5 |
| Biochanin A | Merck | 50 | I. P. | 1.07 | 0.68 | 5 |
| Caffeic acid | Merck | 50 | I. P. | 1.33 | 0.36 | 5 |
| L-Carnitine | Merck | 1690 | I. P. | 1.25 | 0.13 | 14 |
| Chrysin | Merck | 250 | I. P. | 1.17 | 0.23 | 4 |
| L-Citrulline | Merck | 100 | I. P. | 1.18 | 0.47 | 5 |
| Curcumin | Merck | 150 | I. P. | 1.17 | 0.10 | 5 |
| 2,7-Dichlorofluorescein | Merck | 500 | I. P. | 1.40 | 0.35 | 2 |
| Dipentene | Merck | 10 | I. P. | 1.03 | 0.78 | 4 |
| Ebselen | Merck | 16 | I. P. | 1.44 | 0.19 | 4 |
| EGCs | Merck | 150 | Oral | 1.14 | 0.35 | 5 |
| Fenofibrate | Merck | 1600 | I. P. | 1.25 | 0.58 | 5 |
| Ferulic acid | Merck | 50 | I. P. | 1.37 | 0.11 | 5 |
| Fisetin | Merck | 50 | I. P. | 1.18 | 0.62 | 5 |
| Folic acid | Merck | 4 | I. P. | 1.21 | 0.14 | 10 |
| Fumaric acid | Merck | 1000 | I. P. | 1.12 | 0.04 | 5 |
| Genistein | Merck | 10 and 50 | I. P. | 1.45 | 0.01 | 15 |
| [6]-Gingerol | Merck | 10 | I. P. | 1.15 | 0.27 | 4 |
| Glucose | Merck | 2000 | I. P. | 1.08 | 0.62 | 5 |
| Glutamine | Merck | 1000 | I. P. | 1.15 | 0.66 | 5 |
| Glutamine monohydrate | Merck | 1000 | I. P. | 1.10 | 0.47 | 5 |
| Glutathione (GSH-MEE) | Merck | 50 | I. P. | 1.06 | 0.49 | 5 |
| Glycine | Merck | 150 | I. P. | 1.24 | 0.37 | 5 |
| Gossypin | Merck | 500 | I. P. | 1.65 | 0.01 | 14 |
| Histidine | Merck | 800 | I. P. | 1.08 | 0.31 | 5 |
| HMBCa | KOBAYASHI PERFUMERY | 500 mg/kg × 5 times | Oral | 1.44 | 0.02 | 10 |
| Kaempferol | Merck | 500 | I. P. | 1.42 | 0.03 | 4 |
| Luteolin | FUJIFILM | 100 | I. P. | 1.04 | 0.91 | 4 |
| Lysine | Merck | 185 | I. P. | 1.35 | 0.10 | 5 |
| Magnolol | Merck | 10 | I. P. | 1.05 | 0.95 | 5 |
| Maleic acid | Merck | 1000 | I. P. | 1.25 | 0.27 | 5 |
| Methionine | Merck | 187 | I. P. | 1.16 | 0.13 | 5 |
| Mevastatin | Merck | 500 | I. P. | 1.18 | 0.06 | 4 |
| Mn TE-2-PyP | Merck | 10 | I. P. | 1.38 | 0.05 | 5 |
| Morin | Merck | 500 | I. P. | 1.32 | 0.05 | 5 |
| Myricetin | Merck | 500 | I. P. | 1.09 | 0.87 | 5 |
| L-NAME | Dojindo | 10 | I. P. | 1.86 | 0.14 | 5 |
| α-Naphthoflavone | Merck | 250 | I. P. | 1.25 | 0.26 | 4 |
| β-Naphthoflavone | Merck | 250 | I. P. | 1.19 | 0.43 | 5 |
| Naringin | Merck | 500 | I. P. | 1.12 | 0.75 | 5 |
| Nobiletin | Kyoto Univ. | 30 | I. P. | 1.49 | 0.19 | 4 |
| 1-Octanosanol | Merck | 100 | I. P. | 1.08 | 0.14 | 4 |
| Ornithine | Merck | 1000 | I. P. | 1.04 | 0.57 | 5 |
| (-)-2-Oxo-4-thiazolidinecarboxylic acid | Merck | 50 | I. P. | 1.26 | 0.32 | 5 |
| Prednisolone | Merck | 200 | I. P. | 1.20 | 0.27 | 5 |
| Reduced lipoic acid | Merck | 10 | I. P. | 1.25 | 0.37 | 5 |
| Rutin | Merck | 50 | I. P. | 1.10 | 0.57 | 5 |
| Sodium Pyruvate | Merck | 500 | I. P. | 1.21 | 0.28 | 5 |
| Succinic acid | Merck | 1000 | I. P. | 1.07 | 0.69 | 5 |
| Taxifolin | Merck | 500 | I. P. | 1.36 | 0.02 | 5 |
| Tempol | Merck | 250 | I. P. | 1.36 | 0.04 | 5 |
| L-Theanine | Merck | 500 | I. P. | 1.56 | 0.14 | 4 |
| Threonine | Merck | 800 | I. P. | 1.13 | 0.48 | 5 |
| α-tocopherol acetate | Merck | 500 | I. P. | 1.16 | 0.18 | 4 |
| Troglitazone | Merck | 10 | I. P. | 1.41 | 0.01 | 5 |
| Trolox | Merck | 20 | I. P. | 1.41 | 0.01 | 5 |
| Zn-protoporphyrin IX | Merck | 10 | I. P. | 1.62 | 0.15 | 5 |
| Compounds | Supplier | Dosage (mg/kg) | Administration | Relative Change | t-Test | n |
|---|---|---|---|---|---|---|
| Negative effects | ||||||
| Aloe-emodin | Merck | 10 | I. P. | 0.98 | 0.97 | 5 |
| Bee pollen | Merck | 100 | I. P. | 0.88 | 0.20 | 5 |
| Bilirubin | Merck | 200 | I. P. | 0.87 | 0.31 | 5 |
| Capsaicin | Merck | 1 | I. P. | 0.98 | 0.55 | 4 |
| β-carotene | Merck | 500 | I. P. | 0.71 | 0.63 | 4 |
| Citric acid | Merck | 500 | I. P. | 0.86 | 0.02 | 5 |
| Daidzein | Merck | 50 | I. P. | 0.99 | 0.78 | 5 |
| Docosahexaenoic acid | Merck | 100 | I. P. | 0.81 | 0.21 | 4 |
| Eicosapentaenoic acid | Merck | 100 | I. P. | 0.86 | 0.09 | 5 |
| 18β-Glycyrrhetinic acid | Merck | 10 | I. P. | 0.95 | 0.79 | 5 |
| Glycyrrhizic acid ammonium salt | Merck | 10 | I. P. | 0.95 | 0.61 | 7 |
| Ibuprofen | Merck | 75 | I. P. | 0.87 | 0.67 | 4 |
| Indore-3-carbinol | Merck | 100 | I. P. | 0.92 | 0.69 | 4 |
| Isosorbide dinitrate | Merck | 30 | I. P. | 0.87 | 0.19 | 5 |
| Limonin | Merck | 10 | I. P. | 0.75 | 0.12 | 5 |
| Linolenic acid | Merck | 100 | I. P. | 0.87 | 0.91 | 5 |
| N-acetyl-L-cysteine | Merck | 500 | I. P. | 0.92 | 0.53 | 5 |
| Nicotinamide | Merck | 200 | I. P. | 0.76 | 0.03 | 5 |
| 7-Nitroindazole | Merck | 50 | I. P. | 0.97 | 0.51 | 5 |
| Phosphocreatine | Merck | 100 | I. P. | 0.77 | 0.01 | 5 |
| Quercetin | Merck | 3.3 | I. P. | 0.96 | 0.52 | 5 |
| Riboflavin | Merck | 100 | I. P. | 0.77 | 0.12 | 5 |
| Rosmarinic acid | Merck | 50 | I. P. | 0.99 | 0.62 | 5 |
| Sesamin | Merck | 5 | I. P. | 0.88 | 0.28 | 5 |
| Ursolic acid | Merck | 100 | I. P. | 0.97 | 0.33 | 5 |
| Xanthophyll | Merck | 5 | I. P. | 0.97 | 0.55 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibuya, S.; Watanabe, K.; Sakuraba, D.; Abe, T.; Shimizu, T. Natural Compounds That Enhance Motor Function in a Mouse Model of Muscle Fatigue. Biomedicines 2022, 10, 3073. https://doi.org/10.3390/biomedicines10123073
Shibuya S, Watanabe K, Sakuraba D, Abe T, Shimizu T. Natural Compounds That Enhance Motor Function in a Mouse Model of Muscle Fatigue. Biomedicines. 2022; 10(12):3073. https://doi.org/10.3390/biomedicines10123073
Chicago/Turabian StyleShibuya, Shuichi, Kenji Watanabe, Daiki Sakuraba, Takuya Abe, and Takahiko Shimizu. 2022. "Natural Compounds That Enhance Motor Function in a Mouse Model of Muscle Fatigue" Biomedicines 10, no. 12: 3073. https://doi.org/10.3390/biomedicines10123073
APA StyleShibuya, S., Watanabe, K., Sakuraba, D., Abe, T., & Shimizu, T. (2022). Natural Compounds That Enhance Motor Function in a Mouse Model of Muscle Fatigue. Biomedicines, 10(12), 3073. https://doi.org/10.3390/biomedicines10123073