Role of Sirtuins in Physiology and Diseases of the Central Nervous System
Abstract
:1. Introduction
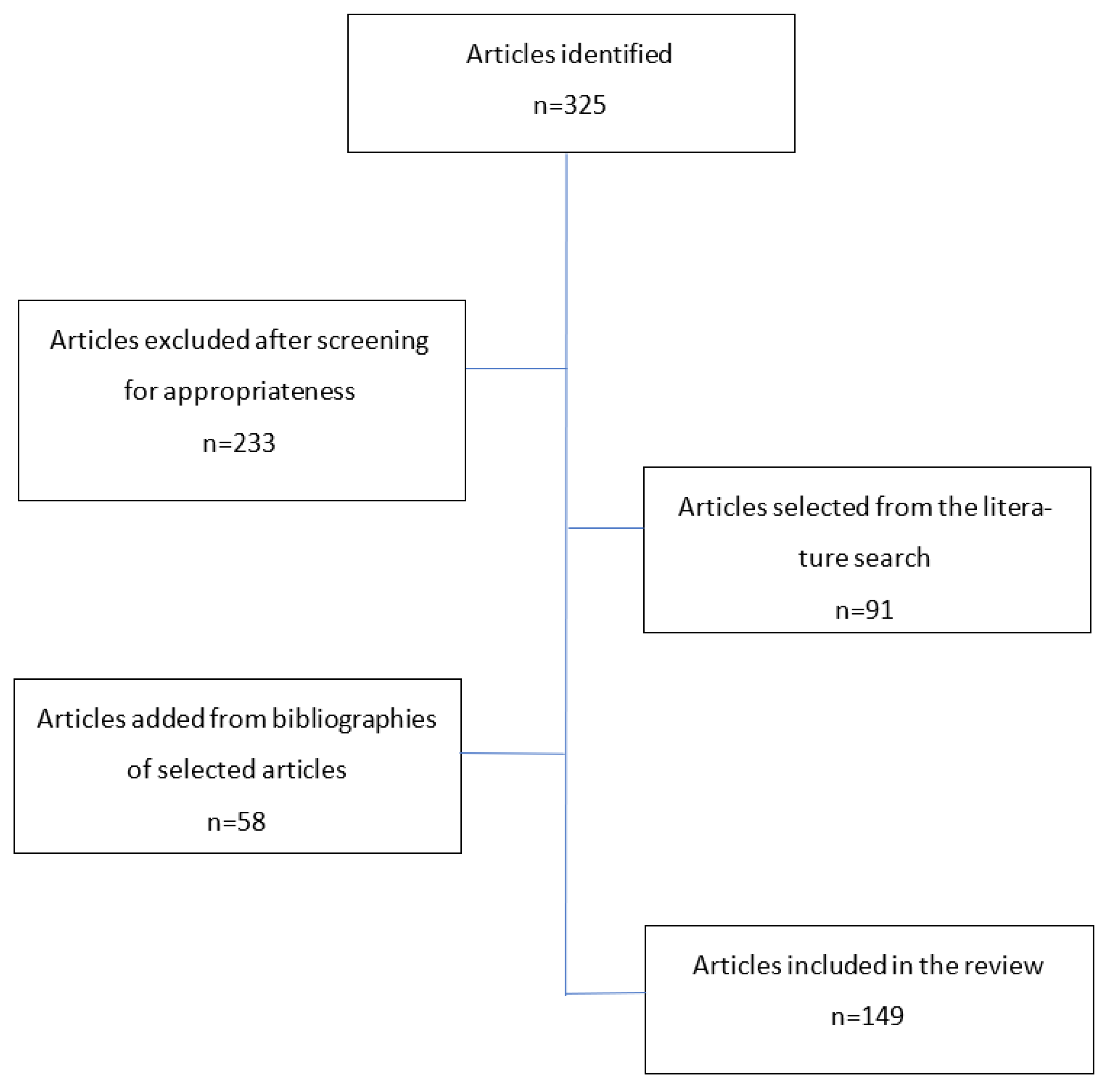
2. Method—Search Strategy
3. SIRTs Functions on a Cellular and Systemic Level
4. SIRTs in the Central Nervous System
5. SIRTs in CNS Diseases
5.1. Traumatic Brain Injury
5.2. Cerebrovascular Disease
5.3. Neurodegenerative Disorders
5.3.1. ALS (Amyotrophic Lateral Sclerosis)
5.3.2. Parkinson’s Disease
5.3.3. Alzheimer’s Disease (AD)
5.3.4. Huntington’s Disease (HD)
5.3.5. Multiple Sclerosis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kratz, E.M.; Sołkiewicz, K.; Kubis-Kubiak, A.; Piwowar, A. Sirtuins as Important Factors in Pathological States and the Role of Their Molecular Activity Modulators. Int. J. Mol. Sci. 2021, 22, 630. [Google Scholar] [CrossRef] [PubMed]
- Foolad, F.; Khodagholi, F.; Javan, M. Sirtuins in Multiple Sclerosis: The crossroad of neurodegeneration, autoimmunity and metabolism. Mult. Scler. Relat. Disord. 2019, 34, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Yamashita, T. Sirtuins in Neuroendocrine Regulation and Neurological Diseases. Front. Neurosci. 2018, 12, 778. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef]
- Libert, S.; Guarente, L. Metabolic and Neuropsychiatric Effects of Calorie Restriction and Sirtuins. Annu. Rev. Physiol. 2013, 75, 669–684. [Google Scholar] [CrossRef]
- Chandramowlishwaran, P.; Vijay, A.; Abraham, D.; Li, G.; Mwangi, S.M.; Srinivasan, S. Role of Sirtuins in Modulating Neuro-degeneration of the Enteric Nervous System and Central Nervous System. Front. Neurosci. 2020, 14, 614331. [Google Scholar] [CrossRef]
- Chen, H.P.; Zhao, Y.T.; Zhao, T.C. Histone Deacetylases and Mechanisms of Regulation of Gene Expression. Crit. Rev. Oncog. 2015, 20, 35–47. [Google Scholar] [CrossRef]
- Flick, F.; Lüscher, B. Regulation of Sirtuin Function by Posttranslational Modifications. Front. Pharmacol. 2012, 3, 29. [Google Scholar] [CrossRef]
- Klar, A.J.; Fogel, S.; Macleod, K. MAR1-a Regulator of the HMa and HMalpha Loci in SACCHAROMYCES CEREVISIAE. Genetics 1979, 93, 37–50. [Google Scholar] [CrossRef]
- Rine, J.; Strathern, J.N.; Hicks, J.B.; Herskowitz, I. A suppressor of mating-type locus mutations in Saccharomyces cerevisiae: Evidence for and identification of cryptic mating-type loci. Genetics 1979, 93, 877–901. [Google Scholar] [CrossRef]
- Khoury, N.; Koronowski, K.B.; Young, J.I.; Perez-Pinzon, M.A. The NAD+-Dependent Family of Sirtuins in Cerebral Ischemia and Preconditioning. Antioxid. Redox Signal. 2018, 28, 691–710. [Google Scholar] [CrossRef] [PubMed]
- Jurkowska, K.; Kratz, E.M.; Sawicka, E.; Piwowar, A. The impact of metalloestrogens on the physiology of male reproductive health as a current problem of the XXI century. J. Physiol. Pharm. Off. J. Pol. Physiol. Soc. 2019, 70, 337–355. [Google Scholar]
- Frye, R.A. Characterization of Five Human cDNAs with Homology to the Yeast SIR2 Gene: Sir2-like Proteins (Sirtuins) Metabolize NAD and May Have Protein ADP-Ribosyltransferase Activity. Biochem. Biophys. Res. Commun. 1999, 260, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Chalkiadaki, A.; Guarente, L. Sirtuins mediate mammalian metabolic responses to nutrient availability. Nat. Rev. Endocrinol. 2012, 8, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Alqarni, M.H.; Foudah, A.I.; Muharram, M.M.; Labrou, N.E. The Pleiotropic Function of Human Sirtuins as Modulators of Metabolic Pathways and Viral Infections. Cells 2021, 10, 460. [Google Scholar] [CrossRef] [PubMed]
- Michan, S.; Sinclair, D. Sirtuins in mammals: Insights into their biological function. Biochem. J. 2007, 404, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Yuan, H.; Brent, M.; Ding, E.C.; Marmorstein, R. SIRT1 Contains N- and C-terminal Regions That Potentiate Deacetylase Activity. J. Biol. Chem. 2012, 287, 2468–2476. [Google Scholar] [CrossRef]
- Herskovits, A.Z.; Guarente, L. SIRT1 in Neurodevelopment and Brain Senescence. Neuron 2014, 81, 471–483. [Google Scholar] [CrossRef]
- Greiss, S.; Gartner, A. Sirtuin/Sir2 phylogeny, evolutionary considerations and structural conservation. Mol. Cells 2009, 28, 407–415. [Google Scholar] [CrossRef]
- Navas, L.E.; Carnero, A. NAD+ metabolism, stemness, the immune response, and cancer. Signal Transduct. Target. Ther. 2021, 6, 2. [Google Scholar] [CrossRef]
- Sadia, K.; Ashraf, M.Z.; Mishra, A. Therapeutic Role of Sirtuins Targeting Unfolded Protein Response, Coagulation, and Inflammation in Hypoxia-Induced Thrombosis. Front. Physiol. 2021, 12, 733453. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Kitada, M.; Koya, D. Sirtuins and Renal Oxidative Stress. Antioxidants 2021, 10, 1198. [Google Scholar] [CrossRef]
- Al-Khaldi, A.; Sultan, S. The expression of sirtuins, superoxide dismutase, and lipid peroxidation status in peripheral blood from patients with diabetes and hypothyroidism. BMC Endocr. Disord. 2019, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R. Superoxide Dismutase in Redox Biology: The Roles of Superoxide and Hydrogen Peroxide. Former. Curr. Med. Chem. Anti Cancer Agents 2011, 11, 341–346. [Google Scholar] [CrossRef]
- Fletcher, M.E.; Boshier, P.R.; Wakabayashi, K.; Keun, H.C.; Smolenski, R.T.; Kirkham, P.A.; Adcock, I.M.; Barton, P.J.; Takata, M.; Marczin, N. Influence of glutathione-S-transferase (GST) inhibition on lung epithelial cell injury: Role of oxidative stress and metabolism. Am. J. Physiol. Cell. Mol. Physiol. 2015, 308, L1274–L1285. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Tang, X.; Chen, H.-Z. Sirtuins and Insulin Resistance. Front. Endocrinol. 2018, 9, 748. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Luo, J. SIRT1 and p53, effect on cancer, senescence and beyond. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2010, 1804, 1684–1689. [Google Scholar] [CrossRef]
- Chen, Y.L.; Fu, L.L.; Wen, X.; Wang, X.Y.; Liu, J.; Cheng, Y.; Huang, J. Sirtuin-3 (SIRT3), a therapeutic target with oncogenic and tu-mor-suppressive function in cancer. Cell Death Dis. 2014, 5, e1047. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Mueller-Steiner, S.; Chen, L.F.; Kwon, H.; Yi, S.; Mucke, L.; Gan, L. SIRT1 Protects against Microglia-dependent Amyloid-β Toxicity through Inhibiting NF-κB Signaling. J. Biol. Chem. 2005, 280, 40364–40374. [Google Scholar] [CrossRef]
- Prozorovski, T.; Schulze-Topphoff, U.; Glumm, R.; Baumgart, J.; Schröter, F.; Ninnemann, O.; Siegert, E.; Bendix, I.; Brüstle, O.; Nitsch, R.; et al. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat. Cell Biol. 2008, 10, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, M.M.; Tomkinson, E.M.; Nobles, J.; Wizeman, J.W.; Amore, A.M.; Quinti, L.; Chopra, V.; Hersch, S.M.; Kazantsev, A.G. The Sirtuin 2 microtubule deacetylase is an abundant neuronal protein that accumulates in the aging CNS. Hum. Mol. Genet. 2011, 20, 3986–3996. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.S.; Lee, E.; Lee, Y.S.; Shin, D.H. SIRT2 Protein Expression in Normal and Aged Rat Brain. J Korean Geriatr. Soc. 2012, 16, 27–33. [Google Scholar]
- Michán, S.; Li, Y.; Chou, M.M.-H.; Parrella, E.; Ge, H.; Long, J.M.; Allard, J.S.; Lewis, K.; Miller, M.; Xu, W.; et al. SIRT1 Is Essential for Normal Cognitive Function and Synaptic Plasticity. J. Neurosci. 2010, 30, 9695–9707. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.R.; Imai, S.I. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 2014, 33, 1321–1340. [Google Scholar] [CrossRef]
- Schwer, B.; Eckersdorff, M.; Li, Y.; Silva, J.C.; Fermin, D.; Kurtev, M.V.; Giallourakis, C.; Comb, M.J.; Alt, F.W.; Lombard, D.B. Calorie restriction alters mitochondrial protein acety-lation. Aging Cell 2009, 8, 604–606. [Google Scholar] [CrossRef]
- Jiang, D.-Q.; Wang, Y.; Li, M.-X.; Ma, Y.-J.; Wang, Y. SIRT3 in Neural Stem Cells Attenuates Microglia Activation-Induced Oxidative Stress Injury Through Mitochondrial Pathway. Front. Cell. Neurosci. 2017, 11, 7. Available online: http://journal.frontiersin.org/article/10.3389/fncel.2017.00007/full (accessed on 13 August 2022). [CrossRef]
- Santos, L.; Escande, C.; Denicola, A. Potential Modulation of Sirtuins by Oxidative Stress. Oxidative Med. Cell. Longev. 2015, 2016, 121925. [Google Scholar] [CrossRef]
- Toorie, A.M.; Nillni, E.A. Minireview: Central Sirt1 regulates energy balance via the melanocortin system and alternate pathways. Mol. Endocrinol. 2014, 28, 1423–1434. [Google Scholar] [CrossRef]
- Shih, J.; Liu, L.; Mason, A.; Higashimori, H.; Donmez, G. Loss of SIRT4 decreases GLT-1-dependent glutamate uptake and increases sensitivity to kainic acid. J. Neurochem. 2014, 131, 573–581. [Google Scholar] [CrossRef]
- Kempuraj, D.; Mehta, T.; Fayyaz, M.; Giler, G.E.; Kaur, H.; Raikwar, S.P.; Kempuraj, D.; Selvakumar, G.P.; Ahmed, M.E.; Thangavel, R.; et al. Current Trends in Biomarkers for Traumatic Brain Injury. Open Access J. Neurol. Neurosurg. 2020, 12, 86–94. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.; Song, R. SIRT2 inhibition exacerbates p53-mediated ferroptosis in mice following experimental traumatic brain injury. NeuroReport 2021, 32, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Deb, P.; Sharma, S.; Hassan, K.M. Pathophysiologic mechanisms of acute ischemic stroke: An overview with emphasis on ther-apeutic significance beyond thrombolysis. Pathophysiology 2010, 17, 197–218. [Google Scholar] [CrossRef] [PubMed]
- She, D.T.; Jo, D.G.; Arumugam, T.V. Emerging Roles of Sirtuins in Ischemic Stroke. Transl. Stroke Res. 2017, 8, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Raval, A.; Lin, H.; Dave, K.; DeFazio, R.; Morte, D.; Kim, E.; Perez-Pinzon, M. Resveratrol and Ischemic Preconditioning in the Brain. Curr. Med. Chem. 2008, 15, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Okamoto, Y.; Nagatsuka, K.; Takahashi, R.; Kalaria, R.N.; Kinoshita, M.; Ihara, M. SIRT1 attenuates severe ischemic damage by preserving cerebral blood flow. NeuroReport 2015, 26, 113–117. [Google Scholar] [CrossRef]
- Hattori, Y.; Okamoto, Y.; Maki, T.; Yamamoto, Y.; Oishi, N.; Yamahara, K.; Nagatsuka, K.; Takahashi, R.; Kalaria, R.N.; Fukuyama, H.; et al. Silent Information Regulator 2 Homolog 1 Counters Cerebral Hypoperfusion Injury by Deacetylating Endothelial Nitric Oxide Synthase. Stroke 2014, 45, 3403–3411. [Google Scholar] [CrossRef]
- Wang, T.; Gu, J.; Wu, P.-F.; Wang, F.; Xiong, Z.; Yang, Y.-J.; Wu, W.-N.; Dong, L.-D.; Chen, J.-G. Protection by tetrahydroxystilbene glucoside against cerebral ischemia: Involvement of JNK, SIRT1, and NF-κB pathways and inhibition of intracellular ROS/RNS generation. Free Radic. Biol. Med. 2009, 47, 229–240. [Google Scholar] [CrossRef]
- Hernández-Jiménez, M.; Hurtado, O.; Cuartero, M.I.; Ballesteros, I.; Moraga, A.; Pradillo, J.M.; McBurney, M.W.; Lizasoain, I.; Moro, M.A. Silent Information Regulator 1 Protects the Brain Against Cerebral Ischemic Damage. Stroke 2013, 44, 2333–2337. [Google Scholar] [CrossRef]
- Esmayel, I.M.; Hussein, S.; Gohar, E.A.; Ebian, H.F.; Mousa, M.M. Plasma levels of sirtuin-1 in patients with cerebrovascular stroke. Neurol. Sci. 2021, 42, 3843–3850. [Google Scholar] [CrossRef]
- Wang, P.; Xu, T.-Y.; Guan, Y.-F.; Tian, W.-W.; Viollet, B.; Rui, Y.-C.; Zhai, Q.-W.; Su, D.-F.; Miao, C.-Y. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1-dependent adenosine monophosphate-activated kinase pathway. Ann. Neurol. 2011, 69, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-F.; Zhang, Y.-L.; Wu, Y.-C. The Role of Sirt1 in Ischemic Stroke: Pathogenesis and Therapeutic Strategies. Front. Neurosci. 2018, 12, 833. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Hong, Y.; Lu, X.; Zhang, J.; Chen, H.; Li, Y.; Ma, Y.; Ying, W. SIRT2 mediates oxidative stress-induced apoptosis of differentiated PC12 cells. NeuroReport 2014, 25, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, L.; Li, C.Y.; Pei, Z.; Zhou, M.; Li, N. SIRT3 protects cells from hypoxia via PGC-1α- and MnSOD-dependent pathways. Neuroscience 2015, 286, 109–121. [Google Scholar] [CrossRef]
- Yin, J.; Han, P.; Tang, Z.; Liu, Q.; Shi, J. Sirtuin 3 Mediates Neuroprotection of Ketones against Ischemic Stroke. J. Cereb. Blood Flow Metab. 2015, 35, 1783–1789. [Google Scholar] [CrossRef]
- Chen, T.; Dai, S.-H.; Li, X.; Luo, P.; Zhu, J.; Wang, Y.-H.; Fei, Z.; Jiang, X.-F. Sirt1-Sirt3 axis regulates human blood-brain barrier permeability in response to ischemia. Redox Biol. 2018, 14, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.K.; Airavaara, M.; Hinzman, J.; Wires, E.M.; Chiocco, M.J.; Howard, D.B.; Shen, H.; Gerhardt, G.; Hoffer, B.J.; Wang, Y. Targeted Over-Expression of Glutamate Transporter 1 (GLT-1) Reduces Ischemic Brain Injury in a Rat Model of Stroke. PLoS ONE 2011, 6, e22135. [Google Scholar] [CrossRef] [PubMed]
- Morris-Blanco, K.C.; Dave, K.R.; Saul, I.; Koronowski, K.B.; Stradecki, H.M.; Perez-Pinzon, M.A. Protein Kinase C Epsilon Promotes Cerebral Ischemic Tolerance Via Modulation of Mitochondrial Sirt5. Sci. Rep. 2016, 6, 29790. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Della-Morte, D.; Wang, L.; Cabral, D.; Beecham, A.; McClendon, M.S.; Luca, C.; Blanton, S.H.; Sacco, R.L.; Rundek, T. Association of the Sirtuin and Mitochondrial Uncoupling Protein Genes with Carotid Plaque. PLoS ONE 2011, 6, e27157. [Google Scholar] [CrossRef]
- Lee, O.-H.; Kim, J.; Kim, J.-M.; Lee, H.; Kim, E.H.; Bae, S.-K.; Choi, Y.; Nam, H.S.; Heo, J.H. Decreased expression of sirtuin 6 is associated with release of high mobility group box-1 after cerebral ischemia. Biochem. Biophys. Res. Commun. 2013, 438, 388–394. [Google Scholar] [CrossRef]
- Hu, Y.; Li, R.; Yang, H.; Luo, H.; Chen, Z. Sirtuin 6 Is Essential for Sodium Sulfide-mediated Cytoprotective Effect in Ische-mia/Reperfusion–Stimulated Brain Endothelial Cells. J. Stroke Cerebrovasc. Dis. 2015, 24, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Hubbi, M.E.; Hu, H.; Kshitiz; Gilkes, D.M.; Semenza, G.L. Sirtuin-7 Inhibits the Activity of Hypoxia-inducible Factors. J. Biol. Chem. 2013, 288, 20768–20775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonsack, F.; Alleyne, C.H.; Sukumari-Ramesh, S. Resveratrol Attenuates Neurodegeneration and Improves Neurological Out-comes after Intracerebral Hemorrhage in Mice. Front. Cell. Neurosci. 2017, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-S.; Wu, Q.; Wu, L.-Y.; Ye, Z.-N.; Jiang, T.-W.; Ling-Yun, W.; Zhuang, Z.; Zhou, M.-L.; Zhang, X.; Hang, C.-H. Sirtuin 1 activation protects against early brain injury after experimental subarachnoid hemorrhage in rats. Cell Death Dis. 2016, 7, e2416. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.-P.; Lv, T.; Hou, P.-P.; Manaenko, A.; Liu, Y.; Jin, Y.; Gao, L.; Jia, F.; Tian, Y.; Li, P.; et al. Sirtuin 5-Mediated Lysine Desuccinylation Protects Mitochondrial Metabolism Following Subarachnoid Hemorrhage in Mice. Stroke 2021, 52, 4043–4053. [Google Scholar] [CrossRef]
- Raval, A.P.; Dave, K.R.; Perez-Pinzon, M.A. Resveratrol Mimics Ischemic Preconditioning in the Brain. J. Cereb. Blood Flow Metab. 2006, 26, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Della, M.D.; Dave, K.R.; DeFazio, R.A.; Bao, Y.C.; Raval, A.P.; Perez-Pinzon, M.A. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1–uncoupling protein 2 pathway. Neuroscience 2009, 159, 993–1002. [Google Scholar] [CrossRef]
- Imai, S.I.; Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef]
- Kane, A.E.; Sinclair, D.A. Sirtuins and NAD+ in the Development and Treatment of Metabolic and Cardiovascular Diseases. Circ. Res. 2018, 123, 868–885. [Google Scholar] [CrossRef]
- Jellinger, K.A. Basic mechanisms of neurodegeneration: A critical update. J. Cell. Mol. Med. 2010, 14, 457–487. [Google Scholar] [CrossRef]
- Pasinetti, G.M.; Bilski, A.E.; Zhao, W. Sirtuins as therapeutic targets of ALS. Cell Res. 2013, 23, 1073–1074. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.C.; Higgins, N.R.; Phung, T.H.; Monteiro, M.J. UBQLN proteins in health and disease with a focus on UBQLN2 in ALS/FTD. FEBS J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Ageta-Ishihara, N.; Nagatsu, S.; Takao, K.; Komine, O.; Endo, F.; Miyakawa, T.; Misawa, H.; Takahashi, R.; Kinoshita, M.; et al. SIRT1 overexpression ameliorates a mouse model of SOD1-linked amyotrophic lateral sclerosis via HSF1/HSP70i chaperone system. Mol. Brain 2014, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Buck, E.; Bayer, H.; Lindenberg, K.S.; Hanselmann, J.; Pasquarelli, N.; Ludolph, A.C.; Weydt, P.; Witting, A. Comparison of Sirtuin 3 Levels in ALS and Huntington’s Disease—Differential Effects in Human Tissue Samples vs. Transgenic Mouse Models. Front. Mol. Neurosci. 2017, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Bause, A.S.; Haigis, M.C. SIRT3 regulation of mitochondrial oxidative stress. Exp. Gerontol. 2013, 48, 634–639. [Google Scholar] [CrossRef]
- Han, Y.S.; Zheng, W.H.; Bastianetto, S.; Chabot, J.G.; Quirion, R. Neuroprotective effects of resveratrol against β-amyloid-induced neurotoxicity in rat hippocampal neurons: Involvement of protein kinase C: Neuroprotective effect of resveratrol. Br. J. Pharmacol. 2004, 141, 997–1005. [Google Scholar] [CrossRef]
- Raynes, R.; Leckey, B.D.; Nguyen, K.; Westerheide, S.D. Heat Shock and Caloric Restriction Have a Synergistic Effect on the Heat Shock Response in a sir2.1-dependent Manner in Caenorhabditis elegans. J. Biol. Chem. 2012, 287, 29045–29053. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Tang, L.; Zhang, N.; Fan, D. Protective effects of resveratrol through the up-regulation of SIRT1 expression in the mutant hSOD1-G93A-bearing motor neuron-like cell culture model of amyotrophic lateral sclerosis. Neurosci. Lett. 2011, 503, 250–255. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador, R.; López-Blanch, R.; Jihad-Jebbar, A.; Vallés, S.L.; Estrela, J.M. Oxidative Stress, Neuroinflammation and Mitochondria in the Pathophysiology of Amyotrophic Lateral Sclerosis. Antioxidants 2020, 9, 901. [Google Scholar] [CrossRef]
- Han, M.-K.; Song, E.-K.; Guo, Y.; Ou, X.; Mantel, C.; Broxmeyer, H.E. SIRT1 Regulates Apoptosis and Nanog Expression in Mouse Embryonic Stem Cells by Controlling p53 Subcellular Localization. Cell Stem Cell 2008, 2, 241–251. [Google Scholar] [CrossRef]
- Mancuso, R.; del Valle, J.; Modol, L.; Martinez, A.; Granado-Serrano, A.B.; Ramirez-Núñez, O.; Pallás, M.; Portero-Otin, M.; Osta, R.; Navarro, X. Resveratrol Improves Motoneuron Function and Extends Survival in SOD1G93A ALS Mice. Neurotherapeutics 2014, 11, 419–432. Available online: http://link.springer.com/10.1007/s13311-013-0253-y (accessed on 13 August 2022). [CrossRef] [PubMed]
- Ntetsika, T.; Papathoma, P.-E.; Markaki, I. Novel targeted therapies for Parkinson’s disease. Mol. Med. 2021, 27, 17. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Pukhalskaia, A.E.; Diatlova, A.S.; Linkova, N.S.; Kvetnoy, I.M. Sirtuins: Role in the Regulation of Oxidative Stress and the Patho-genesis of Neurodegenerative Diseases. Neurosci. Behav. Physiol. 2022, 52, 164–174. [Google Scholar] [CrossRef]
- Singh, P.; Hanson, P.S.; Morris, C.M. SIRT1 ameliorates oxidative stress induced neural cell death and is down-regulated in Par-kinson’s disease. BMC Neurosci. 2017, 18, 46. [Google Scholar] [CrossRef]
- Schwab, A.J.; Sison, S.L.; Meade, M.R.; Broniowska, K.A.; Corbett, J.A.; Ebert, A.D. Decreased Sirtuin Deacetylase Activity in LRRK2 G2019S iPSC-Derived Dopaminergic Neurons. Stem Cell Rep. 2017, 9, 1839–1852. [Google Scholar] [CrossRef]
- Mudò, G.; Mäkelä, J.; Di Liberto, V.D.; Tselykh, T.V.; Olivieri, M.; Piepponen, P.; Eriksson, O.; Mälkiä, A.; Bonomo, A.; Kairisalo, M.; et al. Transgenic expression and activation of PGC-1α protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell. Mol. Life Sci. 2012, 69, 1153–1165. [Google Scholar] [CrossRef]
- Kakefuda, K.; Fujita, Y.; Oyagi, A.; Hyakkoku, K.; Kojima, T.; Umemura, K.; Tsuruma, K.; Shimazawa, M.; Ito, M.; Nozawa, Y.; et al. Sirtuin 1 overexpression mice show a reference memory deficit, but not neuroprotection. Biochem. Biophys. Res. Commun. 2009, 387, 784–788. [Google Scholar] [CrossRef]
- Kida, Y.; Goligorsky, M.S. Sirtuins, Cell Senescence, and Vascular Aging. Can. J. Cardiol. 2016, 32, 634–641. [Google Scholar] [CrossRef]
- De Oliveira, R.M.; Vicente Miranda, H.; Francelle, L.; Pinho, R.; Szegö, É.M.; Martinho, R.; Munari, F.; Lázaro, D.F.; Moniot, S.; Guerreiro, P.; et al. The mechanism of sirtuin 2-mediated exacerbation of alpha-synuclein toxicity in models of Parkinson disease. PLoS Biol. 2017, 15, e2000374. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zhu, K.; Chi, S.; Wang, C.; Xie, A. Emerging Role of Sirtuin 2 in Parkinson’s Disease. Front. Aging Neurosci. 2020, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, D.; Ding, X.; Ying, W. SIRT2 is required for lipopolysaccharide-induced activation of BV2 microglia. NeuroReport 2015, 26, 88–93. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, J.; Yang, Y.; Li, J.; Tu, H. Mitochondrial Sirtuins in Parkinson’s Disease. Neurochem. Res. 2022, 47, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Gleave, J.A.; Perri, P.D.; Nash, J.E. Mitochondrial dysfunction in Parkinson’s disease: A possible target for neuroprotection. Front. Biol. 2014, 9, 489–503. [Google Scholar] [CrossRef]
- Jiang, H.; Khan, S.; Wang, Y.; Charron, G.; He, B.; Sebastian, C.; Du, J.; Kim, R.; Ge, E.; Mostoslavsky, R.; et al. SIRT6 regulates TNF-α secretion through hydrolysis of long-chain fatty acyl lysine. Nature 2013, 496, 110–113. [Google Scholar] [CrossRef]
- Chao, J.; Yu, M.-S.; Ho, Y.-S.; Wang, M.; Chang, R.C.-C. Dietary oxyresveratrol prevents parkinsonian mimetic 6-hydroxydopamine neurotoxicity. Free Radic. Biol. Med. 2008, 45, 1019–1026. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Pradhan, R.; Singh, A.K.; Kumar, P.; Bajpai, S.; Pathak, M.; Chatterjee, P.; Dwivedi, S.; Dey, A.B.; Dey, S. Blood Circulatory Level of Seven Sirtuins in Alzheimer’s Disease: Potent Biomarker Based on Translational Research. Mol. Neurobiol. 2022, 59, 1440–1451. [Google Scholar] [CrossRef]
- Karuppagounder, S.S.; Pinto, J.T.; Xu, H.; Chen, H.-L.; Beal, M.F.; Gibson, G.E. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem. Int. 2009, 54, 111–118. [Google Scholar] [CrossRef]
- Vingtdeux, V.; Giliberto, L.; Zhao, H.; Chandakkar, P.; Wu, Q.; Simon, J.E.; Janle, E.M.; Lobo, J.; Ferruzzi, M.G.; Davies, P.; et al. AMP-activated Protein Kinase Signaling Activation by Resveratrol Modulates Amyloid-β Peptide Metabolism. J. Biol. Chem. 2010, 285, 9100–9113. [Google Scholar] [CrossRef]
- Qin, W.; Yang, T.; Ho, L.; Zhao, Z.; Wang, J.; Chen, L.; Zhao, W.; Thiyagarajan, M.; MacGrogan, D.; Rodgers, J.T.; et al. Neuronal SIRT1 Activation as a Novel Mechanism Underlying the Pre-vention of Alzheimer Disease Amyloid Neuropathology by Calorie Restriction. J. Biol. Chem. 2006, 281, 21745–21754. [Google Scholar] [CrossRef] [PubMed]
- Green, K.N.; Steffan, J.S.; Martínez-Coria, H.; Sun, X.; Schreiber, S.S.; Thompson, L.M.; LaFerla, F.M. Nicotinamide Restores Cognition in Alzheimer’s Disease Transgenic Mice via a Mechanism Involving Sirtuin Inhibition and Selective Reduction of Thr231-Phosphotau. J. Neurosci. 2008, 28, 11500–11510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, S.W.; Cho, S.H.; Zhou, Y.; Schroeder, S.; Haroutunian, V.; Seeley, W.W.; Huang, E.J.; Shen, Y.; Chandrani, M.; Mukherjee, C.; et al. Acetylation of Tau Inhibits Its Degradation and Contributes to Tauopathy. Neuron 2010, 67, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Theendakara, V.; Patent, A.; LPeters Libeu, C.A.; Philpot, B.; Flores, S.; Descamps, O.; Poksay, K.S.; Zhang, Q.; Cailing, G.; Hart, M.; et al. Neuroprotective Sirtuin ratio reversed by ApoE4. Proc. Natl. Acad. Sci. USA 2013, 110, 18303–18308. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R.; Carril, J.C.; Cacabelos, N.; Kazantsev, A.G.; Vostrov, A.V.; Corzo, L.; Cacabelos, P.; Goldgaber, D. Sirtuins in Alzheimer’s Disease: SIRT2-Related GenoPhenotypes and Implications for PharmacoEpiGenetics. Int. J. Mol. Sci. 2019, 20, 1249. [Google Scholar] [CrossRef] [PubMed]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflam. 2017, 14, 1. [Google Scholar] [CrossRef]
- Silva, D.F.; Santana, I.; Esteves, A.R.; Baldeiras, I.; Arduino, D.M.; Oliveira, C.R.; Cardoso, S.M. Prodromal Metabolic Phenotype in MCI Cybrids: Implications for Alzheimer’s Disease. Curr. Alzheimer Res. 2013, 10, 180–190. [Google Scholar] [CrossRef]
- Esteves, A.R.; Arduíno, D.M.; Silva, D.F.; Viana, S.D.; Pereira, F.C.; Cardoso, S.M. Mitochondrial Metabolism Regulates Microtubule Acetylome and Autophagy Trough Sirtuin-2: Impact for Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 1440–1462. [Google Scholar] [CrossRef]
- Kawabata, S. Excessive/Aberrant and Maladaptive Synaptic Plasticity: A Hypothesis for the Pathogenesis of Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 913693. [Google Scholar] [CrossRef]
- Gupta, R.; Ambasta, R.K.; Kumar, P. Multifaced role of protein deacetylase sirtuins in neurodegenerative disease. Neurosci. Biobehav. Rev. 2022, 132, 976–997. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Liu, T.; Hwang, Y.J.; Hyeon, S.J.; Im, H.; Lee, K.; Alvarez, V.E.; McKee, A.C.; Um, S.-J.; et al. SIRT3 deregulation is linked to mitochondrial dysfunction in Alzheimer’s disease. Aging Cell 2017, 17, e12679. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Guo, Q.; Mattson, M.P. Dietary restriction protects hippocampal neurons against the death-promoting action of a pre-senilin-1 mutation. Brain Res. 1999, 842, 224–229. [Google Scholar] [CrossRef]
- Jung, E.S.; Choi, H.; Song, H.; Hwang, Y.J.; Kim, A.; Ryu, H.; Mook-Jung, I. p53-dependent SIRT6 expression protects Aβ42-induced DNA damage. Sci. Rep. 2016, 6, 25628. [Google Scholar] [CrossRef] [PubMed]
- Kaluski, S.; Portillo, M.; Besnard, A.; Stein, D.; Einav, M.; Zhong, L.; Ueberham, U.; Arendt, T.; Mostoslavsky, R.; Sahay, A.; et al. Neuroprotective Functions for the Histone Deacetylase SIRT6. Cell Rep. 2017, 18, 3052–3062. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; Van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S.; et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Wongchitrat, P.; Pakpian, N.; Kitidee, K.; Phopin, K.; Dharmasaroja, P.A.; Govitrapong, P. Alterations in the Expression of Amyloid Precursor Protein Cleaving Enzymes mRNA in Alzheimer Peripheral Blood. Curr. Alzheimer Res. 2018, 16, 29–38. [Google Scholar] [CrossRef]
- Finkbeiner, S. Huntington’s Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a007476. [Google Scholar] [CrossRef]
- Hoppitt, T.; Calvert, M.; Pall, H.; Rickards, H.; Sackley, C. Huntington’s disease. Lancet 2010, 376, 1463–1464. [Google Scholar] [CrossRef]
- Jeong, H.; Cohen, D.E.; Cui, L.; Supinski, A.; Savas, J.N.; Mazzulli, J.R.; Yates, J.R.; Bordone, L.; Guarente, L.; Krainc, D. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat. Med. 2012, 18, 159–165. [Google Scholar] [CrossRef]
- Naia, L.; Rego, A.C. Sirtuins: Double players in Huntington’s disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 2183–2194. [Google Scholar] [CrossRef]
- Naia, L.; Carmo, C.; Campesan, S.; Fão, L.; Cotton, V.E.; Valero, J.; Lopes, C.; Rosenstock, T.R.; Giorgini, F.; Rego, A.C. Mitochondrial SIRT3 confers neuroprotection in Huntington’s disease by regulation of oxidative challenges and mitochondrial dynamics. Free Radic. Biol. Med. 2021, 163, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Manjula, R.; Anuja, K.; Alcain, F.J. SIRT1 and SIRT2 Activity Control in Neurodegenerative Diseases. Front. Pharmacol. 2021, 11, 585821. [Google Scholar] [CrossRef] [PubMed]
- Lazo-Gómez, R.; Ramírez-Jarquín, U.N.; Tovar-y-Romo, L.B.; Tapia, R. Histone Deacetylases and Their Role in Motor Neuron De-Generation. Front. Cell. Neurosci. 2013, 7, 243. Available online: http://journal.frontiersin.org/article/10.3389/fncel.2013.00243/abstract (accessed on 13 August 2022). [CrossRef] [PubMed]
- Süssmuth, S.D.; Haider, S.; Landwehrmeyer, G.B.; Farmer, R.; Frost, C.; Tripepi, G.; Andersen, C.A.; di Bacco, M.; Lamanna, C.; Diodato, E.; et al. An exploratory double-blind, ran-domized clinical trial with selisistat, a SirT1 inhibitor, in patients with Huntington’s disease. Br. J. Clin. Pharmacol. 2015, 79, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Piacente, F.; Bottero, M.; Benzi, A.; Vigo, T.; Uccelli, A.; Bruzzone, S.; Ferrara, G. Neuroprotective Potential of Dendritic Cells and Sirtuins in Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 4352. [Google Scholar] [CrossRef]
- Shindler, K.S.; Ventura, E.; Rex, T.S.; Elliott, P.; Rostami, A. SIRT1 Activation Confers Neuroprotection in Experimental Optic Neuritis. Investig. Opthalmology Vis. Sci. 2007, 48, 3602–3609. [Google Scholar] [CrossRef]
- McDougald, D.S.; Dine, K.E.; Zezulin, A.U.; Bennett, J.; Shindler, K.S. SIRT1 and NRF2 Gene Transfer Mediate Distinct Neuroprotective Effects Upon Retinal Ganglion Cell Survival and Function in Experimental Optic Neuritis. Investig. Opthalmology Vis. Sci. 2018, 59, 1212–1220. [Google Scholar] [CrossRef]
- Dolati, S.; Aghebati-Maleki, L.; Ahmadi, M.; Marofi, F.; Babaloo, Z.; Ayramloo, H.; Jafarisavari, Z.; Oskouei, H.; Afkham, A.; Younesi, V.; et al. Nanocurcumin restores aberrant miRNA expression profile in multiple sclerosis, randomized, double-blind, placebo-controlled trial. J. Cell. Physiol. 2018, 233, 5222–5230. [Google Scholar] [CrossRef]
- Parodi, B.; Rossi, S.; Morando, S.; Cordano, C.; Bragoni, A.; Motta, C.; Usai, C.; Wipke, B.T.; Scannevin, R.H.; Mancardi, G.L.; et al. Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol. 2015, 130, 279–295. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, X.; Zhang, L. Negative regulation of inflammation by SIRT1. Pharmacol. Res. 2013, 67, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.; Kang, S.G.; Ryu, J.K.; Schilling, B.; Fei, M.; Lee, I.S.; Kehasse, A.; Shirakawa, K.; Yokoyama, M.; Schnölzer, M.; et al. SIRT1 deacetylates RORγt and enhances Th17 cell generation. J. Exp. Med. 2015, 212, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Tegla, C.A.; Azimzadeh, P.; Andrian-Albescu, M.; Martin, A.; Cudrici, C.D.; Trippe, R.; Sugarman, A.; Chen, H.; Boodhoo, D.; Vlaicu, S.I.; et al. SIRT1 is decreased during relapses in patients with multiple sclerosis. Exp. Mol. Pathol. 2014, 96, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, G.; Cornelius, C.; Cavallaro, M.; Salinaro, A.T.; Cambria, M.T.; Pennisi, M.; Bella, R.; Milone, P.; Ventimiglia, B.; Migliore, M.; et al. Redox regulation of cellular stress response in multiple sclerosis. Biochem. Pharmacol. 2011, 82, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Ciriello, J.; Tatomir, A.; Hewes, D.; Boodhoo, D.; Anselmo, F.; Rus, V.; Rus, H. Phosphorylated SIRT1 as a biomarker of relapse and response to treatment with glatiramer acetate in multiple sclerosis. Exp. Mol. Pathol. 2018, 105, 175–180. [Google Scholar] [CrossRef]
- Hewes, D.; Tatomir, A.; Kruszewski, A.M.; Rao, G.; Tegla, C.A.; Ciriello, J.; Nguyen, V.; Royal, W., III; Bever, C.; Rus, V.; et al. SIRT1 as a potential biomarker of response to treatment with glatiramer acetate in multiple sclerosis. Exp. Mol. Pathol. 2017, 102, 191–197. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Yan, Y.; Ciric, B.; Ma, C.-G.; Chin, J.; Curtis, M.; Rostami, A.; Zhang, G.-X. LINGO-1-Fc-Transduced Neural Stem Cells Are Effective Therapy for Chronic Stage Experimental Autoimmune Encephalomyelitis. Mol. Neurobiol. 2017, 54, 4365–4378. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Cao, W.; Wei, X.; Chen, J.; Ying, W. SIRT2 Plays Significant Roles in Lipopolysaccharides-Induced Neuroin-flammation and Brain Injury in Mice. Neurochem. Res. 2016, 41, 2490–2500. [Google Scholar] [CrossRef]
- Jastorff, A.M.; Haegler, K.; Maccarrone, G.; Holsboer, F.; Weber, F.; Ziemssen, T.; Turck, C.W. Regulation of proteins mediating neuro-degeneration in experimental autoimmune encephalomyelitis and multiple sclerosis. PROTEOMICS—Clin. Appl. 2009, 3, 1273–1287. [Google Scholar] [CrossRef]
- Lovato, L.; Cianti, R.; Gini, B.; Marconi, S.; Bianchi, L.; Armini, A.; Anghileri, E.; Locatelli, F.; Paoletti, F.; Franciotta, D.; et al. Transketolase and 2′,3′-Cyclic-nucleotide 3′-Phosphodiesterase Type I Isoforms Are Specifically Recognized by IgG Autoantibodies in Multiple Sclerosis Patients. Mol. Cell. Proteom. 2008, 7, 2337–2349. [Google Scholar] [CrossRef]
- Rice, G.I.; Rodero, M.P.; Crow, Y.J. Human Disease Phenotypes Associated With Mutations in TREX1. J. Clin. Immunol. 2015, 35, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Lasigliè, D.; Boero, S.; Bauer, I.; Morando, S.; Damonte, P.; Cea, M.; Monacelli, F.; Odetti, P.; Ballestrero, A.; Uccelli, A.; et al. Sirt6 regulates dendritic cell differentiation, maturation, and function. Aging 2016, 8, 34–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inkster, B.; Strijbis, E.M.M.; Vounou, M.; Kappos, L.; Radue, E.-W.; Matthews, P.M.; Uitdehaag, B.M.; Barkhof, F.; Polman, C.H.; Montana, G.; et al. Histone deacetylase gene variants predict brain volume changes in multiple sclerosis. Neurobiol. Aging 2013, 34, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; Benzi, A.; Sturla, L.; Marubbi, D.; Frumento, D.; Spinelli, S.; Abbotto, E.; Ivaldi, F.; von Holtey, M.; Murone, M.; et al. Sirt6 inhibition delays the onset of experimental au-toimmune encephalomyelitis by reducing dendritic cell migration. J. Neuroinflamm. 2020, 17, 228. [Google Scholar] [CrossRef] [PubMed]
- Burg, N.; Bittner, S.; Ellwardt, E. Role of the epigenetic factor Sirt7 in neuroinflammation and neurogenesis. Neurosci. Res. 2018, 131, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shindler, K.S.; Ventura, E.; Dutt, M.; Elliott, P.; Fitzgerald, D.C.; Rostami, A. Oral Resveratrol Reduces Neuronal Damage in a Model of Multiple Sclerosis. J. Neuro-Ophthalmol. 2010, 30, 328–339. [Google Scholar] [CrossRef]
- Elbaz, E.M.; Senousy, M.A.; El-Tanbouly, D.M.; Sayed, R.H. Neuroprotective effect of linagliptin against cuprizone-induced demy-elination and behavioural dysfunction in mice: A pivotal role of AMPK/SIRT1 and JAK2/STAT3/NF-κB signalling pathway modulation. Toxicol. Appl. Pharmacol. 2018, 352, 153–161. [Google Scholar] [CrossRef]
- Li, W.; Zhang, B.; Tang, J.; Cao, Q.; Wu, Y.; Wu, C.; Guo, J.; Ling, E.-A.; Liang, F. Sirtuin 2, a Mammalian Homolog of Yeast Silent Information Regulator-2 Longevity Regulator, Is an Oligodendroglial Protein That Decelerates Cell Differentiation through Deacetylating-Tubulin. J. Neurosci. 2007, 27, 2606–2616. [Google Scholar] [CrossRef]
- Curry, A.M.; White, D.S.; Donu, D.; Cen, Y. Human Sirtuin Regulators: The “Success” Stories. Front. Physiol. 2021, 12, 752117. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chojdak-Łukasiewicz, J.; Bizoń, A.; Waliszewska-Prosół, M.; Piwowar, A.; Budrewicz, S.; Pokryszko-Dragan, A. Role of Sirtuins in Physiology and Diseases of the Central Nervous System. Biomedicines 2022, 10, 2434. https://doi.org/10.3390/biomedicines10102434
Chojdak-Łukasiewicz J, Bizoń A, Waliszewska-Prosół M, Piwowar A, Budrewicz S, Pokryszko-Dragan A. Role of Sirtuins in Physiology and Diseases of the Central Nervous System. Biomedicines. 2022; 10(10):2434. https://doi.org/10.3390/biomedicines10102434
Chicago/Turabian StyleChojdak-Łukasiewicz, Justyna, Anna Bizoń, Marta Waliszewska-Prosół, Agnieszka Piwowar, Sławomir Budrewicz, and Anna Pokryszko-Dragan. 2022. "Role of Sirtuins in Physiology and Diseases of the Central Nervous System" Biomedicines 10, no. 10: 2434. https://doi.org/10.3390/biomedicines10102434
APA StyleChojdak-Łukasiewicz, J., Bizoń, A., Waliszewska-Prosół, M., Piwowar, A., Budrewicz, S., & Pokryszko-Dragan, A. (2022). Role of Sirtuins in Physiology and Diseases of the Central Nervous System. Biomedicines, 10(10), 2434. https://doi.org/10.3390/biomedicines10102434





