Effect of Ulinastatin on Syndecan-2-Mediated Vascular Damage in IDH2-Deficient Endothelial Cells
Abstract
1. Introduction
2. Experimental Section
2.1. Cell Culture and Transfection
2.2. Mouse Studies
2.3. Immunocytochemistry
2.4. Antibodies and Immunoblotting
2.5. Real-Time Polymerase Chain Reaction (qPCR)
2.6. Cell Viability Assay
2.7. CCK-8 Proliferation Assay
2.8. Cell Scratch Assay
2.9. Mouse Genotyping
2.10. Statistical Analysis
3. Results
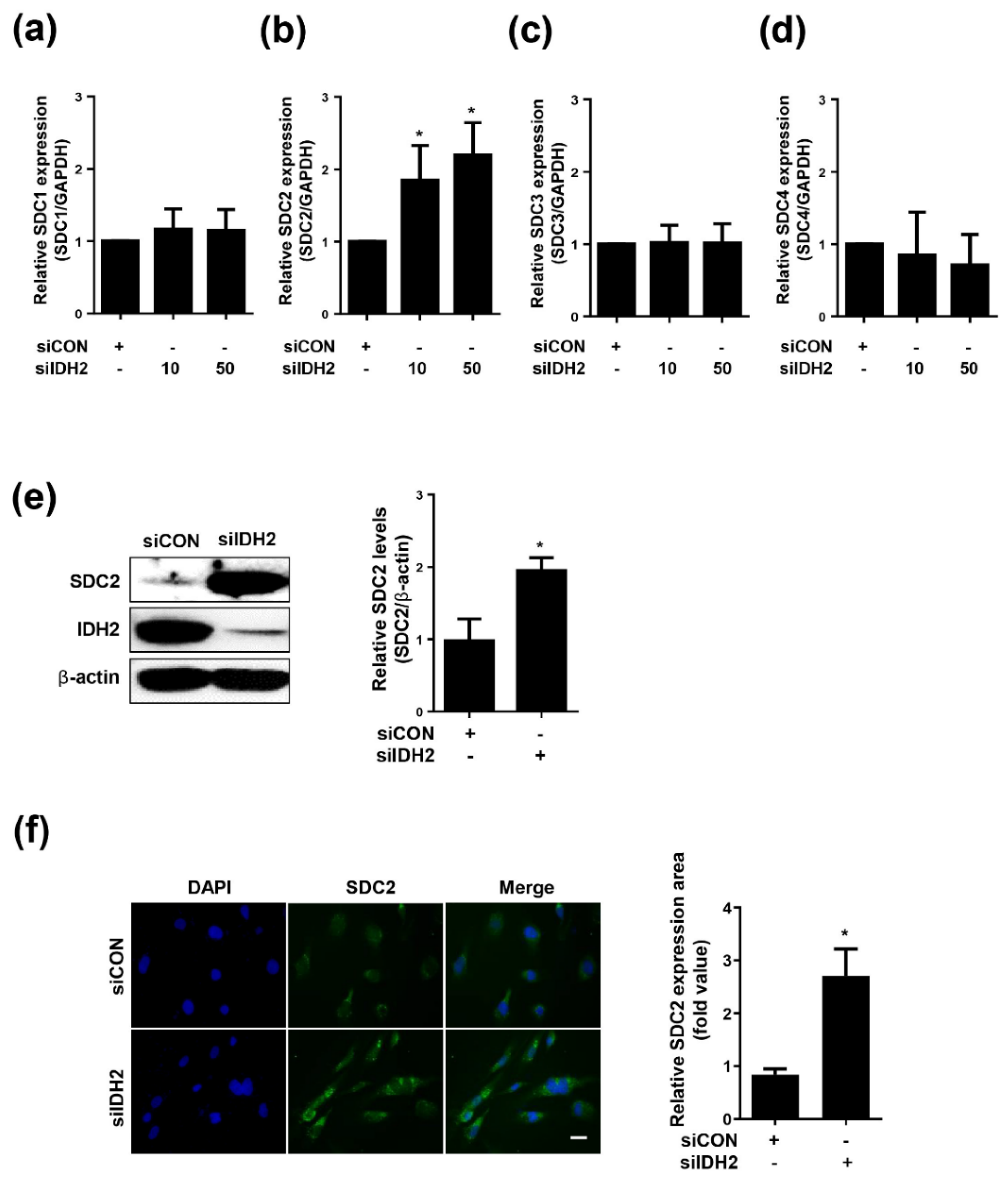
3.1. Expression of SDC2 Is Increased in IDH2-Deficient HUVECs
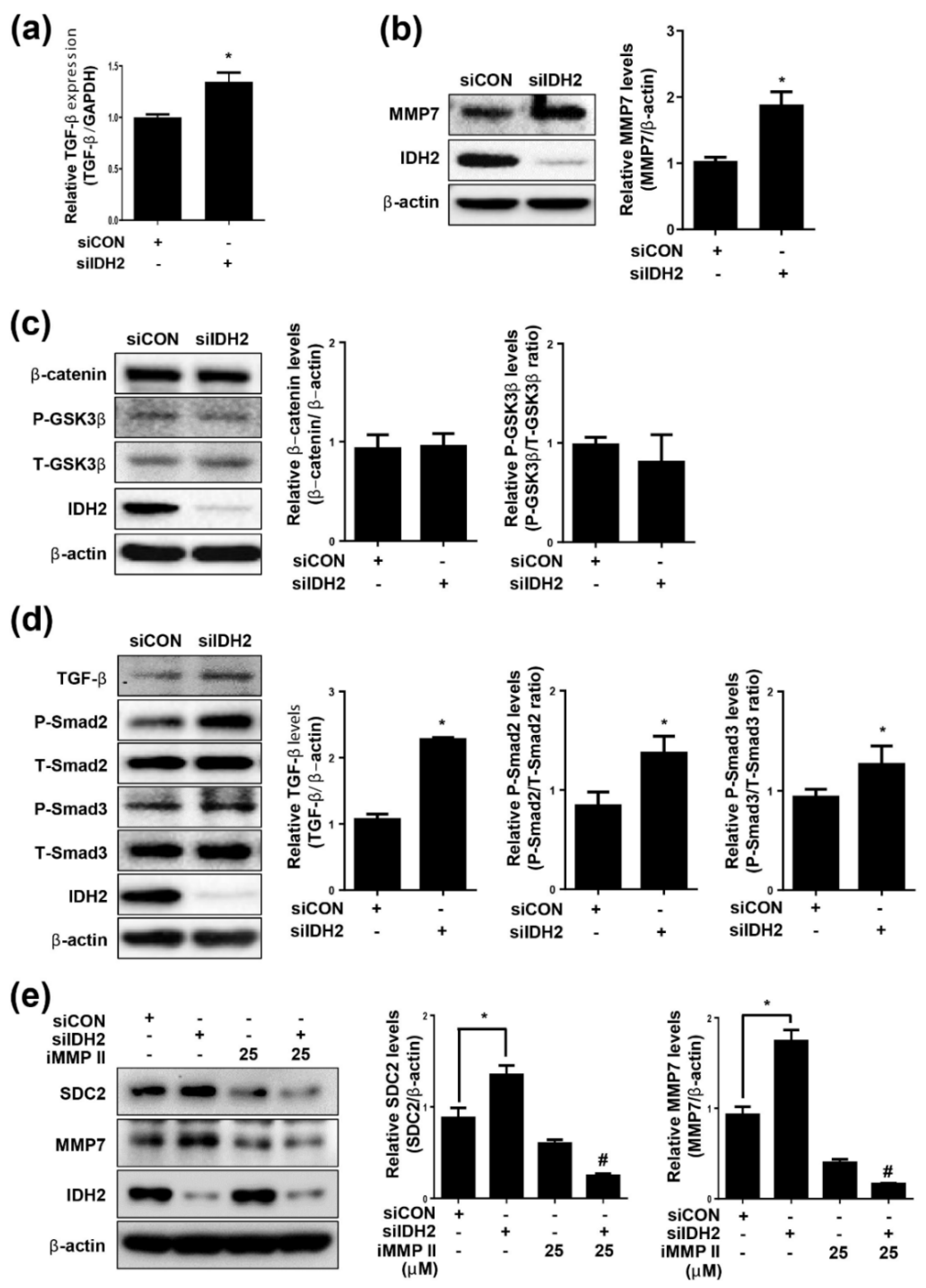
3.2. TGF-β and MMP7 Signaling Correlate with SDC2 Expression in IDH2-Deficient HUVECs
3.3. Effect of UTI on SDC2, MMP7, and TGF-β Signaling in IDH2-Deficient HUVECs
3.4. Effect of UTI on SDC2, MMP7, and TGF-β Signaling in Aorta from IDH2 KO Mice
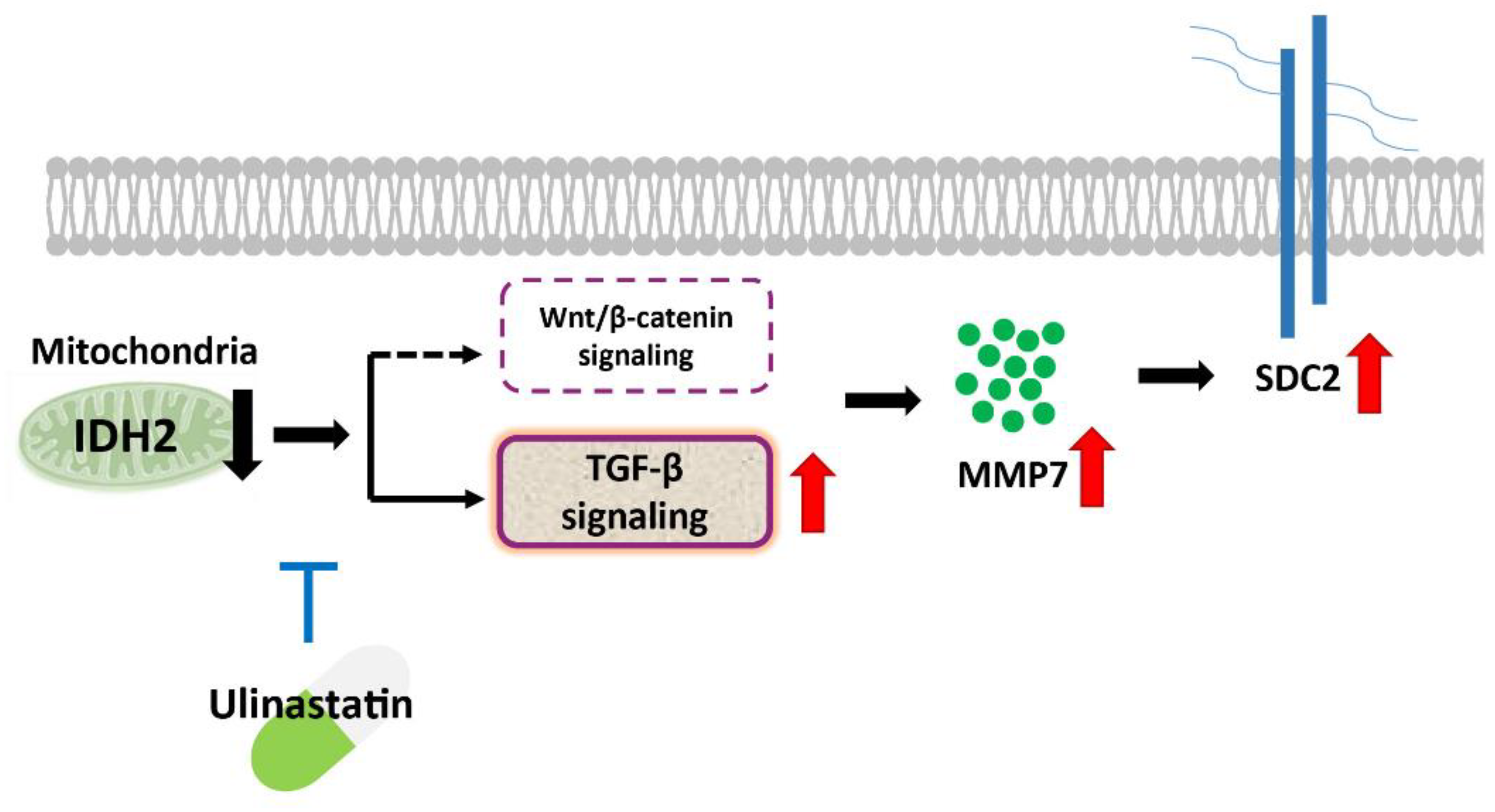
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weinbaum, S.; Tarbell, J.M.; Damiano, E.R. The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 2007, 9, 121–167. [Google Scholar] [CrossRef]
- Tarbell, J.M.; Simon, S.I.; Curry, F.-R.E. Mechanosensing at the vascular interface. Annu. Rev. Biomed. Eng. 2014, 16, 505–532. [Google Scholar] [CrossRef]
- Fu, B.M.; Tarbell, J.M. Mechano-sensing and transduction by endothelial surface glycocalyx: Composition, structure, and function. WIREs Syst. Biol. Med. 2013, 5, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Tarbell, J.M.; Shi, Z.-D.; Dunn, J.; Jo, J. Fluid mechanics, arterial disease, and gene expression. Annu. Rev. Fluid Mech. 2014, 46, 591–614. [Google Scholar] [CrossRef]
- Becker, B.F.; Jacob, M.; Leipert, S.; Salmon, A.H.J.; Chappell, D. Degradation of the endothelial glycocalyx in clinical settings: Searching for the sheddases. Br. J. Clin. Pharmacol. 2015, 80, 389–402. [Google Scholar] [CrossRef]
- Murphy, L.S.; Wickersham, N.; McNeil, J.B.; Shaver, C.M.; May, A.K.; Bastarache, J.A.; Ware, L.B. Endothelial glycocalyx degradation is more severe in patients with non-pulmonary sepsis compared to pulmonary sepsis and associates with risk of ARDS and other organ dysfunction. Ann. Intensiv. Care 2017, 7, 102. [Google Scholar] [CrossRef]
- Zeng, Y.; Ebong, E.E.; Fu, B.M.; Tarbell, J.M. The structural stability of the endothelial glycocalyx after enzymatic removal of glycosaminoglycans. PLoS ONE 2012, 7, e43168. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Koutlas, V.; Pappas, C.; Mitsis, M.; Dounousi, E. The endothelial glycocalyx as a target of ischemia and reperfusion injury in kidney transplantation—Where have we gone so far? Int. J. Mol. Sci. 2021, 22, 2157. [Google Scholar] [CrossRef]
- Afratis, N.; Nikitovic, D.; Multhaupt, H.A.B.; Theocharis, A.D.; Couchman, J.R.; Karamanos, N.K. Syndecans—Key regulators of cell signaling and biological functions. FEBS J. 2017, 284, 27–41. [Google Scholar] [CrossRef]
- Noguer, O.; Reina, M. Is syndecan-2 a key angiogenic element? Sci. World J. 2009, 9, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Chung, H.; Hong, H.; Kim, S.Y.; Kim, S.-E.; Seoh, J.-Y.; Moon, C.M.; Yang, E.G.; Oh, E.-S. Inflammatory hypoxia induces syndecan-2 expression through IL-1beta-mediated FOXO3a activation in colonic epithelia. FASEB J. 2017, 31, 1516–1530. [Google Scholar] [CrossRef] [PubMed]
- Mytilinaiou, M.; Nikitovic, D.; Berdiaki, A.; Kostouras, A.; Papoutsidakis, A.; Tsatsakis, A.; Tzanakakis, G.N. Emerging roles of syndecan 2 in epithelial and mesenchymal cancer progression. IUBMB Life 2017, 69, 824–833. [Google Scholar] [CrossRef]
- Lambaerts, K.; Wilcox-Adelman, S.A.; Zimmermann, P. The signaling mechanisms of syndecan heparan sulfate proteoglycans. Curr. Opin. Cell Biol. 2009, 21, 662–669. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, G.-S.; Dusting, G.J.; Chan, E.C. NADPH oxidase-dependent redox signaling in TGF-beta-mediated fibrotic responses. Redox Biol. 2014, 2, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Lohi, J.; Wilson, C.L.; Roby, J.D.; Parks, W.C.; Kim, W.J.; Lee, S.; Park, M.S.; Jang, Y.K.; Kim, J.B.; Park, S.D. Epilysin, a novel human matrix metalloproteinase (MMP-28) expressed in testis and keratinocytes and in response to injury. J. Biol. Chem. 2001, 276, 10134–10144. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Feng, S.-B.; Cao, Z.-W.; Bei, J.-J.; Chen, Q.; Xu, X.-J.; Zhou, Z.; Yu, Z.-P.; Hu, H.-Y. Up-regulated expression of matrix metalloproteinases in endothelial cells mediates platelet microvesicle-induced angiogenesis. Cell Physiol. Biochem. 2017, 41, 2319–2332. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Jang, B.; Yun, J.-H.; Choi, S.; Park, J.; Shin, D.H.; Lee, S.-T.; Lee, W.; Oh, E.-S. Tyrosine 51 residue of the syndecan-2 extracellular domain is involved in the interaction with and activation of pro-matrix metalloproteinase-7. Sci. Rep. 2019, 9, 10625. [Google Scholar] [CrossRef]
- Li, S.-T.; Dai, Q.; Zhang, S.-X.; Liu, Y.-J.; Yu, Q.-Q.; Tan, F.; Lu, S.-H.; Wang, Q.; Chen, J.-W.; Huang, H.-Q. Ulinastatin attenuates LPS-induced inflammation in mouse macrophage RAW264.7 cells by inhibiting the JNK/NF-kappaB signaling pathway and activating the PI3K/Akt/Nrf2 pathway. Acta Pharmacol. Sin. 2018, 39, 1294–1304. [Google Scholar]
- Cao, C.; Yin, C.; Shou, S.; Wang, J.; Wang, J.; Yu, L.; Li, X.; Chai, Y. Ulinastatin protects against LPS-induced acute lung injury by attenuating TLR4/NF-kappaB pathway activation and reducing inflammatory mediators. Shock 2018, 50, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Jiang, Y.; Jiang, Y.; Yuan, D.; Xiao, L. Ulinastatin attenuates monocyte-endothelial adhesion via inhibiting ROS transfer between the neighboring vascular endothelial cells mediated by Cx43. Am. J. Transl. Res. 2020, 12, 4326–4336. [Google Scholar] [PubMed]
- Han, S.J.; Choi, H.S.; Kim, J.I.; Park, J.-W.; Park, K.M. IDH2 deficiency increases the liver susceptibility to ischemia-reperfusion injury via increased mitochondrial oxidative injury. Redox Biol. 2018, 14, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-J.; Piao, S.; Nagar, H.; Jung, S.-B.; Kim, S.; Lee, I.; Kim, S.-M.; Song, H.-J.; Shin, N.; Kim, D.W.; et al. Isocitrate dehydrogenase 2 deficiency induces endothelial inflammation via p66sh-mediated mitochondrial oxidative stress. Biochem. Biophys. Res. Commun. 2018, 503, 1805–1811. [Google Scholar] [CrossRef]
- Ke, B.; Fan, C.; Yang, L.; Fang, X. Matrix metalloproteinases-7 and kidney fibrosis. Front. Physiol. 2017, 8, 21. [Google Scholar] [CrossRef]
- Burke, B. The role of matrix metalloproteinase 7 in innate immunity. Immunobiology 2004, 209, 51–56. [Google Scholar] [CrossRef]
- Halden, Y.; Rek, A.; Atzenhofer, W.; Szilak, L.; Wabnig, A.; Kungl, A.J. Interleukin-8 binds to syndecan-2 on human endothelial cells. Biochem. J. 2004, 377 Pt 2, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Nagar, H.; Choi, S.; Jung, S.-B.; Kim, H.-W.; Kang, S.K.; Lee, J.W.; Lee, J.H.; Park, J.-W.; Irani, K.; et al. IDH2 deficiency impairs mitochondrial function in endothelial cells and endothelium-dependent vasomotor function. Free Radic. Biol. Med. 2016, 94, 36–46. [Google Scholar] [CrossRef]
- Choi, S.; Kim, J.-Y.; Park, J.H.; Lee, S.-T.; Han, I.-O.; Oh, E.-S. The matrix metalloproteinase-7 regulates the extracellular shedding of syndecan-2 from colon cancer cells. Biochem. Biophys. Res. Commun. 2012, 417, 1260–1264. [Google Scholar] [CrossRef]
- Zhou, S. TGF-beta regulates beta-catenin signaling and osteoblast differentiation in human mesenchymal stem cells. J. Cell Biochem. 2011, 112, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E. The role of Wnt signaling in physiological and pathological angiogenesis. Circ. Res. 2010, 107, 943–952. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, L.; Zhen, G.; Crane, J.L.; Zhou, X.; Cao, X. Transforming growth factor-beta in stem cells and tissue homeostasis. Bone Res. 2018, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Pugia, M.J.; Lott, J.A. Pathophysiology and diagnostic value of urinary trypsin inhibitors. Clin. Chem. Lab. Med. (CCLM) 2005, 43, 1–16. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, J.; Zhang, P.; Ding, W. Ulinastatin attenuates vascular endothelial cell damage in pregnant women with severe pre-eclampsia. An. Acad. Bras. Cienc. 2019, 91, e20180746. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wei, F.; Luo, L.; Xu, S.; Liu, S.; Zhang, D. Ulinastatin attenuates hyper-permeability of vascular endothelialium cells induced by serum from patients with sepsis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2017, 33, 1600–1604. [Google Scholar] [PubMed]
- Li, D.; Ji, H.; Zhao, B.; Xu, C.; Xia, W.; Han, L.; Yu, D.; Ju, Y.; Jin, C. Therapeutic effect of ulinastatin on pulmonary fibrosis via downregulation of TGFbeta1, TNFalpha and NFkappaB. Mol. Med. Rep. 2018, 17, 1717–1723. [Google Scholar]
- Jiang, G.-T.; Chen, X.; Li, D.; An, H.-X.; Jiao, J.-D. Ulinastatin attenuates renal interstitial inflammation and inhibits fibrosis progression in rats under unilateral ureteral obstruction. Mol. Med. Rep. 2014, 10, 1501–1508. [Google Scholar] [CrossRef] [PubMed][Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-j.; Nagar, H.; Lee, J.W.; Kim, S.; Lee, I.; Piao, S.; Jeon, B.H.; Kim, C.-S. Effect of Ulinastatin on Syndecan-2-Mediated Vascular Damage in IDH2-Deficient Endothelial Cells. Biomedicines 2022, 10, 187. https://doi.org/10.3390/biomedicines10010187
Choi S-j, Nagar H, Lee JW, Kim S, Lee I, Piao S, Jeon BH, Kim C-S. Effect of Ulinastatin on Syndecan-2-Mediated Vascular Damage in IDH2-Deficient Endothelial Cells. Biomedicines. 2022; 10(1):187. https://doi.org/10.3390/biomedicines10010187
Chicago/Turabian StyleChoi, Su-jeong, Harsha Nagar, Jun Wan Lee, Seonhee Kim, Ikjun Lee, Shuyu Piao, Byeong Hwa Jeon, and Cuk-Seong Kim. 2022. "Effect of Ulinastatin on Syndecan-2-Mediated Vascular Damage in IDH2-Deficient Endothelial Cells" Biomedicines 10, no. 1: 187. https://doi.org/10.3390/biomedicines10010187
APA StyleChoi, S.-j., Nagar, H., Lee, J. W., Kim, S., Lee, I., Piao, S., Jeon, B. H., & Kim, C.-S. (2022). Effect of Ulinastatin on Syndecan-2-Mediated Vascular Damage in IDH2-Deficient Endothelial Cells. Biomedicines, 10(1), 187. https://doi.org/10.3390/biomedicines10010187







