Clustering Inflammatory Markers with Sociodemographic and Clinical Characteristics of Patients with Diabetes Type 2 Can Support Family Physicians’ Clinical Reasoning by Reducing Patients’ Complexity
Abstract
:1. Background
2. Aim
3. Methods
3.1. Study Population
3.2. Data Collection
3.3. Statistical Analysis
3.4. Cluster Analysis
3.5. Data Analysis Procedure
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and age-related diseases: Role of inflammation triggers and cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.M.; Reeves, G.; Billman, G.E.; Sturmberg, J.P. Inflammation-nature’s way to efficiently respond to all types of challenges: Implications for understanding and managing “the epidemic” of chronic diseases. Front. Med. 2018, 5, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Moni, D.; Capri, M.; Salvioloi, S. The continuum of aging and age-related diseases: Common mechanisms but different rates. Front. Med. 2018, 12, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Pes, G.M.; Lio, D.; Carru, C.; Deiana, L.; Baggio, G.; Franceschi, C.; Ferrucci, L.; Oliveri, F.; Scola, L.; Crivello, A.; et al. Association between longevity and cytokine gene polymorphisms. A study in Sardinian centenarians. Aging Clin. Exp. Res. 2004, 16, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation 2020, 141, 9. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, S.; Malanda, B.; Damasceno, A.; Eckel, R.H.; Gaita, D.; Kotseva, K.; Januzzi, J.L.; Mensah, G.; Plutzky, J.; Prystupiuk, M.; et al. A roadmap on the prevention of cardiovascular disease among people living with diabetes. Glob. Heart 2019, 14, 215–240. [Google Scholar] [CrossRef]
- Das, S.R.; Everett, B.M.; Birtcher, K.K.; Brown, J.M.; Januzzi, J.L., Jr.; Kalyani, R.R.; Kosiborod, M.; Magwire, M.; Morris, P.B.; Neumiller, J.J.; et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: A report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2020, 76, 1117–1145. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration (BMI Mediated Effects); Lu, Y.; Hajifathalian, K.; Ezzati, M.; Woodward, M.; Rimm, E.B.; Danaei, G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: A pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet 2014, 383, 970–983. [Google Scholar]
- Abdelhafiz, A.H.; Brown, S.H.; Bello, A.; El Nahas, M. Chronic kidney disease in older people: Physiology, pathology or both? Nephron Clin. Pract. 2010, 116, c19–c24. [Google Scholar] [CrossRef] [PubMed]
- Forman, D.E.; Maurer, M.S.; Boyd, C.; Brindis, R.; Salive, M.E.; Horne, F. Multimorbidity in older adults with cardiovascular disease. J. Am. Coll. Cardiol. 2018, 71, 2149–2161. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Grasso, M.; Santangelo, R.; Lazzarino, G.; Lunte, S.M.; Caraci, F. Inflammation as the common biological link between depression and cardiovascular diseases: Can carnosine exert a protective role? Curr. Med. Chem. 2020, 27, 1782–1800. [Google Scholar] [CrossRef] [PubMed]
- Trtica Majnarić, L.; Bosnić, Z.; Kurevija, T.; Wittlinger, T. Cardiovascular risk and aging: The need for a more comprehensive understanding. J. Geriatr. Cardiol. 2021, 18, 462–478. [Google Scholar] [PubMed]
- Bekić, S.; Babič, F.; Pavlišková, V.; Paralič, J.; Wittlinger, T.; Majnarić, L.T. Clusters of physical frailty and cognitive impairment and their associated comorbidities in older primary care patients. Healthcare 2021, 9, 891. [Google Scholar] [CrossRef] [PubMed]
- Babič, F.; Trtica Majnarić, L.; Bekić, S.; Holzinger, A. Machine learning for family doctors: A case of cluster analysis for studying aging associated comorbidities and frailty. In Machine Learning and Knowledge Extraction; Holzinger, A., Kieseberg, P., Tjoa, A., Weippl, E., Eds.; CD-MAKE: Canterbury, UK, 2019. [Google Scholar]
- Fried, L.P.; Tangen, C.M.; Walston, J. Cardiovascular health study collaborative research group. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Volpato, S.; Guralnik, J.M.; Ferrucci, L.; Balfour, J.; Chaves, P.; Fried, L.P.; Harris, T.B. Cardiovascular disease, interleukin-6, and risk of mortality in older women: The women’s health and aging study. Circulation 2001, 103, 947–953. [Google Scholar] [CrossRef] [Green Version]
- Angkananard, T.; Anothaisintawee, T.; McEvoy, M.; Attia, J.; Thakkinstian, A. Neutrophil lymphocyte ratio and cardiovascular disease risk: A systematic review and meta-analysis. BioMed Res. Int. 2018, 2018, 2703518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bupp, M.R.G. Sex, the aging immune system, and chronic disease. Cell. Immunol. 2015, 294, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Corriere, T.; Di Marca, S.; Cataudella, E.; Pulvirenti, A.; Alaimo, S.; Stancanelli, B.; Malatino, L. Neutrophil-to-lymphocyte ratio is a strong predictor of atherosclerotic carotid plaques in older adults. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Ambrosy, A.P.; Greene, S.J.; Mentz, R.J.; Subacius, H.P.; Maggioni, A.P.; Swedberg, K.; Nodari, S.; Zannad, F.; Konstam, M.A.; et al. EVEREST trial investigators. Predictive value of low relative lymphocyte counts in patients hospitalized for heart failure with reduced ejection fraction: Insights from the EVEREST trial. Circ. Heart Fail. 2012, 5, 750–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonyali, S.; Ceylan, C.; Yahsi, S.; Karakan, M.S. Does neutrophil to lymphocyte ratio demonstrate deterioration in renal function? Ren. Fail. 2018, 40, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D. Sex differences in inflammation during atherosclerosis. Clin. Med. Insights Cardiol. 2015, 8, 49–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lockhart, C.J.; Hamilton, P.K.; Quinn, C.E.; McVeigh, G.E. End-organ dysfunction and cardiovascular outcomes: The role of the microcirculation. Clin. Sci. 2009, 116, 175–190. [Google Scholar] [CrossRef] [Green Version]
- Howard, R.; Scheiner, A.; Kanetsky, P.A.; Egan, K.M. Sociodemographic and lifestyle factors associated with the neutrophil-to-lymphocyte ratio. Ann. Epidemiol. 2019, 38, 11–21. [Google Scholar] [CrossRef]
- Fest, J.; Ruiter, R.; Ikram, M.A.; Voortman, T.; van Eijck, C.H.J.; Stricker, B.H. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: A population-based prospective cohort study. Sci. Rep. 2018, 8, 10566. [Google Scholar] [CrossRef]
- Iwakura, Y.; Ishigame, H. The IL-23/IL-17 axis in inflammation. J. Clin. Investig. 2006, 116, 1218–1222. [Google Scholar] [CrossRef] [Green Version]
- Trtica Majnarić, L.; Guljaš, S.; Bosnić, Z.; Šerić, V.; Wittlinger, T. Neutrophil-to-lymphocyte ratio as a cardiovascular risk marker may be less efficient in women than in men. Biomolecules 2021, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, Y.J.; Chen, X.; Kwan, T.; Chadban, S.J.; Wu, H. Interleukin 17A promotes diabetic kidney injury. Sci. Rep. 2019, 9, 2264. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Tao, X. Current understanding of IL-37 in human health and disease. Front. Immunol. 2021, 12, 696605. [Google Scholar] [CrossRef]
- Li, T.; Li, H.; Li, W.; Chen, S.; Feng, T.; Jiao, W.; Wu, C.; Dong, J.; Li, Y.; Li, S.; et al. Interleukin-37 sensitize the elderly type 2 diabetic patients to insulin therapy through suppressing the gut microbiota dysbiosis. Mol. Immunol. 2019, 112, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, Y.; Fei, B. Interleukin 37 (IL-37) reduces high glucose-induced inflammation, oxidative stress, and apoptosis of podocytes by inhibiting the STAT3–cyclophilin a (CypA) signaling pathway. Med. Sci. Monit. 2020, 26, e922979-1–e922979-11. [Google Scholar] [CrossRef] [PubMed]
- Krebs, C.F.; Turner, J.-E.; Riedel, J.-H.; Panzer, U. Tissue-specific therapy in immune-mediated kidney diseases: New ARGuments for targeting the IL-23/IL-17 axis. J. Clin. Investig. 2021, 131, e150588. [Google Scholar] [CrossRef]
- López-Bautista, F.; Posadas-Sánchez, R.; Vázquez-Vázquez, C.; Fragoso, J.; Rodríguez-Pérez, J.; Vargas-Alarcón, G. IL-37 Gene and cholesterol metabolism: Association of polymorphisms with the presence of hypercholesterolemia and cardiovascular risk factors. The GEA Mexican study. Biomolecules 2020, 10, 1409. [Google Scholar] [CrossRef]
- Calder, P.; Ahluwalia, N.; Albers, R.; Bosco, N.; Bourdet-Sicard, R.; Haller, D.; Holgate, S.; Jönsson, L.; Latulippe, M.; Marcos, A.; et al. A Consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br. J. Nutr. 2013, 109, S1–S34. [Google Scholar] [CrossRef] [Green Version]
- Bellary, S.; Kyrou, I.; Brown, J.E.; Bailey, C.J. Type 2 diabetes mellitus in older adults: Clinical considerations and management. Nat. Rev. Endocrinol. 2021, 17, 534–548. [Google Scholar] [CrossRef]
- Ljiljana, M.T.; Zvonimir, B.; Nikola, V.; Pınar, Y. Complexity and non-linearity of cardiovascular risk factors in older patients with multimorbidity and reduced renal function. J. Integr. Cardiol. 2020, 3, 1–11. [Google Scholar]
- Zoungas, S.; Woodward, M.; Li, Q.; Cooper, M.E.; Hamet, P.; Harrap, S.; Heller, S.; Marre, M.; Patel, A.; Poulter, N.; et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia 2014, 57, 2465–2474. [Google Scholar] [CrossRef]
- Majnarić-Trtica, L.; Vitale, B. Systems biology as a conceptual framework for research in family medicine; Use in predicting response to influenza vaccination. Prim. Health Care Res. Dev. 2011, 12, 310–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dezelic, G.; Kern, J.; Petrovecki, M.; Ilakovac, V.; Hercigonja-Szekeres, M. Medical informatics in Croatia—A historical survey. Acta Inform. Med. 2014, 22, 49–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šabanovic, Š.; Majnaric Trtica, L.; Babic, F.; Vadovsky, M.; Paralic, J.; Vcev, A.; Holzinger, A. Metabolic syndrome in hypertensive women in the age of menopause: A case study on data from general practice electronic health records. BMC Med. Inform. Decis. Mak. 2018, 18, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, G.; Ganz, T.; Goodnough, L.T. Anemia of inflammation. Blood 2019, 133, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, A.S. A decade after the KDOQI CDK guidelines. Am. J. Kidney Dis. 2012, 60, 683–685. [Google Scholar] [CrossRef]
- National Kidney Fundation. eGFR Calculator. Available online: https://www.kidney.org/professionals/kdoqi/gfr_calculator (accessed on 24 September 2021).
- Barbesino, G. Thyroid function changes in the elderly and their relationship to cardiovascular health: A mini-review. Gerontology 2019, 65, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.; Tel, S.; Rija, J.; Bhat, H.; Raza, M. Neutrophil to lymphocyte ratio and cardiovascular diseases: A review. Expert Rev. Cardiovasc. Ther. 2013, 11, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Bralić Lang, V.; Bergman Marković, B. Prevalence of comorbidity in primary care patients with type 2 diabetes and its association with elevated HbA1c: A cross-sectional study in Croatia. Scand. J. Prim. Health Care 2016, 34, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.W.; Hung, L.C.; Chen, Y.C.; Wang, W.H.; Lin, C.Y.; Tzeng, H.H.; Suen, J.-L.; Chen, Y.-H. Insulin reduces inflammation by regulating the activation of the NLRP3 inflammasome. Front. Immunol. 2021, 11, 587229. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.; DeFronzo, R.A.; Del Prato, S.; Chilton, R.; Singh, R.; Ryder, R.E. Cardiovascular disease and Type 2 diabetes: Has the dawn of a new era arrived? Diabetes Care 2017, 40, 813–820. [Google Scholar] [CrossRef] [Green Version]
- Landi, F.; Russo, A.; Liperoti, R.; Pahor, M.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Midarm muscle circumference, physical performance and mortality: Results from the aging and longevity study in the Sirente geographic area ilSIRENTE study. Clin. Nutr. 2010, 29, 441–447. [Google Scholar] [CrossRef]
- Nardi, R.; Scanelli, G.; Corrao, S.; Iori, I.; Mathieu, G.; Cataldi Amatrian, R. Co-morbidity does not reflect complexity in internal medicine patients. Eur. J. Intern. Med. 2007, 18, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Corrao, S.; Natoli, G.; Nobili, A.; Mannucci, P.M.; Pietrangelo, A.; Perticone, F.; Argano, C.; REPOSI Investigators. Comorbidity does not mean clinical complexity: Evidence from the RePoSI register. Intern. Emerg. Med. 2020, 15, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Whitty, C.J.M.; MacEwen, C.; Goddard, A.; Aldersonm, D.; Marshall, M.; Calderwood, C.; Atherton, F.; McBride, M.; Atherton, J.; Stokes-Lampard, H.; et al. Rising to the challenge of multimorbidity. BMJ 2020, 368, 16964. [Google Scholar] [CrossRef] [Green Version]
- Plsek, P.E.; Greenhalgh, T. Complexity science: The challenge of complexity in health care. BMJ 2001, 323, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Frank, E.; Holmes, G.; Pfahringer, B.; Reutemann, P.; Witten, I.H. The WEKA data mining software: An update. ACM SIGKDD Explor. Newsl. 2009, 11, 10–18. [Google Scholar] [CrossRef]
- WEKA. Weka 3: Data Mining Software in Java. Available online: http://www.cs.waikato.ac.nz/ml/weka (accessed on 21 August 2021).
- Han, J.; Micheline, K. Data Mining: Concepts and Techniques; Morgan Kaufmann: Waltham, MA, USA, 2001. [Google Scholar]
- Majnarić, L.T.; Babič, F.; O’Sullivan, S.; Holzinger, A. AI and big data in healthcare: Towards a more comprehensive research framework for multimorbidity. J. Clin. Med. 2021, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K. Data clustering: 50 years beyond K-means. Pattern Recognit. Lett. 2009, 31, 651–666. [Google Scholar] [CrossRef]
- Tan, P.; Steinbach, M.; Kumar, V. Introduction to Data Mining, Lecture Notes for Chapter 2; University of Minnesota: Minneapolis, MN, USA, 2004. [Google Scholar]
- Bekić, S.; Babič, F.; Filipčić, I.; Majnarić, L. Clustering of mental and physical comorbidity and the risk of frailty in patients aged 60 years or more in primary care. Med. Sci. Monit. 2019, 25, 6820–6835. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, M.; Zghebi, S.S.; Ashcroft, D.M.; Buchan, I.; Chew-Graham, C.; Holt, T.; Mallen, C.; Van Marwijk, H.; Peek, N.; Perera-Salazar, R.; et al. The comorbidity burden of type 2 diabetes mellitus: Patterns, clusters and predictions from a large English primary care cohort. BMC Med. 2019, 17, 145. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- Majnarić, L.T.; Martinović, I.; Šabanović, Š.; Rudan, S.; Babič, F.; Wittlinger, T. The effect of hypertension duration and the age of onset on CV risk factors expression in perimenopausal women. Int. J. Hypertens. 2019, 2019, 9848125. [Google Scholar]
- Nuzzo, A.; Rossi, R.; Modena, M.G. Hypertension alone or related to the metabolic syndrome in postmenopausal women. Expert Rev. Cardiovasc. Ther. 2010, 8, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Pennell, L.M.; Galligan, C.L.; Fish, E.N. Sex affects immunity. J. Autoimmun. 2012, 38, J282–J291. [Google Scholar] [CrossRef] [PubMed]
- Rathod, K.S.; Kapil, V.; Velmurugan, S.; Khambata, R.S.; Siddique, U.; Khan, S.; Van Eijl, S.; Gee, L.C.; Bansal, J.; Pitrola, K.; et al. Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans. J. Clin. Investig. 2017, 127, 169–182. [Google Scholar] [CrossRef]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Curro, D. Thyroid hormones, oxidative stress, and inflammation. Mediat. Inflamm. 2016, 2016, 6757154. [Google Scholar] [CrossRef] [Green Version]
- Regitz-Zagrosek, V.; Lehmkuhl, E.; Weickert, M.O. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clinical research in cardiology. Off. J. Ger. Card. Soc. 2006, 95, 136–147. [Google Scholar]
- Mikić, M.; Šestak, A.; Volarić, M.; Rudan, S.; Majnarić, L.T. Seasonality of the cardiac biomarker troponin in the Eastern Croatian population. J. Clin. Med. 2018, 7, 520. [Google Scholar] [CrossRef] [Green Version]
- Walker, S.R.; Wagner, M.; Tangri, N. Chronic kidney disease, frailty and successful aging: A review. J. Ren. Nutr. 2014, 24, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.J.; Rodriguez-Mañas, L. Diabetes and frailty: Two converging conditions? Can. J. Diabetes 2016, 40, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Kleipool, E.E.; Hoogendijk, E.O.; Trappenburg, M.C.; Handoko, M.L.; Huisman, M.; Peters, M. Frailty in older adults with cardiovascular disease: Cause, effect, or both? Aging Dis. 2018, 9, 489–497. [Google Scholar] [CrossRef] [Green Version]
- Theofilis, P.; Vordoni, A.; Koukoulaki, M.; Vlachopanos, G.; Kalaitzidis, R.G. Dyslipidemia in chronic kidney disease: Contemporary concepts and future therapeutic perspectives. Am. J. Nephrol. 2021, 52, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Starr, K.N.P.; McDonald, S.R.; Bales, C.W. Obesity and physical frailty in older adults: A scoping review of intervention trial. J. Am. Med. Dir. Assoc. 2014, 15, 240–250. [Google Scholar] [CrossRef] [Green Version]
- Nashar, K.; Egan, B.M. Relationship between chronic kidney disease and metabolic syndrome: Current perspectives. Diabetes Metab. Syndr. Obes. 2014, 7, 421–435. [Google Scholar] [CrossRef] [Green Version]
- McAninch, E.A.; Bianco, A.C. Thyroid hormone signaling in energy homeostasis and energy metabolism. Ann. N. Y. Acad. Sci. 2014, 1311, 77–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Corrao, S.; Argano, C.; Natoli, G.; Nobili, A.; Corazza, G.R.; Mannucci, P.M.; Perticone, F.; on behalf of REPOSI Investigators. Sex-differences in the pattern of comorbidities, functional independence, and mortality in elderly inpatients: Evidence from the RePoSI Register. J. Clin. Med. 2019, 8, 81. [Google Scholar] [CrossRef] [Green Version]
- Samson, L.D.; Boots, A.M.H.; Ferreira, J.A. In-depth immune cellular profiling reveals sex-specific associations with frailty. Immun. Ageing 2020, 17, 20. [Google Scholar]
- Frangogiannis, N. Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Valensin, S.; Bonafè, M.; Paolisso, G.; Yashin, A.; Monti, D.; De Benedictis, G. The network and the remodeling theories of aging: Historical background and new perspectives. Exp. Gerontol. 2000, 35, 879–896. [Google Scholar] [CrossRef]




| No | Variables and Their Abbreviations | Variable Description | |
|---|---|---|---|
| 1 | Gender | Categorical (0,1) Category frequency Males = 98 Females = 76 | |
| 2 | Age (years) | Numerical Mean: 67.03 Max = 88, Min = 50 | |
| 3 | Number of comorbidities (<3, ≥3) | Categorical (0,1) Category frequency 0 = 8 1 = 166 | |
| 4 | Body mass index (BMI) (kg/m2) | Numerical Mean: 30.53 Max = 51.37, Min = 19.4 | |
| 5 | Midarm circumference (mac) (cm) | Numerical Mean: 29.974 Max = 39, Min = 21 | |
| 6 | Marker of inflammation-related anemia Hemoglobin (Hb) (g/L) | Numerical Mean: 142.27 Max = 166, Min = 93 | |
| 7 | Estimated glomerular filtration rate (eGFR) (mL/min/1.73 m2) | Numerical Mean: 80.723 Max = 163, Min = 29 | |
| 8 | eGFR levels | Nominal 1,2,3,4 | |
| Category | Frequency | ||
| 1 | 55 | ||
| 2 | 64 | ||
| 3 | 43 | ||
| 4 | 12 | ||
| 9 | Triglycerides (mmol/L) | Numerical Mean: 1.981 Max = 8.7, Min = 0.53 | |
| 10 | HDL cholesterol (mmol/L) | Numerical Mean: 2.209 Max = 141, Min = 2.49 | |
| 11 | Thyroid-stimulating hormone (TSH) (mU/L) | Numerical Mean: 2.905 Max = 9.45, Min = 0.15 | |
| 12 | Traditional marker of inflammation C-reactive protein (CRP) (mg/L) | Numerical Mean: 2.877 Max = 27.2, Min = 0.2 | |
| 13 | Frailty index | Categorical 0,1,2 | |
| Category | Frequency | ||
| 0 | 101 | ||
| 1 | 42 | ||
| 2 | 31 | ||
| 14 | Diabetes mellitus type 2 (DM2) duration (years) | Numerical Mean: 8.809 Max = 30, Min = 1 | |
| 15 | Hypertension | Categorical (0 = No, 1 = Yes) Category frequency 0 = 19 1 = 155 | |
| 16 | Hypertension duration (years) | Numerical Mean: 10.48 Max = 25, Min = 0 | |
| 17 | Diagnosis of chronic heart disease (CHD) | Categorical (0 = No, 1 = Yes) Category frequency 0 = 92 1 = 82 | |
| 18 | Diagnosis of coronary artery disease (CAD) | Categorical (0 = No, 1 = Yes) Category frequency 0 = 114 1 = 60 | |
| 19 | Diagnosis of periphery artery disease (PAD) | Categorical (0 = No, 1 = Yes) Category frequency 0 = 137 1 = 37 | |
| 20 | Diagnosis of osteoporosis | Categorical (0 = No, 1 = Yes) Category frequency 0 = 135 1 = 39 | |
| 21 | Diagnosis of severe osteoarthritis | Categorical (0 = No, 1 = Yes) Category frequency 0 = 89 1 = 85 | |
| 22 | Diagnosis of low back pain | Categorical (0 = No, 1 = Yes) Category frequency 0 = 115 1 = 59 | |
| 23 | Diagnosis of anxiety/depression | Categorical (0 = No, 1 = Yes) Category frequency 0 = 105 1 = 69 | |
| 24 | New treatment option Dipeptidyl peptidase-4 inhibitor (DPP4) | Categorical (0 = No, 1 = Yes) Category frequency 0 = 143 1 = 31 | |
| 25 | New treatment option Sodium glucose cotransporter-2 inhibitors (SGLT2) | Categorical (0 = No, 1 = Yes) Category frequency 0 = 167 1 = 7 | |
| 26 | New treatment option Glucagon-likepeptide-1 receptor agonists (GLP1r) | Categorical (0 = No, 1 = Yes) Category frequency 0 = 158 1 = 16 | |
| 27 | Therapy with insulin | Categorical (0 = No, 1 = Yes) Category frequency 0 = 134 1 = 40 | |
| 28 | Emerging marker of inflammation Neutrophil-to-lymphocyte ratio (NLR) | Numerical Mean: 1.718 Max = 5.4, Min = 0.6 | |
| 29 | Emerging marker of inflammation Il-17A (pg/mL) | Numerical Mean: 3.291 Max = 75.52 Min = 0.01 | |
| 30 | Emerging marker of inflammation Il-37 (pg/mL) | Numerical Mean: 42.679 Max = 1788.4 Min = 0.14 | |
| K Value | Number of Iterations | Within-Cluster Sum of Squared Errors |
|---|---|---|
| 3 | 8 | 2712 |
| 4 | 7 | 2689 |
| 5 | 8 | 2593 |
| 6 | 7 | 2592 |
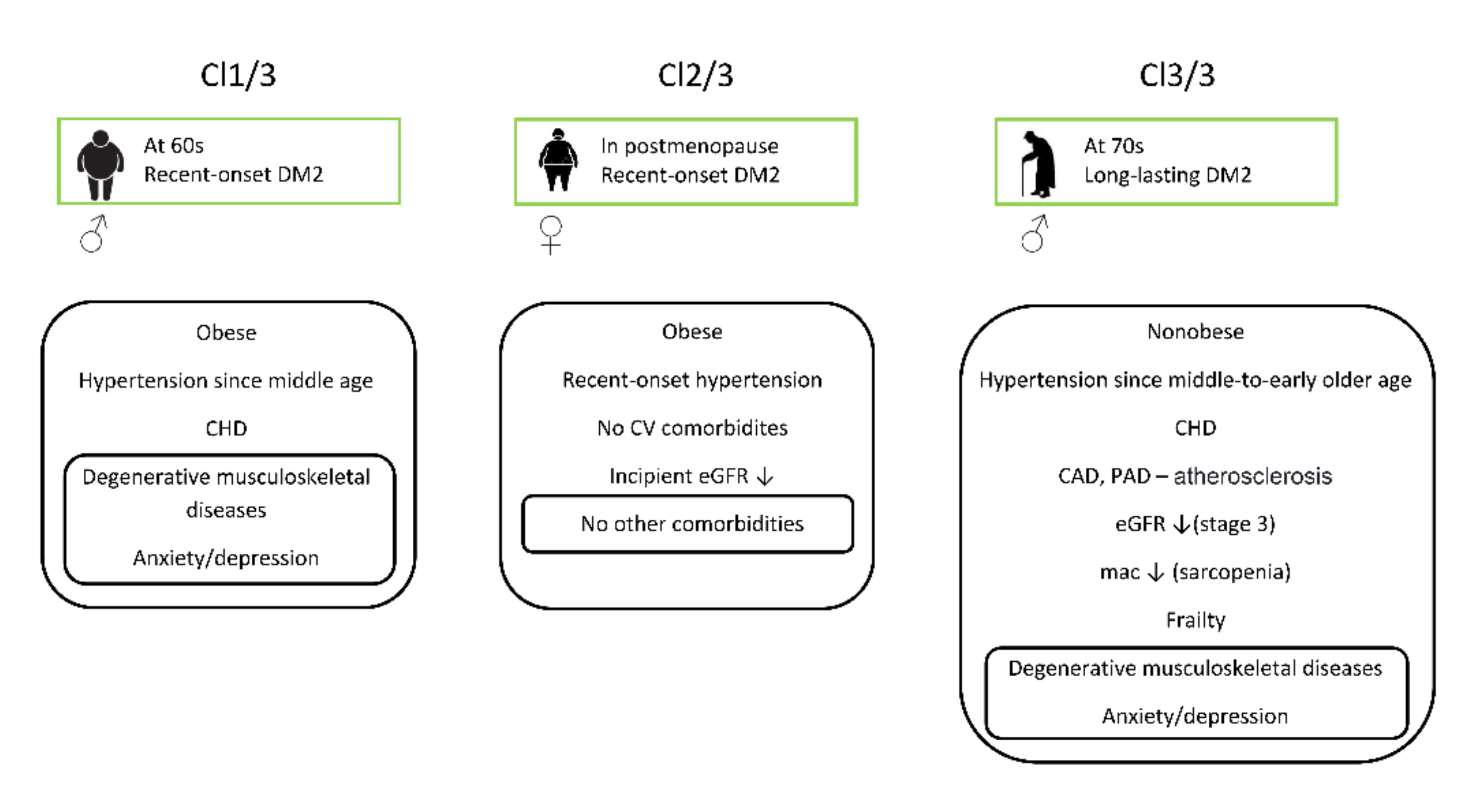
| Variable | Cl 1/3 | Cl 2/3 | Cl 3/3 |
|---|---|---|---|
| Gender (M,F) (0,1) | 1 | 0 | 1 |
| Age (years) | 61 | 63 | 72 |
| Number of comorbidities (<3, ≥3) (0,1) | 1 | 1 | 1 |
| BMI (kg/m2) | 37 | 32 | 27 |
| mac (cm) | 29 | 30 | 28 |
| Hb (g/L) | 148 | 142 | 123 |
| eGFR (mL/min/1.73 m2) | 82 | 59 | 29 |
| eGFR levels (1–4) | 2 | 1 | 3 |
| Triglycerides (mmol/L) | 1.5 | 1.6 | 2.1 |
| HDL cholesterol (mmol/L) | 1.4 | 1.3 | 1.2 |
| TSH (mU/L) | 3.3 | 2.2 | 1.2 |
| CRP (mg/L) | 1.2 | 1.1 | 0.8 |
| Frailty index (0,1,2) | 0 | 0 | 2 |
| DM2 duration (years) | 1 | 2 | 10 |
| Hypertension (0 = No, 1 = Yes) | 1 | 1 | 1 |
| Hypertension duration (years) | 10 | 0 | 15 |
| CHD (0 = No, 1 = Yes) | 1 | 0 | 1 |
| CAD (0 = No, 1 = Yes) | 0 | 0 | 1 |
| PAD (0 = No, 1 = Yes) | 0 | 0 | 1 |
| Osteoporosis (0 = No, 1 = Yes) | 0 | 0 | 0 |
| Severe osteoarthritis (0 = No, 1 = Yes) | 1 | 0 | 1 |
| Low back pain (0 = No, 1 = Yes) | 1 | 0 | 1 |
| Anxiety/depression (0 = No, 1 = Yes) | 1 | 0 | 1 |
| DPP4 therapy (0 = No, 1 = Yes) | 0 | 0 | 0 |
| SGLT2 therapy (0 = No, 1 = Yes) | 0 | 0 | 0 |
| GLP1r therapy (0 = No, 1 = Yes) | 0 | 0 | 0 |
| Insulin therapy (0 = No, 1 = Yes) | 0 | 0 | 0 |
| NLR | 1.1 | 1.7 | 1.7 |
| Il-17A (pg/mL) | 0.68 | 1.42 | 0.68 |
| Il-37 (pg/mL) | 0.24 | 0.8 | 13.4 |
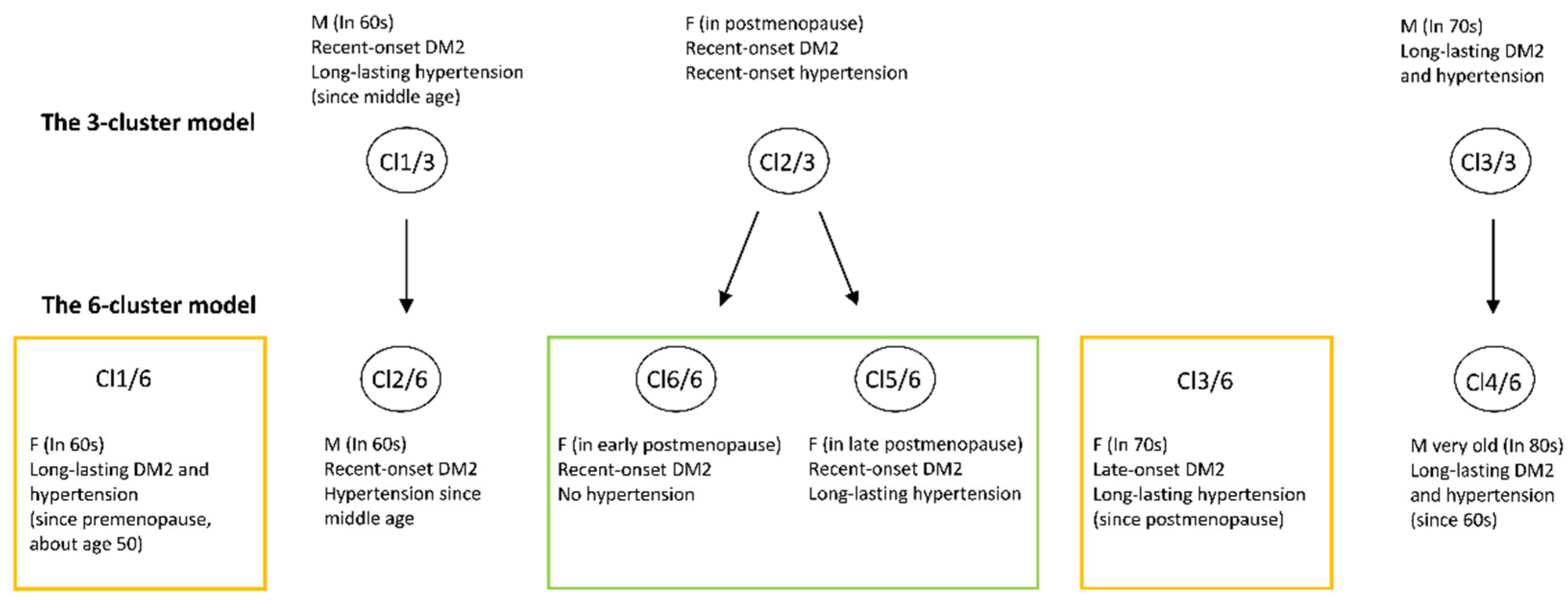
| Variable | Cl 1/6 | Cl 2/6 | Cl 3/6 | Cl 4/6 | Cl 5/6 | Cl 6/6 |
|---|---|---|---|---|---|---|
| Gender (M,F) (0,1) | 0 | 1 | 0 | 1 | 0 | 0 |
| Age (years) | 61 | 64 | 72 | 80 | 67 | 61 |
| Number of comorbidities (<3, ≥3) (0,1) | 1 | 1 | 1 | 1 | 1 | 1 |
| BMI (kg/m2) | 32 | 31.64 | 28.4 | 24.03 | 26 | 26.78 |
| mac (cm) | 32 | 30 | 33 | 27 | 29 | 28 |
| Hb (g/L) | 157 | 142 | 123 | 134 | 129 | 162 |
| eGFR (mL/min/1.73 m 2) | 79 | 87 | 29 | 59 | 59 | 58 |
| eGFR levels (1–4) | 1 | 2 | 3 | 3 | 2 | 1 |
| Triglycerides (mmol/L) | 1.5 | 1.2 | 1.2 | 1.9 | 2.1 | 1 |
| HDL cholesterol (mmol/L) | 1.3 | 1.2 | 1.2 | 1.2 | 1.5 | 1.6 |
| TSH (mU/L) | 2.1 | 2.2 | 2.39 | 3.3 | 1.9 | 2.2 |
| CRP (mg/L) | 1.1 | 0.9 | 0.6 | 0.8 | 1.1 | 1.2 |
| Frailty index (0,1,2) | 0 | 0 | 0 | 2 | 0 | 0 |
| DM2 duration (years) | 10 | 2 | 1 | 12 | 3 | 2 |
| Hypertension (0 = No, 1 = Yes) | 1 | 1 | 1 | 1 | 1 | 0 |
| Hypertension duration (years) | 10 | 7 | 10 | 15 | 15 | 0 |
| CHD (0 = No, 1 = Yes) | 0 | 0 | 1 | 1 | 0 | 0 |
| CAD (0 = No, 1 = Yes) | 0 | 0 | 1 | 1 | 0 | 0 |
| PAD (0 = No, 1 = Yes) | 0 | 0 | 1 | 1 | 0 | 0 |
| Osteoporosis (0 = No, 1 = Yes) | 0 | 0 | 0 | 1 | 0 | 0 |
| Severe osteoarthritis (0 = No, 1 = Yes) | 0 | 1 | 1 | 1 | 0 | 0 |
| Low back pain (0 = No, 1 = Yes) | 1 | 1 | 1 | 1 | 0 | 0 |
| Anxiety/depression (0 = No, 1 = Yes) | 1 | 1 | 1 | 1 | 0 | 0 |
| DPP4 therapy (0 = No, 1 = Yes) | 0 | 0 | 0 | 0 | 0 | 0 |
| SGLT2 therapy (0 = No, 1 = Yes) | 0 | 0 | 0 | 0 | 0 | 0 |
| GLP1r therapy (0 = No, 1 = Yes) | 0 | 0 | 0 | 0 | 0 | 0 |
| Insulin therapy (0 = No, 1 = Yes) | 0 | 0 | 0 | 0 | 0 | 0 |
| NLR | 107 | 1.1 | 1 | 1.6 | 1.3 | 1.1 |
| Il-17A (pg/mL) | 1.53 | 0.68 | 0.01 | 0.68 | 1.47 | 1.42 |
| Il-37 (pg/mL) | 0.24 | 10.2 | 0.22 | 16.4 | 0.8 | 3.4 |
| NLR = −0.2602 × Gender + 0.0156 ∗ BMI + −0.0077 × eGFR in mL/min/1.73m2 + −0.058 × eGFR levels + −0.0813 ∗ Triglycerides + −0.081 × TSH + 0.0182 × CRP + 0.3491 × Severe osteoarthritis + 0.0149 × Il-17A + −0.0007 × Il-37 + 2.2697 |
| Il-17A = 0.0584 × age in years + −1.1963 × eGFR levels + 0.0459x − xHDL + 0.3422 × CRP + 0.0385 × Il-37 + −0.8856 |
| Il-37 = 17.377 × eGFR levels + −0.883 × HDL + −6.4164 × TSH + −4.9867 × CRP + 17.3077 × Il-17A + −15.2037 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosnic, Z.; Yildirim, P.; Babič, F.; Šahinović, I.; Wittlinger, T.; Martinović, I.; Majnaric, L.T. Clustering Inflammatory Markers with Sociodemographic and Clinical Characteristics of Patients with Diabetes Type 2 Can Support Family Physicians’ Clinical Reasoning by Reducing Patients’ Complexity. Healthcare 2021, 9, 1687. https://doi.org/10.3390/healthcare9121687
Bosnic Z, Yildirim P, Babič F, Šahinović I, Wittlinger T, Martinović I, Majnaric LT. Clustering Inflammatory Markers with Sociodemographic and Clinical Characteristics of Patients with Diabetes Type 2 Can Support Family Physicians’ Clinical Reasoning by Reducing Patients’ Complexity. Healthcare. 2021; 9(12):1687. https://doi.org/10.3390/healthcare9121687
Chicago/Turabian StyleBosnic, Zvonimir, Pinar Yildirim, František Babič, Ines Šahinović, Thomas Wittlinger, Ivo Martinović, and Ljiljana Trtica Majnaric. 2021. "Clustering Inflammatory Markers with Sociodemographic and Clinical Characteristics of Patients with Diabetes Type 2 Can Support Family Physicians’ Clinical Reasoning by Reducing Patients’ Complexity" Healthcare 9, no. 12: 1687. https://doi.org/10.3390/healthcare9121687






