Enhanced Brain Responses to Pain-Related Words in Chronic Back Pain Patients and Their Modulation by Current Pain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. Verbal Stimuli
2.3. Experimental Procedure
2.4. Analysis of Behavioral Data
2.5. fMRI-Data Acquisition and Analysis
3. Results
3.1. Questionnaire and Behavioral Data
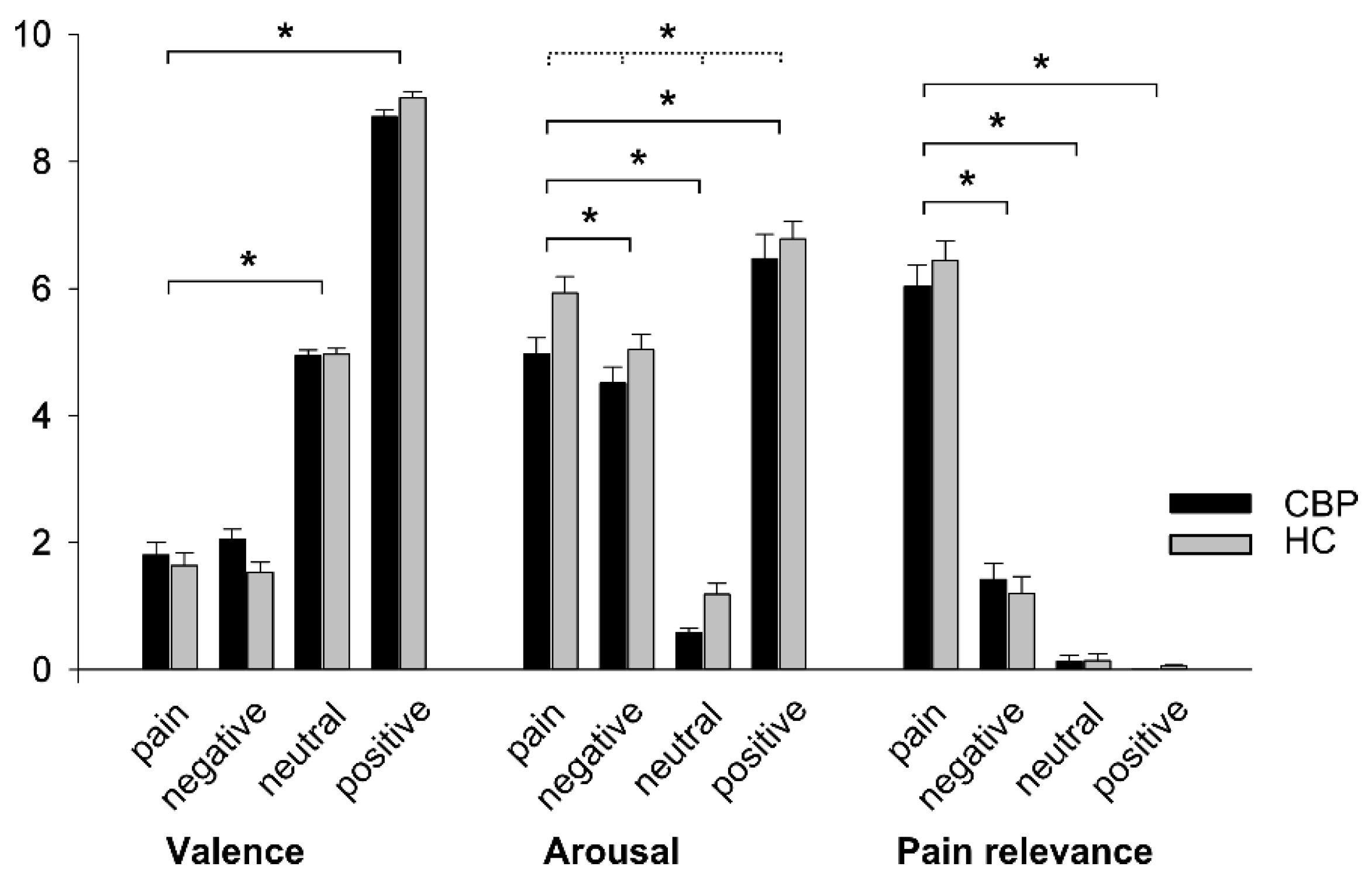
H1: Behavioral Effects of Group and Word Category
3.2. Imaging
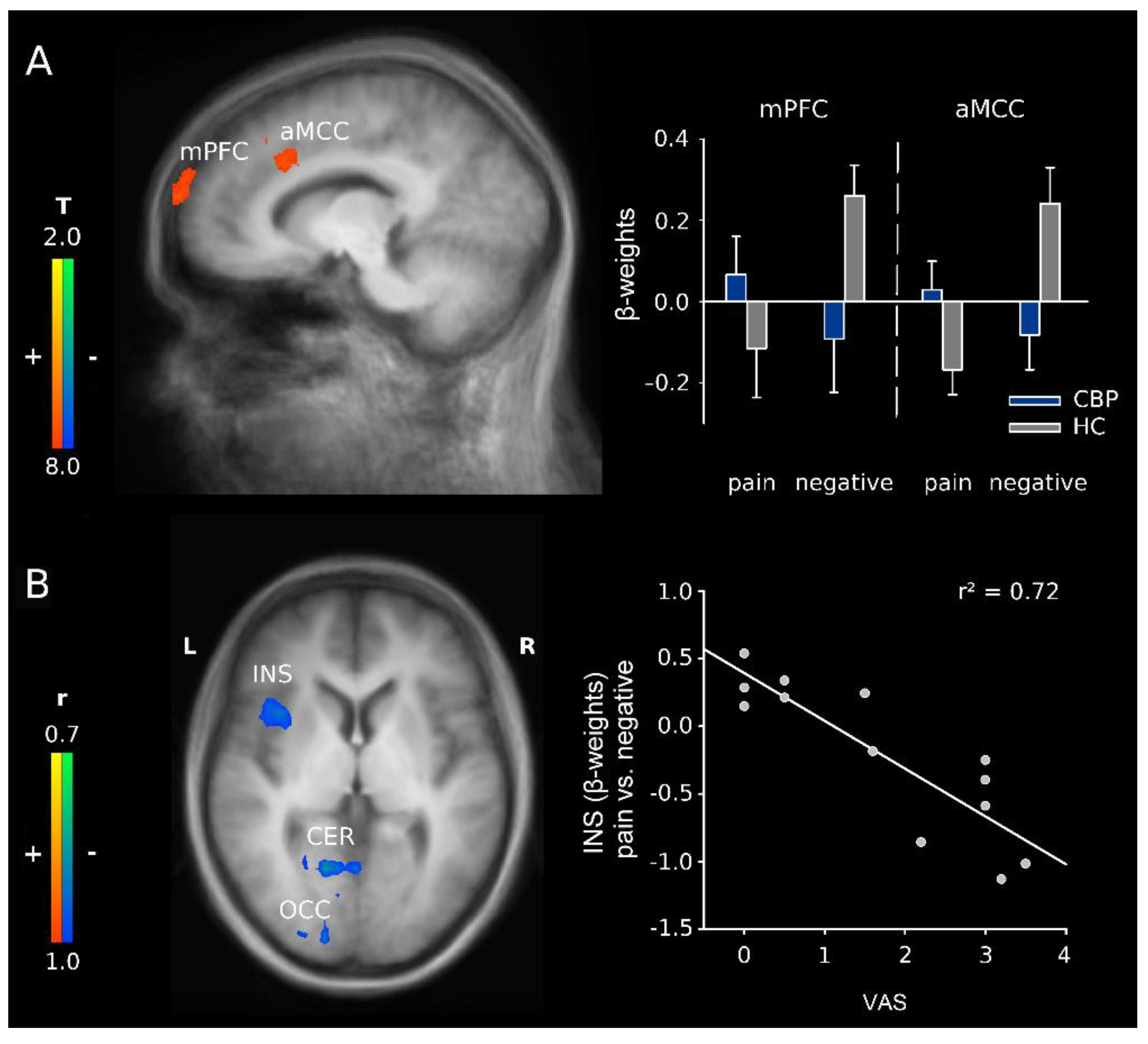
3.2.1. H2: Effects of Group and Word Category
3.2.2. H3: Correlation Analyses of Word Category in CBP Patients
4. Discussion
4.1. H1: Behavioral Effects of Group and Word Category
4.2. H2: Effects of Group and Word Category
4.3. H3: Relationship to Current Pain
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CBP | chronic back pain |
| HC | healthy control |
| fMRI | functional magnetic resonance imaging |
| BOLD | blood oxygen level-dependent |
| DLPFC | dorsolateral prefrontal cortex |
| ERP | event-related potential |
| VAS | visual analogue scale |
| NRS | numerical rating scale |
| mPFC | medial prefrontal cortex |
| INS | insula |
| CER | cerebellum |
| OCC | occipital cortex |
| SMA | supplementary motor area |
| MI | primary motor cortex |
| ACC | anterior cingulate cortex |
| PCC | posterior cingulate cortex |
| MCC | midcingular cortex |
| sACC | subgenual anterior cingulate cortex |
| aMCC | anterior midcingulate cortex |
References
- Kenntner-Mabiala, R.; Weyers, P.; Pauli, P. Independent effects of emotion and attention on sensory and affective pain perception. Cogn. Emot. 2007, 21, 1615–1629. [Google Scholar] [CrossRef]
- Villemure, C.; Slotnick, B.M.; Bushnell, M.C. Effects of odors on pain perception: Deciphering the roles of emotion and attention. Pain 2003, 106, 101–108. [Google Scholar] [CrossRef]
- Seminowicz, D.A.; Davis, K.D. Interactions of pain intensity and cognitive load: The brain stays on task. Cereb. Cortex 2007, 17, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Valet, M.; Sprenger, T.; Boecker, H.; Willoch, F.; Rummeny, E.; Conrad, B.; Erhard, P.; Tolle, T.R. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain—An FMR1 analysis. Pain 2004, 109, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Loggia, M.L.; Mogil, J.S.; Bushnell, M.C. Empathy hurts: Compassion for another increases both sensory and affective components of pain perception. Pain 2008, 136, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Rainville, P.; Bao, Q.V.H.; Chretien, P. Pain-related emotions modulate experimental pain perception and autonomic responses. Pain 2005, 118, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Godinho, F.; Magnin, M.; Frot, M.; Perchet, C.; Garcia-Larrea, L. Emotional modulation of pain: Is it the sensation or what we recall? J. Neurosci. 2006, 26, 11454–11461. [Google Scholar] [CrossRef] [PubMed]
- Decety, J.; Jackson, P.L.; Brunet, E.; Meltzoff, A.N. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia 2006, 44, 752–761. [Google Scholar]
- Kenntner-Mabiala, R.; Pauli, P. Affective modulation of brain potentials to painful and nonpainful stimuli. Psychophysiology 2005, 42, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Singer, T.; Seymour, B.; O’Doherty, J.; Kaube, H.; Dolan, R.J.; Frith, C.D. Empathy for pain involves the affective but not sensory components of pain. Science 2004, 303, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Coghill, R.C.; Koyama, T.; McHaffie, J.G.; Laurienti, P.J. The subjective experience of pain: Where expectations become reality. Proc. Natl. Acad. Sci. USA 2005, 102, 12950–12955. [Google Scholar]
- Wager, T.D.; Rilling, J.K.; Smith, E.E.; Sokolik, A.; Casey, K.L.; Davidson, R.J.; Kosslyn, S.M.; Rose, R.M.; Cohen, J.D. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science 2004, 303, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Miltner, W.H.R.; Braun, C.; Arnold, M.; Witte, H.; Taub, E. Coherence of gamma-band eeg activity as a basis for associative learning. Nature 1999, 397, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Weiss, T.; Miltner, W.H.R.; Dillmann, J. The influence of semantic priming on event-related potentials to painful laser-heat stimuli in migraine patients. Neurosci. Lett. 2003, 340, 135–138. [Google Scholar] [CrossRef]
- Iannetti, G.D.; Hughes, N.P.; Lee, M.C.; Mouraux, A. Determinants of laser-evoked EEG responses: Pain perception or stimulus saliency? J. Neurophysiol. 2008, 100, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Iannetti, G.D.; Mouraux, A. From the neuromatrix to the pain matrix (and back). Exp. Brain Res. 2010, 205, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Legrain, V.; Iannetti, G.D.; Plaghki, L.; Mouraux, A. The pain matrix reloaded: A salience detection system for the body. Prog. Neurobiol. 2011, 93, 111–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apkarian, A.V.; Bushnell, M.C.; Treede, R.D.; Zubieta, J.K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 2005, 9, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R. From the gate to the neuromatrix. Pain 1999, S121–S126. [Google Scholar] [CrossRef]
- Bower, G.H. Mood and memory. Am. Psychol. 1981, 36, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Hebb, D.O. The Organization of Behavior: A Neuropsychological Theory; Wiley: New York, NY, USA, 1949; p. 335. [Google Scholar]
- Dutt-Gupta, J.; Bown, T.; Cyna, A.M. Effect of communication on pain during intravenous cannulation: A randomized controlled trial. Br. J. Anaesth. 2007, 99, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Ott, J.; Aust, S.; Nouri, K.; Promberger, R. An everyday phrase may harm your patients the influence of negative words on pain during venous blood sampling. Clin. J. Pain 2012, 28, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Han, S. Neural substrates underlying evaluation of pain in actions depicted in words. Behav. Brain Res. 2007, 181, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Osaka, N.; Osaka, M.; Morishita, M.; Kondo, H.; Fukuyama, H. A word expressing affective pain activates the anterior cingulate cortex in the human brain: An fMRI study. Behav. Brain Res. 2004, 153, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Apkarian, A.V.; Sosa, Y.; Sonty, S.; Levy, R.M.; Harden, R.N.; Parrish, T.B.; Gitelman, D.R. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J. Neurosci. 2004, 24, 10410–10415. [Google Scholar] [CrossRef] [PubMed]
- Flor, H.; Braun, C.; Elbert, T.; Birbaumer, N. Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci. Lett. 1997, 224, 5–8. [Google Scholar] [CrossRef]
- Baliki, M.N.; Chialvo, D.R.; Geha, P.Y.; Levy, R.M.; Harden, R.N.; Parrish, T.B.; Apkarian, A.V. Chronic pain and the emotional brain: Specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J. Neurosci. 2006, 26, 12165–12173. [Google Scholar] [CrossRef] [PubMed]
- Wand, B.M.; Parkitny, L.; O’Connell, N.E.; Luomajoki, H.; McAuley, J.H.; Thacker, M.; Moseley, G.L. Cortical changes in chronic low back pain: Current state of the art and implications for clinical practice. Man. Ther. 2011, 16, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Siddall, P.J.; Stanwell, P.; Woodhouse, A.; Somorjai, R.L.; Dolenko, B.; Nikulin, A.; Bourne, R.; Himmelreich, U.; Lean, C.; Cousins, M.J.; et al. Magnetic resonance spectroscopy detects biochemical changes in the brain associated with chronic low back pain: A preliminary report. Anesth. Analg. 2006, 102, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Lutzenberger, W.; Flor, H.; Birbaumer, N. Enhanced dimensional complexity of the eeg during memory for personal pain in chronic pain patients. Neurosci. Lett. 1997, 226, 167–170. [Google Scholar] [CrossRef]
- Richter, M.; Eck, J.; Straube, T.; Miltner, W.H.R.; Weiss, T. Do words hurt? Brain activation during the processing of pain-related words. Pain 2010, 148, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Craig, K.D.; Miltner, W.H.R.; Rainville, P. Brain responses to dynamic facial expressions of pain. Pain 2006, 126, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Eck, J.; Richter, M.; Straube, T.; Miltner, W.H.R.; Weiss, T. Affective brain regions are activated during the processing of pain-related words in migraine patients. Pain 2011, 152, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Flor, H.; Knost, B.; Birbaumer, N. Processing of pain- and body-related verbal material in chronic pain patients: Central and peripheral correlates. Pain 1997, 73, 413–421. [Google Scholar] [CrossRef]
- Oldfield, R.C. Assessment and analysis of handedness - edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Nikendei, C.; Dengler, W.; Wiedemann, G.; Pauli, P. Selective processing of pain-related word stimuli in subclinical depression as indicated by event-related brain potentials. Biol. Psychol. 2005, 70, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Hautzinger, M.; Kühner, C.; Keller, F. Bdi-ii Beck-Depressions-Inventar; Harcourt Test Services: Frankfurt, Germany, 2006. [Google Scholar]
- Sullivan, M.J.L.; Bishop, S.R.; Pivik, J. The pain catastrophizing scale: Development and validation. Psychol. Assess. 1995, 7, 524–532. [Google Scholar] [CrossRef]
- Meyer, K.; Sprott, H.; Mannion, A.F. Cross-cultural adaptation, reliability, and validity of the german version of the pain catastrophizing scale. J. Psychosom. Res. 2008, 64, 469–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guy, M.E.; Mccarter, R.E. Scale to measure emotive imagery. Percept. Mot. Skill 1978, 46, 1267–1274. [Google Scholar] [CrossRef]
- Talairach, J.; Tournoux, P. Coplanar Stereotaxic atlas of the Human Brain; Thieme: Stuttgart, Germany, 1988. [Google Scholar]
- Forman, S.D.; Cohen, J.D.; Fitzgerald, M.; Eddy, W.F.; Mintun, M.A.; Noll, D.C. Improved assessment of significant activation in functional magnetic-resonance-imaging (fmri)—Use of a cluster-size threshold. Magn. Reson. Med. 1995, 33, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Goebel, R.; Esposito, F.; Formisano, E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum. Brain Mapp. 2006, 27, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Straube, T.; Schmidt, S.; Weiss, T.; Mentzel, H.J.; Miltner, W.H.R. Sex differences in brain activation to anticipated and experienced pain in the medial prefrontal cortex. Hum. Brain Mapp. 2009, 30, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Bantick, S.J.; Wise, R.G.; Ploghaus, A.; Clare, S.; Smith, S.M.; Tracey, I. Imaging how attention modulates pain in humans using functional MRI. Brain 2002, 125, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Bornhovd, K.; Quante, M.; Glauche, V.; Bromm, B.; Weiller, C.; Buchel, C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: A single-trial fMRI study. Brain 2002, 125, 1326–1336. [Google Scholar] [CrossRef] [PubMed]
- Tolle, T.R.; Kaufmann, T.; Siessmeier, T.; Lautenbacher, S.; Berthele, A.; Munz, F.; Zieglgansberger, W.; Willoch, F.; Schwaiger, M.; Conrad, B.; et al. Region-specific encoding of sensory and affective components of pain in the human brain: A positron emission tomography correlation analysis. Ann. Neurol. 1999, 45, 40–47. [Google Scholar] [CrossRef]
- Euston, D.R.; Gruber, A.J.; McNaughton, B.L. The role of medial prefrontal cortex in memory and decision making. Neuron 2012, 76, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Bingel, U.; Schoell, E.; Buchel, C. Imaging pain modulation in health and disease. Curr. Opin. Neurol. 2007, 20, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, P.; Ingvar, M. Imaging cognitive modulation of pain processing. Pain 2002, 95, 1–5. [Google Scholar] [CrossRef]
- Tracey, I.; Mantyh, P.W. The cerebral signature and its modulation for pain perception. Neuron 2007, 55, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M.; Chen, J.I.; Lautenbacher, S.; Vachon-Presseau, E.; Rainville, P. Cerebral regulation of facial expressions of pain. J. Neurosci. 2011, 31, 8730–8738. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M.; Lautenbacher, S.; LeBlanc, N.; Rainville, P. Are both the sensory and the affective dimensions of pain encoded in the face? Pain 2012, 153, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Pulvermuller, F. Brain embodiment of syntax and grammar: Discrete combinatorial mechanisms spelt out in neuronal circuits. Brain Lang. 2010, 112, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Pulvermuller, F.; Fadiga, L. Active perception: Sensorimotor circuits as a cortical basis for language. Nat. Rev. Neurosci. 2010, 11, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Pulvermuller, F. Brain reflections of words and their meaning. Trends Cogn. Sci. 2001, 5, 517–524. [Google Scholar] [CrossRef]
- Hugues, S.; Garcia, R. Reorganization of learning-associated prefrontal synaptic plasticity between the recall of recent and remote fear extinction memory. Learn. Mem. 2007, 14, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Brighina, F.; De Tommaso, M.; Giglia, F.; Scalia, S.; Cosentino, G.; Puma, A.; Panetta, M.; Giglia, G.; Fierro, B. Modulation of pain perception by transcranial magnetic stimulation of left prefrontal cortex. J. Headache Pain 2011, 12, 185–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenz, J.; Minoshima, S.; Casey, K.L. Keeping pain out of mind: The role of the dorsolateral prefrontal cortex in pain modulation. Brain 2003, 126, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Ung, H.; Brown, J.E.; Johnson, K.A.; Younger, J.; Hush, J.; Mackey, S. Multivariate classification of structural MRI data detects chronic low back pain. Cereb. Cortex 2014, 24, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, M.C.; Ceko, M.; Low, L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013, 14, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Shackman, A.J.; Salomons, T.V.; Slagter, H.A.; Fox, A.S.; Winter, J.J.; Davidson, R.J. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011, 12, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Misra, G.; Coombes, S.A. Neuroimaging evidence of motor control and pain processing in the human midcingulate cortex. Cereb. Cortex 2015, 25, 1906–1919. [Google Scholar] [CrossRef] [PubMed]
- Peyron, R.; Faillenot, I.; Mertens, P.; Laurent, B.; Garcia-Larrea, L. Motor cortex stimulation in neuropathic pain. Correlations between analgesic effect and hemodynamic changes in the brain. A pet study. Neuroimage 2007, 34, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Vogt, B.A. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 2005, 6, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Peyron, R.; Laurent, B.; Garcia-Larrea, L. Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol. Clin. 2000, 30, 263–288. [Google Scholar] [CrossRef]
- Wiech, K.; Lin, C.S.; Brodersen, K.H.; Bingel, U.; Ploner, M.; Tracey, I. Anterior insula integrates information about salience into perceptual decisions about pain. J. Neurosci. 2010, 30, 16324–16331. [Google Scholar] [CrossRef] [PubMed]
- Diers, M.; Koeppe, C.; Diesch, E.; Stolle, A.M.; Holzl, R.; Schiltenwolf, M.; van Ackern, K.; Flor, H. Central processing of acute muscle pain in chronic low back pain patients: An EEG mapping study. J. Clin. Neurophysiol. 2007, 24, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Reinert, A.; Treede, R.; Bromm, B. The pain inhibiting pain effect: An electrophysiological study in humans. Brain Res. 2000, 862, 103–110. [Google Scholar] [CrossRef]

| CBP | HC | ||||
|---|---|---|---|---|---|
| Sex | |||||
| Male/Female | 2/11 | 2/11 | |||
| Age (in years) | 44.31 ± 12.15 | 46.46 ± 10.19 | |||
| Range | 23–56 | 24–58 | |||
| Pain history | |||||
| 6–12 months | N = 2 | N = 0 | |||
| 2–5 years | N = 4 | N = 0 | |||
| >5 years | N = 7 | N = 0 | |||
| Pain intensity | t | df | p | ||
| Mean pain intensity (VAS a recent 4 weeks) | 3.31 ± 1.83 | 0.09 ± 0.30 | 5.72 | 10.53 | <0.001 |
| Strongest pain (VAS recent 4 weeks) | 5.14 ± 1.85 | 0.27 ± 0.90 | 6.27 | 12.76 | <0.001 |
| Current pain (VAS post scanning) | 1.72 ± 1.34 | 0.05 ± 0.15 | 4.15 | 10.25 | 0.002 |
| BDI b score | 7.77 ± 5.13 | 2.62 ± 1.76 | 3.50 | 12.12 | 0.004 |
| Pain Catastrophizing Scale (PCS) | 14.08 ± 6.11 | 11.82 ± 7.04 | 0.61 | 24 | 0.550 |
| Rumination | 5.46 ± 3.46 | 5.09 ± 3.27 | 0.12 | 24 | 0.905 |
| Helplessness | 4.85 ± 3.11 | 4.00 ± 2.61 | 0.49 | 24 | 0.632 |
| Magnification | 3.77 ± 2.32 | 2.73 ± 2.19 | 0.98 | 24 | 0.351 |
| Task difficulty c | 1.38 ± 1.50 | 1.38 ± 1.50 | 0 | 24 | 1 |
| χ2 | |||||
| Correct word categorization d | 15.66 ± 0.79 | 15.45 ± 1.65 | 0.04 | 1 | 0.865 |
| x | y | z | Cluster Size | t-Value | Brain Region | Laterality | Brodmann Area |
|---|---|---|---|---|---|---|---|
| −20 | 13 | 35 | 76 | 4.92 | anterior cingulate cortex/dorsolateral prefrontal cortex | R/L | 32 |
| −10 | −76 | 64 | 54 | 3.97 | medial prefrontal cortex | L | 10 |
| x | y | z | Cluster Size | r-Value | Brain Region | Laterality | Brodmann Area |
|---|---|---|---|---|---|---|---|
| 39 | 13 | 2 | 48 | −0.83 | insula | R | 13/44 |
| 45 | −65 | 21 | 56 | −0.84 | medial temporal cortex | R | 39 |
| −16 | −62 | −11 | 108 | −0.84 | cerebellum | L | |
| 5 | −87 | 18 | 193 | −0.88 | occipital cortex | R | 17/18/23 |
| −43 | −15 | 41 | 40 | −0.90 | precentral cortex (MI) | L | 4 |
| −4 | −77 | 39 | 106 | −0.91 | parietal cortex/occipital cortex | L | 19/7 |
| 14 | −55 | −8 | 211 | −0.96 | cerebellum | R | 19 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritter, A.; Franz, M.; Puta, C.; Dietrich, C.; Miltner, W.H.R.; Weiss, T. Enhanced Brain Responses to Pain-Related Words in Chronic Back Pain Patients and Their Modulation by Current Pain. Healthcare 2016, 4, 54. https://doi.org/10.3390/healthcare4030054
Ritter A, Franz M, Puta C, Dietrich C, Miltner WHR, Weiss T. Enhanced Brain Responses to Pain-Related Words in Chronic Back Pain Patients and Their Modulation by Current Pain. Healthcare. 2016; 4(3):54. https://doi.org/10.3390/healthcare4030054
Chicago/Turabian StyleRitter, Alexander, Marcel Franz, Christian Puta, Caroline Dietrich, Wolfgang H. R. Miltner, and Thomas Weiss. 2016. "Enhanced Brain Responses to Pain-Related Words in Chronic Back Pain Patients and Their Modulation by Current Pain" Healthcare 4, no. 3: 54. https://doi.org/10.3390/healthcare4030054






