Association of Coagulopathy and Inflammatory Biomarkers with Severity in SARS-CoV-2-Infected Individuals of the Al-Qunfudhah Region of Saudi Arabia
Abstract
:1. Introduction
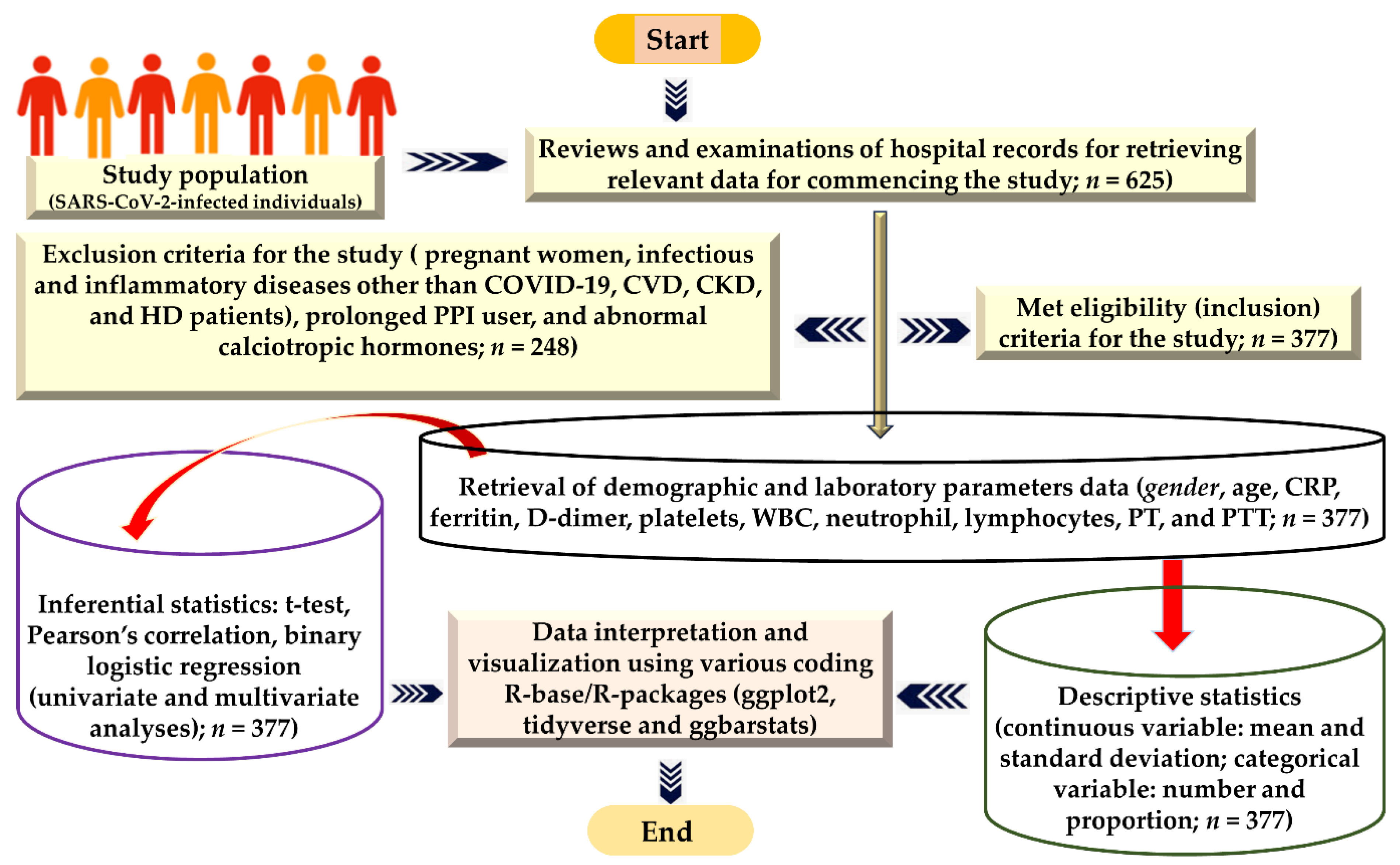
2. Methods
2.1. Design of the Investigation and Target Study Population
2.2. Ethical Approval Statement
2.3. Sample Size Computations
2.4. Eligibility Criteria
2.5. Laboratory Investigation Technique and Primary Data Generation Strategies
2.6. Quality Assurance of the Data
2.7. Analytical Processes for Data Interpretation
3. Results
3.1. Baseline Features of the Research Participants
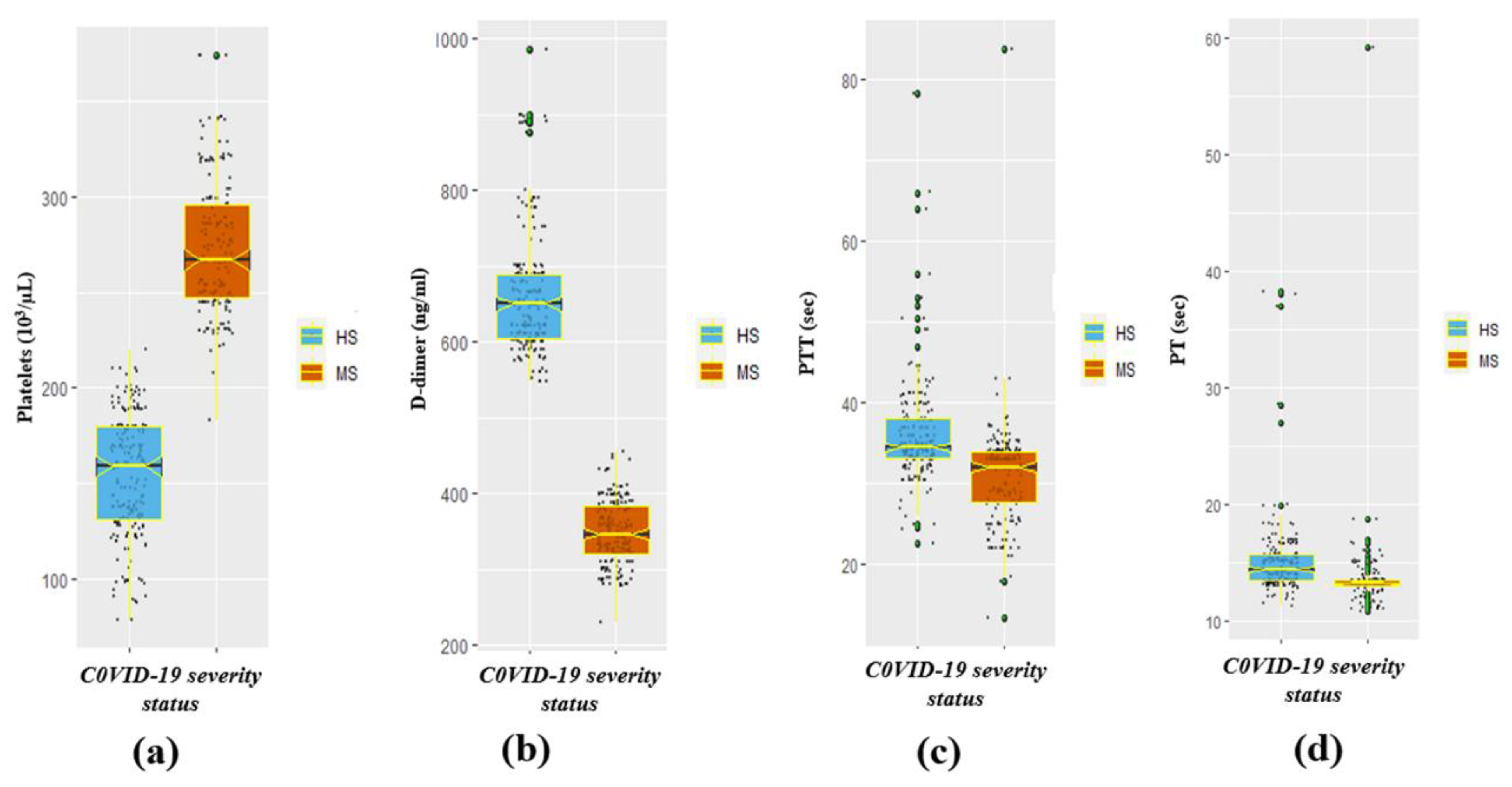
3.2. The Significant Difference in Coagulation-Disorder and Inflammatory Biomarkers by Severity Status
3.3. The Significant Difference in Coagulation-Disorder and Inflammatory Biomarkers by Gender
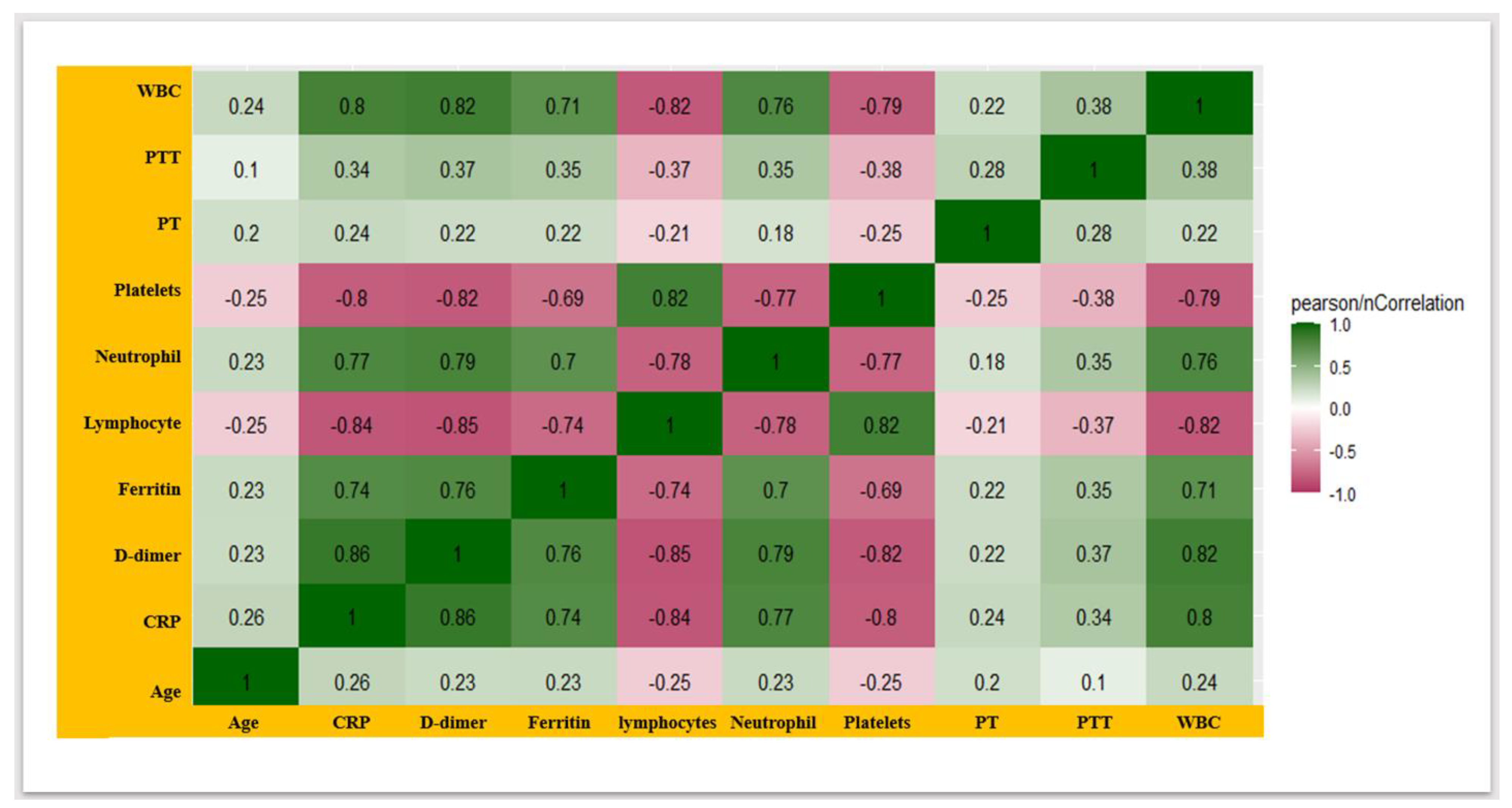
3.4. COVID-19 Severity-Associated Modulation in the Coagulation-Disorder Markers and Inflammatory Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, S.; Hossain, M.J.; Nahar, Z.; Shahriar, M.; Bhuiyan, M.A.; Islam, M.R. Emerging SARS-CoV-2 variants and subvariants: Challenges and opportunities in the context of COVID-19 pandemic. Environ. Health Insights 2022, 16, 11786302221129396. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.; Trent, E.; Goncalves, B.D.S.; Pereira, D.G.; Puri, R.; Frazier, N.A.; Sodhi, K.; Pillai, S.S. Cognitive dysfunction associated with COVID-19: Prognostic role of circulating biomarkers and microRNAs. Front. Aging Neurosci. 2022, 14, 1020092. [Google Scholar] [CrossRef] [PubMed]
- Hulkoti, V.S.; Acharya, S.; Kumar, S.; Talwar, D.; Khanna, S.; Annadatha, A.; Madaan, S.; Verma, V.; Sagar, V. Association of serum ferritin with COVID-19 in a cross-sectional study of 200 intensive care unit patients in a rural hospital: Is ferritin the forgotten biomarker of mortality in severe COVID-19? J. Fam. Med. Prim. Care 2022, 11, 2045. [Google Scholar]
- Üstündağ, H.; Mertoğlu, C.; Huyut, M.T. Oxyhemoglobin Dissociation Curve in COVID-19 Patients. Meandros Med. Dent. J. 2023, 24, 58. [Google Scholar] [CrossRef]
- Dai, W.; Rao, R.; Sher, A.; Tania, N.; Musante, C.J.; Allen, R. A prototype QSP model of the immune response to SARS-CoV-2 for community development. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.F.; Ho, Y.-C. SARS-CoV-2: A storm is raging. J. Clin. Investig. 2020, 130, 2202–2205. [Google Scholar] [CrossRef] [PubMed]
- Lillicrap, D. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J. Thromb. Haemost. 2020, 18, 786–787. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, K.; Kaur, H.; Sarma, P.; Bhattacharyya, A.; Sharma, D.J.; Prajapat, M.; Pathak, M.; Kothari, A.; Kumar, S.; Rana, S. Serum ferritin as a predictive biomarker in COVID-19. A systematic review, meta-analysis and meta-regression analysis. J. Crit. Care 2022, 67, 172–181. [Google Scholar] [CrossRef]
- Lindsley, A.W.; Schwartz, J.T.; Rothenberg, M.E. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J. Allergy Clin. Immunol. 2020, 146, 1–7. [Google Scholar] [CrossRef]
- Hu, R.; Han, C.; Pei, S.; Yin, M.; Chen, X. Procalcitonin levels in COVID-19 patients. Int. J. Antimicrob. Agents 2020, 56, 106051. [Google Scholar] [CrossRef]
- Lin, Z.; Long, F.; Yang, Y.; Chen, X.; Xu, L.; Yang, M. Serum ferritin as an independent risk factor for severity in COVID-19 patients. J. Infect. 2020, 81, 647–679. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Fu, Z.; Luo, M.; Zhang, Z.; Zhang, K.; He, Y.; Wan, D.; Zhang, L.; Wang, J. Potential factors for prediction of disease severity of COVID-19 patients. MedRxiv 2020, 2020-03. [Google Scholar] [CrossRef]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef]
- Motoc, N.S.; Man, M.A.; Urda, A.E.C.; Ruta, V.M.; Todea, D.A.; Pop, C.M. Neutrophil-to-Lymphocyte Ratio and Platelets-to-Lymphocytes Ratio in severe COPD exacerbation: The importance of obstructive sleep apnea. Eur. Respir. J. 2019, 54, PA2582. [Google Scholar] [CrossRef]
- Xie, G.; Ding, F.; Han, L.; Yin, D.; Lu, H.; Zhang, M. The role of peripheral blood eosinophil counts in COVID-19 patients. Allergy 2021, 76, 471–482. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; Yuan, S.; Kok, K.-H.; To, K.K.-W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.-Y.; Poon, R.W.-S. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef]
- Ponti, G.; Maccaferri, M.; Ruini, C.; Tomasi, A.; Ozben, T. Biomarkers associated with COVID-19 disease progression. Crit. Rev. Clin. Lab. Sci. 2020, 57, 389–399. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Karp Leaf, R.S.; Dzik, W.H.; Carlson, J.C.; Fogerty, A.E.; Waheed, A.; Goodarzi, K.; Bendapudi, P.K.; Bornikova, L.; Gupta, S. COVID-19 and coagulation: Bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood J. Am. Soc. Hematol. 2020, 136, 489–500. [Google Scholar] [CrossRef]
- Chiappetta, S.; Sharma, A.M.; Bottino, V.; Stier, C. COVID-19 and the role of chronic inflammation in patients with obesity. Int. J. Obes. 2020, 44, 1790–1792. [Google Scholar] [CrossRef]
- Bisoendial, R.J.; Kastelein, J.J.; Levels, J.H.; Zwaginga, J.J.; van den Bogaard, B.; Reitsma, P.H.; Meijers, J.C.; Hartman, D.; Levi, M.; Stroes, E.S. Activation of inflammation and coagulation after infusion of C-reactive protein in humans. Circ. Res. 2005, 96, 714–716. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, W.; Yan, X.; Guo, T.; Wang, B.; Xia, H.; Ye, L.; Xiong, J.; Jiang, Z.; Liu, Y. Prognostic value of C-reactive protein in patients with coronavirus 2019. Clin. Infect. Dis. 2020, 71, 2174–2179. [Google Scholar] [CrossRef]
- Alamdari, N.M.; Afaghi, S.; Rahimi, F.S.; Tarki, F.E.; Tavana, S.; Zali, A.; Fathi, M.; Besharat, S.; Bagheri, L.; Pourmotahari, F. Mortality risk factors among hospitalized COVID-19 patients in a major referral center in Iran. Tohoku J. Exp. Med. 2020, 252, 73–84. [Google Scholar] [CrossRef]
- Kurian, S.J.; Mathews, S.P.; Paul, A.; Viswam, S.K.; Nagri, S.K.; Miraj, S.S.; Karanth, S. Association of serum ferritin with severity and clinical outcome in COVID-19 patients: An observational study in a tertiary healthcare facility. Clin. Epidemiol. Glob. Health 2023, 21, 101295. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Carcillo, J.A.; Sward, K.; Halstead, E.S.; Telford, R.; Jimenez-Bacardi, A.; Shakoory, B.; Simon, D.; Hall, M.; Eunice Kennedy Shriver National Institute of Child Health. A systemic inflammation mortality risk assessment contingency table for severe sepsis. Pediatr. Crit. Care Med. 2017, 18, 143–150. [Google Scholar] [CrossRef]
- Fu, S.; Fu, X.-Y.; Song, Y.; Li, M.; Pan, P.-H.; Tang, T.; Zhang, C.-H.; Jiang, T.-J.; Tan, D.-M.; Fan, X.-G. Virologic and clinical characteristics for prognosis of severe COVID-19: A retrospective observational study in Wuhan, China. MedRxiv 2020. [Google Scholar]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Edeas, M.; Saleh, J.; Peyssonnaux, C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? Int. J. Infect. Dis. 2020, 97, 303–305. [Google Scholar] [CrossRef]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.D.; Ageno, W.; Madjid, M.; Guo, Y. COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Zhang, W.; Sang, L.; Shi, J.; Zhong, M.; Jiang, L.; Song, B.; Kang, L.; Zhang, Y.; Zhang, D.; Yu, Y. Association of D-dimer elevation with inflammation and organ dysfunction in ICU patients with COVID-19 in Wuhan, China: A retrospective observational study. Aging 2021, 13, 4794. [Google Scholar] [CrossRef]
- Sukrisman, L.; Sinto, R. Coagulation profile and correlation between D-dimer, inflammatory markers, and COVID-19 severity in an Indonesian national referral hospital. J. Int. Med. Res. 2021, 49, 03000605211059939. [Google Scholar] [CrossRef]
- Samadizadeh, S.; Masoudi, M.; Rastegar, M.; Salimi, V.; Shahbaz, M.B.; Tahamtan, A. COVID-19: Why does disease severity vary among individuals? Respir. Med. 2021, 180, 106356. [Google Scholar] [CrossRef]
- Kumar, A.; Prasoon, P.; Kumari, C.; Pareek, V.; Faiq, M.A.; Narayan, R.K.; Kulandhasamy, M.; Kant, K. SARS-CoV-2-specific virulence factors in COVID-19. J. Med. Virol. 2021, 93, 1343–1350. [Google Scholar] [CrossRef]
- Yamane, T. Statistics an Industry Analysis, 2nd ed.; Harper & Row: New York, NY, USA, 1967. [Google Scholar]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Sugiyama, M. Tools and factors predictive of the severity of COVID-19. Glob. Health Med. 2023, 5, 78–84. [Google Scholar] [CrossRef]
- Alroomi, M.; Rajan, R.; Omar, A.A.; Alsaber, A.; Pan, J.; Fatemi, M.; Zhanna, K.D.; Aboelhassan, W.; Almutairi, F.; Alotaibi, N.; et al. Ferritin level: A predictor of severity and mortality in hospitalized COVID-19 patients. Immun. Inflamm. Dis. 2021, 9, 1648–1655. [Google Scholar] [CrossRef]
- Varikasuvu, S.R.; Varshney, S.; Dutt, N.; Munikumar, M.; Asfahan, S.; Kulkarni, P.P.; Gupta, P. D-dimer, disease severity, and deaths (3D-study) in patients with COVID-19: A systematic review and meta-analysis of 100 studies. Sci. Rep. 2021, 11, 21888. [Google Scholar] [CrossRef]
- Liao, D.; Zhou, F.; Luo, L.; Xu, M.; Wang, H.; Xia, J.; Gao, Y.; Cai, L.; Wang, Z.; Yin, P.; et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: A retrospective cohort study. Lancet Haematol. 2020, 7, e671–e678. [Google Scholar] [CrossRef]
- Chi, L.; Wang, S.; Wang, X.; Yang, C.; Luo, J. Predictive value of C-reactive protein for disease severity and survival in COVID-19 patients: A systematic review and meta-analysis. Clin. Exp. Med. 2023, 23, 2001–2008. [Google Scholar] [CrossRef]
- Huyut, M.T.; Üstündağ, H. Prediction of diagnosis and prognosis of COVID-19 disease by blood gas parameters using decision trees machine learning model: A retrospective observational study. Med. Gas Res. 2022, 12, 60–66. [Google Scholar] [CrossRef]
- Huyut, M.T.; Velichko, A. Diagnosis and Prognosis of COVID-19 Disease Using Routine Blood Values and LogNNet Neural Network. Sensors 2022, 22, 4820. [Google Scholar] [CrossRef]
- Huyut, M.T. Automatic Detection of Severely and Mildly Infected COVID-19 Patients with Supervised Machine Learning Models. Ing. Rech. Biomed. 2023, 44, 100725. [Google Scholar] [CrossRef]
- Che, K.; Zeng, Z.; Hong, C.; Peng, D.; Liu, A.; He, Y. Association between serum C-reactive protein (CRP) and Omicron variant COVID-19 pneumonia in cancer patients: A multicenter cross-sectional study at the end of 2022 in China. Medicine 2024, 103, e36965. [Google Scholar] [CrossRef]
- Villoteau, A.; Asfar, M.; Otekpo, M.; Loison, J.; Gautier, J.; Annweiler, C.; GERIA-COVID Study Group. Elevated C-reactive protein in early COVID-19 predicts worse survival among hospitalized geriatric patients. PLoS ONE 2021, 16, e0256931. [Google Scholar] [CrossRef]
- Li, F.; He, M.; Zhou, M.; Lai, Y.; Zhu, Y.; Liu, Z.; Wang, Y.; Wang, Y. Association of C-reactive protein with mortality in COVID-19 patients: A secondary analysis of a cohort study. Sci. Rep. 2023, 13, 20361. [Google Scholar] [CrossRef]
- Saputro, T.A.; Purwaningsih, N.V.; Ainutajriani, A.; Watoyani, T. Correlation between Corona Viruses Disease (COVID-19) and C-Reactive Protein (CRP) in Patients at Haji Hospital Surabaya. Medicra 2022, 5, 11–16. [Google Scholar] [CrossRef]
- Ali, N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J. Med. Virol. 2020, 92, 2409–2411. [Google Scholar] [CrossRef]
- Mertoglu, C.; Huyut, M.T.; Olmez, H.; Tosun, M.; Kantarci, M.; Coban, T.A. COVID-19 is more dangerous for older people and its severity is increasing: A case-control study. Med. Gas Res. 2022, 12, 51–54. [Google Scholar] [CrossRef]
- Tahery, N.; Khodadost, M.; Jahani Sherafat, S.; Rezaei Tavirani, M.; Ahmadi, N.; Montazer, F.; Rezaei Tavirani, M.; Naderi, N. C-reactive protein as a possible marker for severity and mortality of COVID-19 infection. Gastroenterol. Hepatol. Bed Bench 2021, 14 (Suppl. S1), S118–S122. [Google Scholar]
- Tahir Huyut, M.; Huyut, Z.; İlkbahar, F.; Mertoğlu, C. What is the impact and efficacy of routine immunological, biochemical, and hematological biomarkers as predictors of COVID-19 mortality? Int. Immunopharmacol. 2022, 105, 108542. [Google Scholar] [CrossRef]
- Huyut, M.T.; Huyut, Z. Forecasting of Oxidant/Antioxidant levels of COVID-19 patients by using Expert models with biomarkers used in the Diagnosis/Prognosis of COVID-19. Int. Immunopharmacol. 2021, 100, 108127. [Google Scholar] [CrossRef]
- Huyut, M.T.; Velichko, A.; Belyaev, M. Detection of Risk Predictors of COVID-19 Mortality with Classifier Machine Learning Models Operated with Routine Laboratory Biomarkers. Appl. Sci. 2022, 12, 12180. [Google Scholar] [CrossRef]
- Velichko, A.; Huyut, M.T.; Belyaev, M.; Izotov, Y.; Korzun, D. Machine Learning Sensors for Diagnosis of COVID-19 Disease Using Routine Blood Values for Internet of Things Application. Sensors 2022, 22, 7886. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; Zhang, Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef]
- Huyut, M.T.; İlkbahar, F. The effectiveness of blood routine parameters and some biomarkers as a potential diagnostic tool in the diagnosis and prognosis of COVID-19 disease. Int. Immunopharmacol. 2021, 98, 107838. [Google Scholar] [CrossRef]
- Vargas-Vargas, M.; Cortés-Rojo, C. Ferritin levels and COVID-19. Rev. Panam. Salud Pública 2020, 44, e72. [Google Scholar] [CrossRef]
- Fazal, M. C-reactive protein a promising biomarker of COVID-19 severity. Korean J. Clin. Lab. Sci. 2021, 53, 201–207. [Google Scholar] [CrossRef]
- Ishikura, T.; Nakano, T.; Kitano, T.; Tokuda, T.; Sumi-Akamaru, H.; Naka, T. Serum ferritin level during hospitalization is associated with Brain Fog after COVID-19. Sci. Rep. 2023, 13, 13095. [Google Scholar] [CrossRef]
- Cheng, L.; Li, H.; Li, L.; Liu, C.; Yan, S.; Chen, H.; Li, Y. Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Clin. Lab. Anal. 2020, 34, e23618. [Google Scholar] [CrossRef] [PubMed]
- Nemec, H.M.; Ferenczy, A.; Christie, B.D., 3rd; Ashley, D.W.; Montgomery, A. Correlation of D-dimer and Outcomes in COVID-19 Patients. Am. Surg. 2022, 88, 2115–2118. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Li, X.; Chen, J.; Ouyang, M.; Zhang, H.; Zhao, X.; Tang, L.; Luo, Q.; Xu, M.; Yang, L. Evaluation of variation in D-dimer levels among COVID-19 and bacterial pneumonia: A retrospective analysis. J. Thromb. Thrombolysis 2020, 50, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, K.; Wei, H.; Chen, W.; Wang, W.; Jia, L.; Liu, Q.; Zhang, J.; Shan, T.; Peng, Z. Dynamic relationship between D-dimer and COVID-19 severity. Br. J. Haematol. 2020, 190, e24. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, A.; Prosch, H.; Zehetmayer, S.; Gysan, M.R.; Bernitzky, D.; Vonbank, K.; Idzko, M.; Gompelmann, D. Impact of persistent D-dimer elevation following recovery from COVID-19. PLoS ONE 2021, 16, e0258351. [Google Scholar] [CrossRef]
- Yu, C.; Lei, Q.; Li, W.; Wang, X.; Li, W.; Liu, W. Epidemiological and clinical characteristics of 1663 hospitalized patients infected with COVID-19 in Wuhan, China: A single-center experience. J. Infect. Public Health 2020, 13, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Harmouch, F.; Shah, K.; Hippen, J.T.; Kumar, A.; Goel, H. Is it all in the heart? Myocardial injury as major predictor of mortality among hospitalized COVID-19 patients. J. Med. Virol. 2021, 93, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, O.; Andres, M.; Leon-Ramirez, J.M.; Sánchez-Payá, J.; Rodríguez, J.C.; Sánchez, R.; García-Sevila, R.; Boix, V.; Gil, J.; Merino, E. Experience with tocilizumab in severe COVID-19 pneumonia after 80 days of follow-up: A retrospective cohort study. J. Autoimmun. 2020, 114, 102523. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, X.; Jiao, H.; Liu, X. Coagulopathy in patients with COVID-19: A systematic review and meta-analysis. Aging 2020, 12, 24535–24551. [Google Scholar] [CrossRef]
- Mertoglu, C.; Huyut, M.T.; Arslan, Y.; Ceylan, Y.; Coban, T.A. How do routine laboratory tests change in coronavirus disease 2019? Scand. J. Clin. Lab. Investig. 2021, 81, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Huyut, M.T.; Huyut, Z. Effect of ferritin, INR, and D-dimer immunological parameters levels as predictors of COVID-19 mortality: A strong prediction with the decision trees. Heliyon 2023, 9, e14015. [Google Scholar] [CrossRef] [PubMed]

| Overall | Males | Females | ||||
|---|---|---|---|---|---|---|
| Characteristic | Mean ± SD N = 377 | Median (IQR) N = 377 | Mean ± SD N = 213 | Median (IQR) N = 213 | Mean ± SD N = 164 | Median (IQR) N = 164 |
| CRP (mg/L) | 63.9 ± 44.0 | 65.0 (22.0, 113.0) | 74.0 ± 43.1 | 84.0 (25.0, 115.0) | 50.8 ± 41.8 | 26.5 (17.4, 88.0) |
| D-dimer (ng/mL) | 512.8 ± 169.4 | 576.0 (350.0, 650.0) | 549.0 ± 168.5 | 601.0 (380.0, 656.0) | 465.8 ± 159.1 | 388.0 (333.8, 644.3) |
| WBC (103/µL) | 12.3 ± 4.2 | 12.0 (8.4, 16.1) | 13.2 ± 4.1 | 14.0 (8.8, 16.6) | 11.2 ± 4.1 | 8.9 (8.1, 15.3) |
| Neutrophil (%) | 78.7 ± 10.8 | 78.0 (69.0, 89.0) | 80.9 ± 10.5 | 83.0 (69.0, 89.0) | 75.9 ± 10.5 | 71.0 (68.0, 86.0) |
| Lymphocyte (%) | 16.6 ± 9.1 | 11.0 (8.5, 23.8) | 14.6 ± 8.7 | 9.5 (8.3, 22.0) | 19.1 ± 9.1 | 21.0 (8.7, 24.8) |
| Platelets (103/µL) | 211.1 ± 68.7 | 199.0 (152.0, 266.0) | 197.9 ± 61.0 | 181.0 (150.0, 250.0) | 228.2 ± 74.3 | 245.5 (161.0, 286.0) |
| Ferritin (µg/L) | 741.7 ± 174.7 | 689.0 (594.0, 890.0) | 778.8 ± 181.6 | 790.0 (634.0, 897.0) | 693.5 ± 152.8 | 654.0 (576.8, 798.3) |
| Age (in years) | 46.2 ± 23.4 | 46.0 (25.0, 66.0) | 43.7 ± 22.8 | 44.0 (23.0, 62.0) | 49.4 ± 23.9 | 50.0 (28.0, 67.0) |
| PT (sec) | 14.4 ± 3.9 | 13.4 (13.0, 15.1) | 15.0 ± 4.9 | 13.9 (13.1, 15.2) | 13.7 ± 1.6 | 13.3 (13.0, 14.1) |
| PTT (sec) | 33.8 ± 7.0 | 33.7 (31.0, 36.0) | 34.8 ± 7.1 | 33.5 (31.8, 37.0) | 32.5 ± 6.5 | 34.0 (29.0, 35.1) |
| By Gender | By Severity Status | |||||
|---|---|---|---|---|---|---|
| Laboratory Profile | Female N = 164 1 | Male N = 213 1 | p-Value 2 | HS N = 198 1 | MS N = 179 1 | p-Value 2 |
| CRP (mg/L) | 50.8 ± 41.8 | 74.0 ± 43.1 | <0.001 | 102.4 ± 22.9 | 21.3 ± 6.9 | <0.001 |
| D-dimer (ng/mL) | 465.8 ± 159.1 | 549.0 ± 168.5 | <0.001 | 661.1 ± 80.6 | 348.7 ± 42.9 | <0.001 |
| WBC (103/µL) | 11.2 ± 4.1 | 13.2 ± 4.1 | <0.001 | 15.9 ± 2.2 | 8.4 ± 1.5 | <0.001 |
| Neutrophil (%) | 75.9 ± 10.5 | 80.9 ± 10.5 | <0.001 | 87.4 ± 7.2 | 69.1 ± 3.6 | <0.001 |
| Lymphocyte (%) | 19.1 ± 9.1 | 14.6 ± 8.7 | <0.001 | 8.6 ± 1.5 | 25.4 ± 4.8 | <0.001 |
| Platelets (103/µL) | 228.2 ± 74.3 | 197.9 ± 61.0 | <0.001 | 154.0 ± 31.8 | 274.2 ± 34.9 | <0.001 |
| Ferritin (µg/L) | 693.5 ± 152.8 | 778.8 ± 181.6 | <0.001 | 875.8 ± 126.8 | 593.4 ± 67.3 | <0.001 |
| Age (in years) | 49.4 ± 23.9 | 43.7 ± 22.8 | 0.021 | 51.5 ± 24.5 | 40.3 ± 20.7 | >0.05 |
| PT (sec) | 13.7 ± 1.6 | 15.0 ± 4.9 | <0.001 | 15.3 ± 3.9 | 13.5 ± 3.6 | <0.001 |
| PTT (sec) | 32.5 ± 6.5 | 34.8 ± 7.1 | 0.001 | 36.4 ± 6.5 | 31.0 ± 6.3 | <0.001 |
| Univariate Binary Logistic Regression | Multivariate Binary Logistic Regression | |||||
|---|---|---|---|---|---|---|
| Characteristic | COR 2 | 95% CI 1 | p-Value | AOR 1 | 95% CI 1 | p-Value |
| CRP (mg/L) | 1.295 | 1.14, 1.5 | <0.001 | 1.308 | 1.137, 1.50 | <0.001 |
| D-dimer(ng/mL) | 1.40 | 1.26, 1.59 | <0.05 | 1.3 | 1.24, 1.49 | <0.05 |
| WBC (103/µL) | 4.745 | 3.1, 7.2 | <0.001 | 4.612 | 2.98, 7.13 | <0.001 |
| Neutrophil (%) | 1.55 | 1.41, 1.69 | <0.001 | 1.54 | 1.39, 1.68 | <0.001 |
| Lymphocyte (%) | =0.1 | 0.014, 1.49 | <0.001 | 0.153 | 0.015, 1.55 | =0.112 |
| Platelets (103/µL) | 0.823 | 0.76, 0.89 | <0.001 | 0.813 | 0.734, 0.899 | <0.001 |
| Ferritin (µg/L) | 1.05 | 1.032, 1.068 | <0.001 | 1.048 | 1.03, 1.066 | <0.001 |
| PT (sec) | 1.492 | 1.284, 1.73 | <0.001 | 1.347 | 1.15, 1.57 | <0.001 |
| PTT (sec) | 1.23 | 1.16, 1.3 | <0.001 | 1.234 | 1.16, 1.314 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izhari, M.A.; Hadadi, M.A.A.; Alharbi, R.A.; Gosady, A.R.A.; Sindi, A.A.A.; Dardari, D.M.M.; Alotaibi, F.E.; Klufah, F.; Albanghali, M.A.; Alharbi, T.H. Association of Coagulopathy and Inflammatory Biomarkers with Severity in SARS-CoV-2-Infected Individuals of the Al-Qunfudhah Region of Saudi Arabia. Healthcare 2024, 12, 729. https://doi.org/10.3390/healthcare12070729
Izhari MA, Hadadi MAA, Alharbi RA, Gosady ARA, Sindi AAA, Dardari DMM, Alotaibi FE, Klufah F, Albanghali MA, Alharbi TH. Association of Coagulopathy and Inflammatory Biomarkers with Severity in SARS-CoV-2-Infected Individuals of the Al-Qunfudhah Region of Saudi Arabia. Healthcare. 2024; 12(7):729. https://doi.org/10.3390/healthcare12070729
Chicago/Turabian StyleIzhari, Mohammad Asrar, Mansoor A. A. Hadadi, Raed A. Alharbi, Ahmed R. A. Gosady, Abdulmajeed Abdulghani A. Sindi, Daifallah M. M. Dardari, Foton E. Alotaibi, Faisal Klufah, Mohammad A Albanghali, and Tahani H Alharbi. 2024. "Association of Coagulopathy and Inflammatory Biomarkers with Severity in SARS-CoV-2-Infected Individuals of the Al-Qunfudhah Region of Saudi Arabia" Healthcare 12, no. 7: 729. https://doi.org/10.3390/healthcare12070729







