The Significance of Coronary Artery Calcification for Percutaneous Coronary Interventions
Abstract
1. Introduction
2. Diagnosis
2.1. Computed Tomography (CT)
2.2. Coronary Angiography
2.3. Intravascular Imaging: IVUS (Intravascular Ultrasound) and OCT (Optical Coherence Tomography)
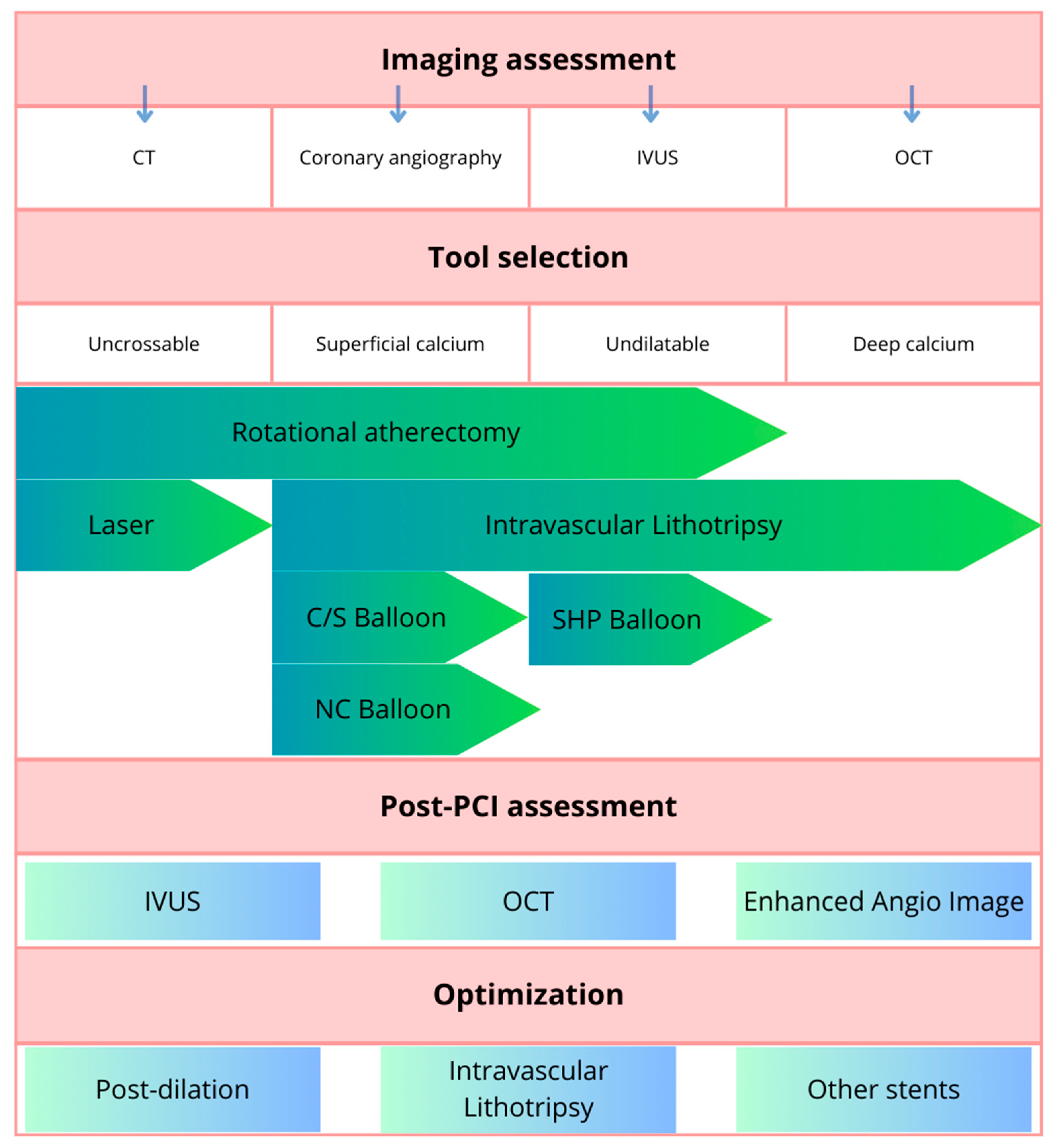
3. Plaque Modification Techniques in Calcified Lesions
- Cutting balloons and scoring balloons. A cutting balloon is a non-compliant balloon (not expandable over their nominal diameter with overinflation) with microblades (three or four) placed on its surface longitudinally, cutting the plaque [36]. Scoring balloons can be either semi-compliant or non-compliant depending on the manufacturer and model and have scoring elements on its surface (helical elements, cutting the plaque with minimal risk of balloon displacement). The presence of these elements enables effective dilation at lower inflation pressures, which decrease the risk of dissection.
- High- and super-high-pressure balloons (HPB and SHP). The expansion profile of high-pressure balloons compared to semi-compliant balloons is more uniform and limited which prevents the dogboning effect (a situation in which there is both under- and overexpansion of the balloon which can lead to vessel dissection or perforation) [21]. A super-high-pressure balloon (OPN, SIS Medical AG) has a twin-layer construction that enables inflations up to 35 atm (in selected cases even up to 40–50 atm) with a low risk of balloon rupture [37]. The limitation of these balloons is their stiffness that can make advancing them across the lesion difficult.
- Intravascular lithotripsy (IVL). The Shockwave Medical coronary IVL system is a fluid-filled balloon angioplasty catheter with two lithotripsy emitters incorporated into the shaft of the 12 mm long balloon (which is 0.014″ guidewire compatible). The balloon is delivered across the lesion and then expanded to 4 atm to enable the energy transfer. The fluid inside the balloon is vaporized using an electrical discharge from the emitters, which as a result creates a rapidly expanding and collapsing bubble that generates sonic pressure waves. The shockwaves pass through the balloon and into the calcified plaque, causing it to crack or fracture. The shockwaves from the lithotripsy emitters disrupt the structure of the calcified plaque without causing significant trauma to the surrounding healthy tissue. The goal is to modify the plaque, making the stent implantation easier. The system comes in 2.5, 3.0, 3.5, and 4.0 mm balloon diameters and should be sized 1:1 to the reference vessel diameter. When the balloon is positioned and expanded to 4 atmospheres, the cycle of 10 IVL pulses is delivered (which is followed by optional brief inflation to 6 atm). Up to 80 pulses per balloon or 120 with the latest generation Shockwave C2+ system can be delivered, with deflation between cycles to allow distal perfusion. The potential drawback of this technique is the bulkiness of the balloon which may hinder the delivery across the lesion. The Disrupt CAD studies demonstrated high procedural success and low 30 day major adverse cardiac event rate [38].
- Atherectomy techniques: rotational atherectomy (RA, rotablation) and orbital atherectomy (OA). The principle of these techniques is to ablate the calcific plaque while also creating plaque fractures and fissures.Rotational atherectomy was first described in 1987. It employs an olive-shaped burr with diamond chips embedded into it. The rotational atherectomy system consists of a console (which regulates the flow of air to the advancer, controlling burr rotation speed and also displays procedural parameters such as burr speed, duration of atherectomy, decelerations which are sudden drops in rotational speed), an advancer (which is used to control movement of the burr) and the burr itself which is introduced to the coronary vessel over a dedicated guidewire. The burr rotates at high speeds (140,000 to 180,000 rpm) and ablates the calcified tissue in a mechanism known as differential cutting (which preferentially ablates the inelastic tissue without damaging the vessel). The operator should use a pecking motion of the burr, which is a quick back and forth movement of the burr to the lesion and back. Decelerations in burr rotational speed should be avoided in order to prevent the burr stall which is serious complication (a situation in which the burr is stuck in the lesion and can no longer be moved or rotated). The particles of debris are <5 μm in diameter and can pass to the systemic circulation without causing distal embolization, however, some considerations are important to prevent the no-flow or slow-flow phenomenon (short burr runs, pauses between runs, appropriate rotational speed, avoidance of decelerations, appropriate pharmacotherapy, i.e., verapamil, nitrates, proper periprocedural anticoagulation). The burrs come in different sizes (from 1.25 mm up to 2.5 mm) to make it suitable for different vessel diameters (burr size should be <0.7 of a reference vessel diameter). PREPARE-CALC (patients were randomized to a lesion modification with use of either RA or cutting/scoring balloons) and ROTAXUS (randomization to groups with RA followed by stenting or stenting without RA) trials indicated that RA before stent implantation is feasible and effective nearly in all patients with heavily calcified lesions [39,40]. A schematic representation of the RA burr is shown in Figure 3.Orbital atherectomy uses two physical mechanisms: differential sanding and centrifugal forces. The crown rotates eccentrically (off-center), creating an “orbital” motion as it spins around the catheter shaft. Compared to RA, there is only one size of the crown, but use of different speed settings makes it suitable for different vessel sizes (the range of the orbital motion is higher with higher speeds). Two speed settings are available; low speed (80,000 rpm) is often used for the first pass, while the higher speed (120,000 rpm) can be utilized in certain lesions, especially in vessels with a larger diameter. Contrary to RA, OA works bidirectionally both when it is advanced and retracted. The ORBIT II trial has proved the safety of the procedure by indicating a low rate of adverse ischemic events [41]. A large, randomized ECLIPSE trial (OA vessel preparation compared with high-pressure balloons angioplasty and/or cutting balloons) is ongoing [42].
- Excimer laser coronary angioplasty (ELCA) is a relatively rarely-used method. Photoablation (a process based on the emission of monochromatic coherent light in the ultraviolet range) involves the breaking of molecular bonds within the plaque material, causing it to vaporize or ablate. ELCA catheters also have different sizes available and the ratio between the catheter size and the reference vessel diameter should be 0.5–0.6. The clinical use of ELCA is limited. It is mainly used to tackle lesions that are non-dilatable with conventional methods [43]. In calcified lesions it can be used either as a standalone method or after failed RA. ELCA is the only possible option if the lesion is uncrossable with a microcatheter or a guidewire (such as in chronic total occlusions). A specific application of this method is within underexpanded stents (however, the indication is off-label) [21]. One study of 81 cases confirmed ELCA to be superior to predilation with high-pressure balloon in patients with stent restenosis due to stent underexpansion (OCT confirmed cracks in calcium behind the struts achieved using ELCA) [44].
4. Complications
- -
- Entrapped equipment. There is no universal, straightforward technique to retrieve retained equipment due to a large number of possible scenarios. In the case of an entrapped wire or stent it is possible, for example, to pull them out with a special snare, drag them into the guidewire or guide extension with a balloon or, in some cases, pin them to the vessel wall with a stent. A technique with two or three coronary guidewires entangling the entrapped stent to retrieve it is also feasible in some situations [46]. A specific and especially dangerous situation is the entrapment of the RA burr (burr stall); thus, it is important to avoid decelerations (a sudden drop in rotational speed) during RA. A rare scenario is when all methods to retrieve entrapped equipment fail and cardiac surgery is needed.
- -
- No-reflow. The mechanism of this phenomenon is not fully understood (endothelial dysfunction, microvascular obstruction, arteriolar spasm, or distal embolization with microparticles may contribute). It is most common in ST-segment–elevation myocardial infarction and procedures performed in degenerated vein grafts, but also after the use of atherectomy devices [47]. Various pharmacological agents such as adenosine, nitroprusside, nicardipine, and verapamil may be administered. Verapamil, heparine and nitroglycerine are commonly used in flush solutions during RA [48].
- -
- Dissection. It is essential to promptly identify and to properly manage coronary dissection, since major dissections may potentially result in the obstruction of coronary flow and hemodynamic collapse. Establishing or maintaining blood flow in the artery with dissection is crucial. In most cases, a dissection needs to be covered with a stent. If intravascular imaging is used, IVUS is preferable over OCT. During OCT passage an antegrade injection of contrast is needed, which may further aggravate a dissection or cause a new intimal tear.
- -
- Perforation of the vessel. Perforations are generally rare, however, the presence of CAC and use of atheroablative devices or cutting balloons are well-known predictors of perforation [49]. The strategy of management is determined by the location and severity of coronary perforation. Most commonly, prolonged balloon inflation at the site of perforation is performed. If it fails, a covered stent must be implanted. In distal perforations coil embolization is possible. Transthoracic echocardiography has to be completed to assess for cardiac tamponade and need of pericardiocentesis.
5. Long-Term Management and Antiplatelet Treatment
6. Ongoing Research and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, N.D.; Kouwabunpat, D.; Vo, A.N.; Detrano, R.C.; Eisenberg, H.; Goel, M.; Tobis, J.M. Coronary calcium and atherosclerosis by ultrafast computed tomography in asymptomatic men and women: Relation to age and risk factors. Am. Heart J. 1994, 127, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.P.; Farb, A.; Malcom, G.; Virmani, R. Effect of menopause on plaque morphologic characteristics in coronary athero-sclerosis. Am. Heart J. 2001, 141, S58–S62. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Torii, S.; Kutyna, M.; Sakamoto, A.; Finn, A.V.; Virmani, R. Coronary Artery Calcification and its Progression: What Does it Really Mean? J. Am. Coll. Cardiol. Cardiovasc. Imaging 2018, 11, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.S.; Shanahan, C.M. Vascular calcification and hypertension: Cause and effect. Ann. Med. 2012, 44 (Suppl. 1), S85–S92. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, M.V.; Tarigopula, M.; Mintz, G.S.; Maehara, A.; Stone, G.W.; Généreux, P. Coronary Artery Calcification: Pathogenesis and Prognostic Implications. J. Am. Coll. Cardiol. 2014, 63, 1703–1714. [Google Scholar] [CrossRef] [PubMed]
- Demer, L.L.; Tintut, Y. Vascular calcification: Pathobiology of a multifaceted disease. Circulation 2008, 117, 2938–2948. [Google Scholar] [CrossRef]
- Abedin, M.; Tintut, Y.; Demer, L.L. Vascular calcification: Mechanisms and clinical ramifications. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Cleary, P.A.; Orchard, T.J.; Genuth, S.; Wong, N.D.; Detrano, R.; Backlund, J.Y.C.; Zinman, B.; Jacobson, A.; Sun, W.; Lachin, J.M.; et al. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Com-plications (DCCT/EDIC) Study. Diabetes 2006, 55, 3556–3565. [Google Scholar] [CrossRef]
- Carson, A.P.; Steffes, M.W.; Carr, J.J.; Kim, Y.; Gross, M.D.; Carnethon, M.R.; Reis, J.P.; Loria, C.M.; Jacobs, D.R., Jr.; Lewis, C.E. Hemoglobin a1c and the progression of coronary artery calcification among adults without diabetes. Diabetes Care 2015, 38, 66–71. [Google Scholar] [CrossRef]
- Johnson, R.C.; Leopold, J.A.; Loscalzo, J. Vascular calcification: Pathobiological mechanisms and clinical implications. Circ. Res. 2006, 99, 1044–1059. [Google Scholar] [CrossRef]
- Abedin, M.; Omland, T.; Ueland, T.; Khera, A.; Aukrust, P.; Murphy, S.A.; Jain, T.; Gruntmanis, U.; McGuire, D.K.; de Lemos, J.A. Relation of osteoprotegerin to coronary calcium and aortic plaque (from the Dallas Heart Study). Am. J. Cardiol. 2007, 99, 513–518. [Google Scholar] [CrossRef]
- Maldonado, N.; Kelly-Arnold, A.; Vengrenyuk, Y.; Laudier, D.; Fallon, J.T.; Virmani, R.; Cardoso, L.; Weinbaum, S.; Stanley, W.C.; Keehan, K.H.; et al. A mechanistic analysis of the role of microcalcifications in atherosclerotic plaque stability: Potential implications for plaque rupture. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H619–H628. [Google Scholar] [CrossRef]
- Ferencik, M.; Blankstein, R.; Nasir, K. Unravelling the coronary artery calcium paradox: Benefits of plaques of stone. Eur. Heart J.—Cardiovasc. Imaging 2019, 20, 1305–1306. [Google Scholar] [CrossRef]
- Criqui, M.H.; Denenberg, J.O.; Ix, J.H.; McClelland, R.L.; Wassel, C.L.; Rifkin, D.E.; Carr, J.J.; Budoff, M.J.; Allison, M.A. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA 2014, 311, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Cainzos-Achirica, M.; Agrawal, T. Implications of the Plaque Density Paradox for Risk Assessment in 2020. J. Am. Coll. Cardiol. Cardiovasc. Imaging 2021, 14, 243–245. [Google Scholar] [CrossRef]
- Jin, H.-Y.; Weir-McCall, J.R.; Leipsic, J.A.; Son, J.-W.; Sellers, S.L.; Shao, M.; Blanke, P.; Ahmadi, A.; Hadamitzky, M.; Kim, Y.-J.; et al. The Relationship Between Coronary Calcification and the Natural History of Coronary Artery Disease. J. Am. Coll. Cardiol. Cardiovasc. Imaging 2021, 14, 233–242. [Google Scholar] [CrossRef]
- Puri, R.; Nicholls, S.J.; Shao, M.; Kataoka, Y.; Uno, K.; Kapadia, S.R.; Tuzcu, E.M.; Nissen, S.E. Impact of statins on serial coronary calcification during atheroma progression and regression. J. Am. Coll. Cardiol. 2015, 65, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Räber, L.; Taniwaki, M.; Zaugg, S.; Kelbæk, H.; Roffi, M.; Holmvang, L.; Noble, S.; Pedrazzini, G.; Moschovitis, A.; Lüscher, T.F.; et al. Effect of high-intensity statin therapy on atherosclerosis in non-infarct-related coronary arteries (IBIS-4): A serial intravascular ultrasonography study. Eur. Heart J. 2015, 36, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Okura, H.; Kume, T.; Yamada, R.; Kobayashi, Y.; Fukuhara, K.; Koyama, T.; Nezuo, S.; Neishi, Y.; Hayashida, A.; et al. Impact of Target Lesion Coronary Calcification on Stent Expansion. Circ. J. 2014, 78, 2209–2214. [Google Scholar] [CrossRef]
- Alexopoulos, N.; Raggi, P. Calcification in atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 681–688. [Google Scholar] [CrossRef]
- Barbato, E.; Gallinoro, E.; Abdel-Wahab, M.; Andreini, D.; Carrié, D.; Di Mario, C.; Dudek, D.; Escaned, J.; Fajadet, J.; Guagliumi, G.; et al. Management strategies for heavily calcified coronary stenoses: An EAPCI clinical consensus statement in collaboration with the EURO4C-PCR group. Eur. Heart J. 2023, 44, 4340–4356. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-J.; Lin, F.Y.; Lee, S.-E.; Andreini, D.; Bax, J.; Cademartiri, F.; Chinnaiyan, K.; Chow, B.J.; Conte, E.; Cury, R.C.; et al. Coronary Atherosclerotic Precursors of Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2018, 71, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Burke, A.P.; Kolodgie, F.D.; Farb, A. Pathology of the Thin-Cap Fibroatheroma: A Type of Vulnerable Plaque. J. Interv. Cardiol. 2003, 16, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, S.; Ito, H.; Sarai, M.; Kondo, T.; Kawai, H.; Nagahara, Y.; Harigaya, H.; Kan, S.; Anno, H.; Takahashi, H.; et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J. Am. Coll. Cardiol. 2015, 66, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Nerlekar, N.; Ha, F.J.; Cheshire, C.; Rashid, H.; Cameron, J.D.; Wong, D.T.; Seneviratne, S.; Brown, A.J. Computed Tomographic Coronary Angiography–Derived Plaque Characteristics Predict Major Adverse Cardiovascular Events. Circ. Cardiovasc. Imaging 2018, 11, e006973. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.C.; Moss, A.J.; Dweck, M.; Adamson, P.D.; Alam, S.; Hunter, A.; Shah, A.S.; Pawade, T.; Weir-McCall, J.R.; Roditi, G.; et al. Coronary Artery Plaque Characteristics Associated with Adverse Outcomes in the SCOT-HEART Study. J. Am. Coll. Cardiol. 2019, 73, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, S.; Sarai, M.; Harigaya, H.; Anno, H.; Inoue, K.; Hara, T.; Naruse, H.; Ishii, J.; Hishida, H.; Wong, N.D.; et al. Computed Tomographic Angiography Characteristics of Atherosclerotic Plaques Subsequently Resulting in Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2009, 54, 49–57. [Google Scholar] [CrossRef]
- Achenbach, S. Imaging the Vulnerable Plaque on Coronary CTA. J. Am. Coll. Cardiol. Cardiovasc. Imaging 2020, 13, 1418–1421. [Google Scholar] [CrossRef]
- Carroll, J.D.; Chen, S.-Y.J. The Use of CTCA for Planning PCI: Using the 3D Coronary Tree Information*. JACC Cardiovasc. Interv. 2020, 13, 2571–2573. [Google Scholar] [CrossRef]
- Takagi, H.; Ishikawa, Y.; Orii, M.; Ota, H.; Niiyama, M.; Tanaka, R.; Morino, Y.; Yoshioka, K. Optimized interpretation of fractional flow reserve derived from computed tomography: Comparison of three interpretation methods. J. Cardiovasc. Comput. Tomogr. 2019, 13, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Caixeta, A.; Stone, G. A Guide to Calculating SYNTAX Score. Interv. Cardiol. 2012, 7, 21–23. [Google Scholar] [CrossRef]
- Räber, L.; Mintz, G.S.; Koskinas, K.C.; Johnson, T.W.; Holm, N.R.; Onuma, Y.; Radu, M.D.; Joner, M.; Yu, B.; Jia, H.; et al. Clinical use of intracoronary imaging. Part 1: Guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur. Heart J. 2018, 39, 3281–3300. [Google Scholar] [CrossRef] [PubMed]
- Fujino, A.; Mintz, G.S.; Matsumura, M.; Lee, T.; Kim, S.-Y.; Hoshino, M.; Usui, E.; Yonetsu, T.; Haag, E.S.; Shlofmitz, R.A.; et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention 2018, 13, 2182–2189. [Google Scholar] [CrossRef]
- Yahagi, K.; Joner, M.; Virmani, R. The Mystery of Spotty Calcification: Can We Solve It by Optical Coherence Tomography? Circ. Cardiovasc. Imaging 2016, 9, e004252. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, X.; Kan, J.; Ge, Z.; Han, L.; Lu, S.; Tian, N.; Lin, S.; Lu, Q.; Wu, X.; et al. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: The ULTIMATE Trial. J. Am. Coll. Cardiol. 2018, 72, 3126–3137. [Google Scholar] [CrossRef]
- Costa, J.R.; Mintz, G.S.; Carlier, S.G.; Mehran, R.; Teirstein, P.; Sano, K.; Liu, X.; Lui, J.; Na, Y.; Castellanos, C.; et al. Nonrandomized comparison of coronary stenting under intravascular ultrasound guidance of direct stenting without predilation versus conventional predilation with a semi-compliant balloon versus pre-dilation with a new scoring balloon. Am. J. Cardiol. 2007, 100, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Secco, G.G.; Ghione, M.; Mattesini, A.; Dall’ara, G.; Ghilencea, L.; Kilickesmez, K.; De Luca, G.; Fattori, R.; Parisi, R.; Marino, P.N.; et al. Very high-pressure dilatation for undilatable coronary lesions: Indications and results with a new dedicated balloon. EuroIntervention 2016, 12, 359–365. [Google Scholar] [CrossRef]
- Kereiakes, D.J.; Hill, J.M.; Shlofmitz, R.A.; Klein, A.J.; Riley, R.F.; Price, M.J.; Herrmann, H.C.; Bachinsky, W.; Waksman, R.; Stone, G.W.; et al. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Arteries. JACC Cardiovasc. Interv. 2023, 16, 2472–2474. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Toelg, R.; Byrne, R.A.; Geist, V.; El-Mawardy, M.; Allali, A.; Rheude, T.; Robinson, D.R.; Abdelghani, M.; Sulimov, D.S.; et al. High-Speed Rotational Atherectomy Versus Modified Balloons Prior to Drug-Eluting Stent Implantation in Severely Calcified Coronary Lesions. Circ. Cardiovasc. Interv. 2018, 11, e007415. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Richardt, G.; Büttner, H.J.; Toelg, R.; Geist, V.; Meinertz, T.; Schofer, J.; King, L.; Neumann, F.-J.; Khattab, A.A. High-Speed Rotational Atherectomy Before Paclitaxel-Eluting Stent Implantation in Complex Calcified Coronary Lesions: The Randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) Trial. JACC Cardiovasc. Interv. 2013, 6, 10–19. [Google Scholar] [CrossRef]
- Chambers, J.W.; Feldman, R.L.; Himmelstein, S.I.; Bhatheja, R.; Villa, A.E.; Strickman, N.E.; Shlofmitz, R.A.; Dulas, D.D.; Arab, D.; Khanna, P.K.; et al. Pivotal Trial to Evaluate the Safety and Efficacy of the Orbital Atherectomy System in Treating De Novo, Severely Calcified Coronary Lesions (ORBIT II). JACC Cardiovasc. Interv. 2014, 7, 510–518. [Google Scholar] [CrossRef]
- Généreux, P.; Kirtane, A.J.; Kandzari, D.E.; Armstrong, E.J.; Krucoff, M.W.; Redfors, B.; Ben-Yehuda, O.; Lerew, D.R.; Ali, Z.A.; Maehara, A.; et al. Randomized evaluation of vessel preparation with orbital atherectomy prior to drug-eluting stent implantation in severely calcified coronary artery lesions: Design and rationale of the ECLIPSE trial. Am. Heart J. 2022, 249, 1–11. [Google Scholar] [CrossRef]
- Cubero-Gallego, H.; Tizón-Marcos, H.; Vaquerizo, B. Current options for the management of calcified lesions. REC Intervent. Cardiol. 2020, 2, 129–139. [Google Scholar] [CrossRef]
- Lee, T.; Shlofmitz, R.A.; Song, L.; Tsiamtsiouris, T.; Pappas, T.; Madrid, A.; Jeremias, A.; Haag, E.S.; Ali, Z.A.; Moses, J.W.; et al. The effectiveness of excimer laser angioplasty to treat coronary in-stent restenosis with peri-stent calcium as assessed by optical coherence tomography. EuroIntervention 2019, 15, e279–e288. [Google Scholar] [CrossRef]
- De Maria, G.L.; Scarsini, R.; Banning, A.P. Management of Calcific Coronary Artery Lesions: Is it Time to Change Our Interventional Therapeutic Approach? JACC Cardiovasc. Interv. 2019, 12, 1465–1478. [Google Scholar] [CrossRef] [PubMed]
- Doll, J.A.; Hira, R.S.; Kearney, K.E.; Kandzari, D.E.; Riley, R.F.; Marso, S.P.; Grantham, J.A.; Thompson, C.A.; McCabe, J.M.; Karmpaliotis, D.; et al. Management of Percutaneous Coronary Intervention Complications: Algorithms From the 2018 and 2019 Seattle Percutaneous Coronary Intervention Complications Conference. Circ. Cardiovasc. Interv. 2020, 13, e008962. [Google Scholar] [CrossRef] [PubMed]
- Rezkalla, S.H.; Stankowski, R.V.; Hanna, J.; Kloner, R.A. Management of No-Reflow Phenomenon in the Catheterization Laboratory. JACC Cardiovasc. Interv. 2017, 10, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.bostonscientific.com/content/dam/bostonscientific/Interventional%20Cardiology/portfolio-group/Plaque-Modification/Rotablator-System-Reference-Guide_IC-193906-AA.pdf (accessed on 18 February 2024).
- Harries, I.; Graham, A.; Ramcharitar, S. Tools & Techniques-Clinical: Management of coronary perforation. EuroIntervention 2014, 10, 646–647. [Google Scholar] [CrossRef] [PubMed]
- Forero, M.T.F.T.; Sardella, G.; Salvi, N.; Cortese, B.; di Palma, G.; Werner, N.; Aksoy, A.; Escaned, J.; Salazar, C.S.; Gonzalo, N.; et al. Coronary lithotripsy for the treatment of underexpanded stents: The international multicentre CRUNCH registry. EuroIntervention 2022, 18, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Bastante, T.; Rivero, F.; Cuesta, J.; Alfonso, F. Calcified Neoatherosclerosis Causing “Undilatable” In-Stent Restenosis: Insights of Optical Coherence Tomography and Role of Rotational Atherectomy. JACC Cardiovasc. Interv. 2015, 8, 2039–2040. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Giustino, G.; Redfors, B.; Palmerini, T.; Witzenbichler, B.; Weisz, G.; Stuckey, T.D.; Maehara, A.; Mehran, R.; Kirtane, A.J.; et al. Impact of percutaneous coronary intervention extent, complexity and platelet reactivity on outcomes after drug-eluting stent implantation. Int. J. Cardiol. 2018, 268, 61–67. [Google Scholar] [CrossRef]
- Dangas, G.; Baber, U.; Sharma, S.; Giustino, G.; Mehta, S.; Cohen, D.J.; Angiolillo, D.J.; Sartori, S.; Chandiramani, R.; Briguori, C.; et al. Ticagrelor with or without Aspirin after Complex PCI. J. Am. Coll. Cardiol. 2020, 75, 2414–2424. [Google Scholar] [CrossRef]
- Lhermusier, T.; Motreff, P.; Bataille, V.; Cayla, G.; Farah, B.; Roncalli, J.; Elbaz, M.; Boudou, N.; Campello-Parada, F.; Bouisset, F.; et al. TIcagrelor in Rotational Atherectomy to Reduce TROPonin Enhancement: The TIRATROP Study, A Randomized Controlled Trial. J. Clin. Med. 2023, 12, 1445. [Google Scholar] [CrossRef] [PubMed]
- Kini, A.; Reich, D.; Marmur, J.D.; Mitre, C.A.; Sharma, S.K. Reduction in periprocedural enzyme elevation by abciximab after rotational atherectomy of type B2 lesions: Results of the Rota ReoPro randomized trial. Am. Heart J. 2001, 142, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Tomey, M.I.; Teirstein, P.S.; Kini, A.S.; Reitman, A.B.; Lee, A.C.; Généreux, P.; Chambers, J.W.; Grines, C.L.; Himmelstein, S.I.; et al. North American Expert Review of Rotational Atherectomy. Circ. Cardiovasc. Interv. 2019, 12, e007448. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.F.; Patel, M.P.; Abbott, J.D.; Bangalore, S.; Brilakis, E.S.; Croce, K.J.; Doshi, D.; Kaul, P.; Kearney, K.E.; Kerrigan, J.L.; et al. SCAI Expert Consensus Statement on the Management of Calcified Coronary Lesions. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 101259. [Google Scholar] [CrossRef]
- Scalamogna, M.; Abdel-Wahab, M.; Mashayekhi, K.; Fusaro, M.; Leistner, D.M.; Ayoub, M.; Xhepa, E.; Joner, M.; Kastrati, A.; Cassese, S.; et al. Randomized ComparIson of Strategies to PrepAre SeveRely CALCified Coronary Lesions 2: Design and Rationale of the ISAR-CALC 2 Trial. Cardiovasc. Revasc. Med. 2023, 49, 22–27. [Google Scholar] [CrossRef]
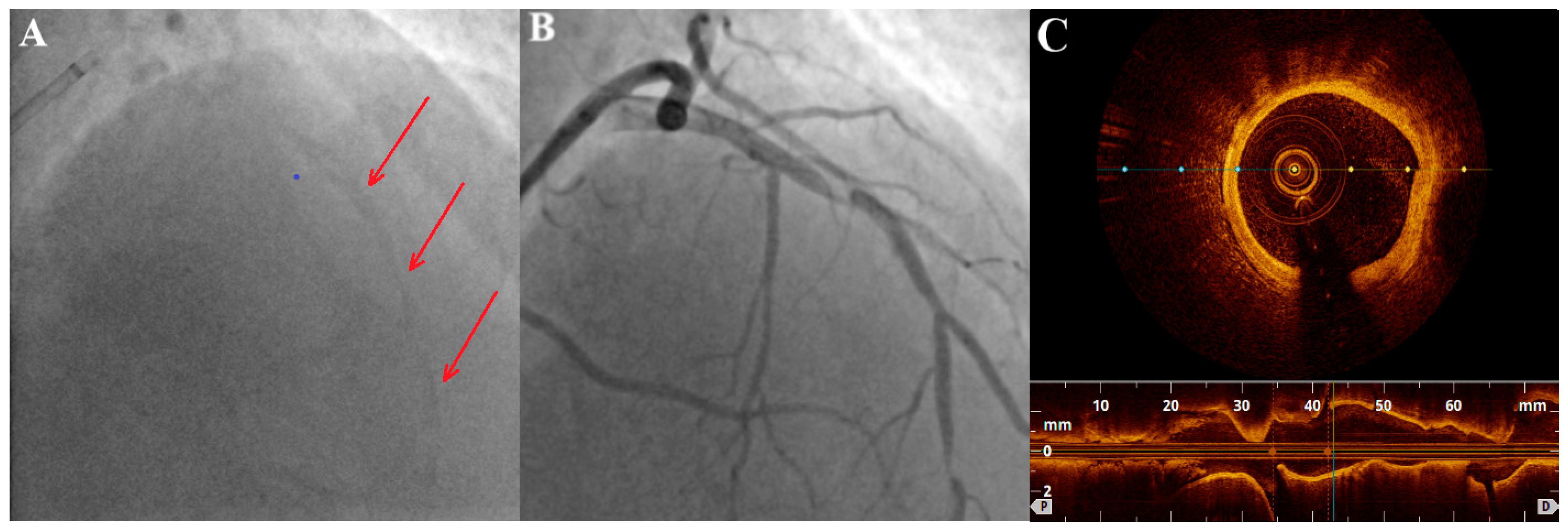
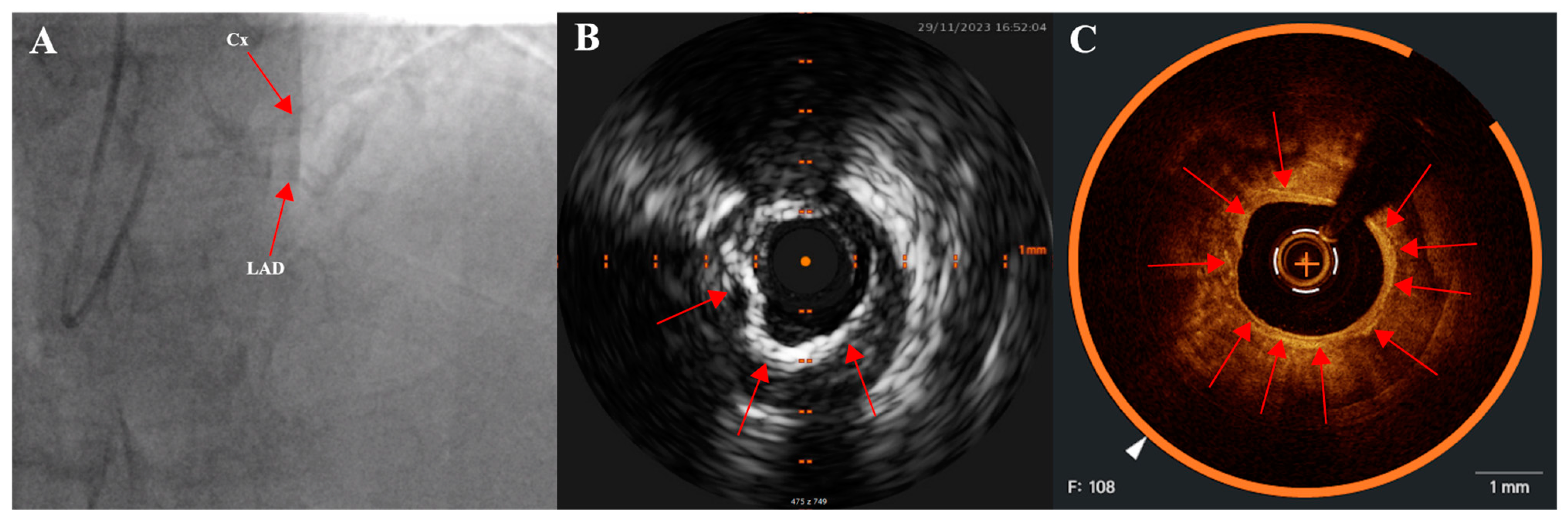


| IVUS | OCT | |
|---|---|---|
| Spatial resolution | 50–200 μm | 15–20 μm |
| Need of contrast injection | No | Yes |
| Duration of data aquisition | 2–4 min | <10 s |
| Visualization of severe CAC | +++ | +++ |
| Visualization of mild/moderate CAC | ++ | +++ |
| Deep calcium | +++ | ++ |
| Calcium arch | +++ | +++ |
| Calcium thickness | - | +++ |
| Longitudinal calcium length | + | +++ |
| Non-homogenous plaque/necrotic core | +++ | + |
| Super-High Pressure Ballons | Cutting Balloons | Scoring Ballons | RA | OA | IVL | ELCA | |
|---|---|---|---|---|---|---|---|
| Physical mechanism | High pressure with uniform expansion | Radial incision | Focal force/slice | Differential cutting | Differential sanding and pulsatile forces | Pulsatile mechanical energy | Photoablation (vaporization) |
| Guidewire | 0.014″ | 0.014″ | 0.014″ | 0.009″ (Rotawire Floppy/ Extra support) | 0.012″/0.014″ (Viperwire) | 0.014″ | 0.014″ |
| Pressure | Up to 45 atm | Rated burst pressure 12 atm | Rated burst pressure 16 atm | Typically 4–6 atm | |||
| Ablation direction | unidirectional | bidirectional | |||||
| Rotational speed | 140,000–180,000 rpm | 80,000/120,000 rpm | |||||
| Particle size | 5–10 μm | <2 μm | |||||
| Specific considerations/applications | - balloon uncrossable lesions - late in-stent restenosis with calcified neoatherosclerosis | - long diffuse calcification - large diameter vessels - aorto-ostial lesion - more than one lesion requiring modification, with a difference in reference vessel diameter | - focal lesion - bifurcation with both branches requiring calcium modification - stent underexpansion with calcium outside the stent (off-label) | - uncrossable lesions - thrombotic calcified lesions - stent underexpansion with calcium outside the stent (off-label) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lis, P.; Rajzer, M.; Klima, Ł. The Significance of Coronary Artery Calcification for Percutaneous Coronary Interventions. Healthcare 2024, 12, 520. https://doi.org/10.3390/healthcare12050520
Lis P, Rajzer M, Klima Ł. The Significance of Coronary Artery Calcification for Percutaneous Coronary Interventions. Healthcare. 2024; 12(5):520. https://doi.org/10.3390/healthcare12050520
Chicago/Turabian StyleLis, Paweł, Marek Rajzer, and Łukasz Klima. 2024. "The Significance of Coronary Artery Calcification for Percutaneous Coronary Interventions" Healthcare 12, no. 5: 520. https://doi.org/10.3390/healthcare12050520
APA StyleLis, P., Rajzer, M., & Klima, Ł. (2024). The Significance of Coronary Artery Calcification for Percutaneous Coronary Interventions. Healthcare, 12(5), 520. https://doi.org/10.3390/healthcare12050520






