1. Introduction
Chronic diseases place a significant burden on global health [
1]. According to the World Health Organization (WHO), chronic diseases are characterized by certain key attributes, including their long-lasting nature, resulting residual disabilities, non-reversible pathological changes as their underlying cause, the need for specialized patient training for rehabilitation, or extended periods of supervision, observation, or care [
2,
3].
In developed countries, there is a high prevalence of individuals suffering from multiple chronic conditions, known as multi-morbidity [
4], which tends to increase with age [
4,
5]. The management of chronic diseases poses a substantial challenge to healthcare systems worldwide, as these systems have traditionally focused on addressing acute episodes rather than delivering organized, long-term care for patients dealing with chronic conditions [
6]. A key characteristic of chronic diseases is the necessity for ongoing supervision, care, and observation. Primary care, which is defined by qualities such as continuity, coordination, and addressing complexity, is well-suited to handle the management of chronic conditions [
7]. There is growing evidence underscoring the importance of reorienting health policies and healthcare systems toward chronic care, with an emphasis on strengthening primary care [
8]. Countries with robust primary care systems typically achieve better health outcomes at a lower cost [
9].
In the realm of liver disease, experts have extensively explored the topic of drug-induced liver injury over the past three decades [
10,
11,
12].
Chronic drug use is known to cause persistent damage, where the liver’s ability to regenerate eventually becomes dysfunctional, leading to liver scarring and cirrhosis. Despite recent therapeutic advances and significant development in modern medicine, liver diseases remain a global health problem, affecting over 10% of the global population [
13]. Chronic hepatic disorders remain a significant concern globally [
14] due to the high mortality rate associated with liver cirrhosis in Western nations.
Nutritional supplements are products designed to provide essential nutrients that may be lacking in sufficient quantities in a person’s diet. Natural hepatoprotective products derived from herbs, such as
Silybum marianum L. Gaertn.,
Cynara scolymus L.,
Cichorium intybus L.,
Capparis spinosa L.,
Glycyrrhiza glabra L.,
Ganoderma lucidum Karst.,
Chlorella ssp., and other species containing various bioactive compounds, can have a significant positive impact on liver metabolic parameters in patients with chronic diseases [
14,
15,
16]. These naturally hepatoprotective substances, with different structures, but sharing the same therapeutic activity, can act as active agents in various chronic diseases. Several phyto molecules, including flavonoids, alkaloids, glycosides, and saponins obtained from various plant sources, have been reported as potent hepatoprotective agents [
17].
Studies have shown beneficial effects of certain isolated bioactive compounds, such as polyphenolic compounds like curcumin [
18], resveratrol [
19], and quercetin [
20], on metabolic parameters in these patients. Similarly, other natural products like grapes [
21], green tea, orange juice [
22], hibiscus [
23], aloe vera [
24], wild bitter, berberine [
25], barberry [
26], and withaferin A [
27,
28] have demonstrated similar effects on metabolism.
Both in ancient and modern phytotherapy, extremely versatile and valuable plants with hepatoprotective properties are used and studied continuously. Examples of such plants include milk thistle (
Silybum marianum L. Gaertn.), artichoke (
Cynara cardunculus L.), and chicory (
Cichorium intybus L.) [
29]. These plants have been specifically chosen due to the global concern surrounding liver diseases, as all parts of these plants have potential applications. Artichoke and chicory are commonly consumed as foods or dietary supplements, with less frequent use as phytomedicines [
30]. Milk thistle, renowned for its hepatoprotective properties, is recognized for its ability to support liver functions, and dietary supplements and phytomedicines derived from milk thistle fruits/seeds are considered effective remedies in this regard [
31]. Currently, dietary supplements based on milk thistle rank among the top 40 best-selling herbal supplements [
32]. Over the past decade, there has been a significant rise in the demand for phytomedicines and dietary supplements with hepatoprotective properties. This surge can be attributed to the abundance of information available on their therapeutic significance.
Bioactive compounds can be used as strategies for the prevention and treatment of metabolic disorders, specific to many chronic conditions, by improving the inflammatory state and other comorbidities, such as obesity, dyslipidemias, and cardiovascular diseases [
33].
The aim of the study is to see the frequency of HP consumption as adjuvant treatments among patients with chronic diseases (other than chronic liver disorders) and analyzes the habits related to the consumption of nutritional supplements among these patients. Additionally, the differences in nutritional status, assessed by measuring body composition between consumers and non-consumers of nutritional supplements, were evaluated.
2. Materials and Methods
2.1. Study Design
In the first part of this cross-sectional study, we analyzed frequency of HP consumption among 954 patients with chronic diseases from Bihor County, Romania, in addition to prescribed medications for their conditions. Data from four pharmacies in the city of Oradea, Bihor County, Romania were analyzed over 12 months (from October 2021 to October 2022). Patients with medical prescriptions for chronic diseases were recruited based on the diagnosis provided by the current doctor. The selection criteria were as follows: age over 18 years, expressing the agreement to participate in the study, the presence of one or more chronic diseases, and the prescribed treatment containing one or more allopathic drugs. The exclusion criteria were the patient’s refusal to participate in the study, the presence of acute diseases, the presence of chronic liver diseases, and psychiatric and oncologic disorders. Having chronic diseases, patients are treated with one or more drugs for a long time without interruption. The 954 patients selected and included in the analysis were categorized into seven groups according to the number of drugs prescribed for the chronic disease/diseases (from Group 1 with one medicine, to Group 7 with seven or more medicines) (
Table 1). Data processing and statistical analysis were conducted to examine frequency in use of HP across the study groups, as well as the types of nutritional supplements administered.
All patients included in the analysis were invited to complete a questionnaire that evaluates the habits related to the consumption of food supplements and to participate in a nutritional assessment. One hundred ninety-five patients consented to participate in the survey, and 65 patients agreed to undergo a nutritional status evaluation.
2.2. Evaluation of Supplement Usage Habits
An examination tool was developed as a questionnaire of seven questions, both open-ended and closed-ended, in the Romanian language (translated as
Supplementary Material S1).
The package of questions, as well as the answer options, was chosen and established following consultations with professionally experienced pharmacists (in the community pharmacy), for more than 5 years. Items were developed for identifying the habits regarding the consumption of food supplements among the population with chronic diseases. The questions were formulated in such a way as to be as simple as possible and easy to understand to result in a sufficiently relevant questionnaire, but also to be as short as possible.
The first section of the questionnaire establishes the socio-demographic criteria of the participants, the habit of consuming food supplements or not, the purpose of their use, the type of supplements consumed, and the way in which the used supplement was chosen. The second section establishes whether the recommended doses are respected, the duration of use, and the self-assessed impact on health.
A number of 20 patients with chronic diseases answered the questions from the initial draft of the questionnaire (pilot test). In the final form, there were minor editorial changes, and the items were supported by the initial draft. Experts from the College of Pharmacists from Bihor County (the representative forum of pharmacists for Bihor County, which was also the county professional organization of pharmacists and a sub-branch of the College of Pharmacists from Romania) evaluated the final version of the questionnaire for content validity. After that, face-to-face interview with trained interviewers was used to collect the data from the 195 patients who participated in the survey. Internal consistency of the questionnaire was estimated using the coefficient Cronbach’s alpha [
34]. The average value of Cronbach’s Alpha test for the seven questionnaire items was 0.886, falling into the “good” category.
2.3. Assessment of Nutritional Status
Evaluation of the nutritional status was conducted using the Tanita MC 780 MA body bioelectrical impedance analyzer (BIA) from Tokyo, Japan [
35,
36]. The collected data were evaluated using the GMON 3.4.1 medical software from Chemnitz, Germany.
BIA body analyzers, approved by the World Public Health Nutrition Association (WPHNA), were employed to determine body composition with high precision, including measurements of BMI (body mass index), fat mass, muscle mass, sarcopenic index, and total body water (TBW) hydration status. The margin of error for these measurements was 0.1 kg.
Regarding BMI, the following classification was used:
18.49 or less—Underweight;
18.50 to 24.99—Normal weight;
25.00 to 29.99—Overweight;
30.00 to 34.99—Obesity (degree I);
35.00 to 39.99—Obesity (degree II);
40.00 or more—Obesity (degree III) or morbid obesity.
Both men and women were included in these categories.
For fat mass, normal range for women is between 23% and 34%, and for men, it is between 13% and 25% (marked as 1 in our study). If the subject had a fat mass below the normal range, it was marked as “0” in both cases. In men, a range between 25% and 30% is considered excessive (marked as 2), and over 30% is classified as very high (marked as 3). In women, the excessive range is between 34% and 40% (marked as 2), and over 40% is classified as very high fat mass (marked as 3).
According to body analyzer, the normal ranges for muscle mass are as follows:
Ages < 65: 75–89% for men, 63–75.5% for women.
Ages 65–70: 73–86% for men, 62–73.5% for women.
Ages > 70: 70–84% for men, 60–72.5% for women.
In our study, these intervals were scored as 1. Individuals with muscle mass below the normal range were marked as 0, while those with muscle mass above the normal limit were marked as 2.
For the sarcopenic index, normal values for women should be above 5.85, and for men, it was above 7.00. High values of sarcopenic index are associated with the infiltration of fats between actin and myosin, which can block insulin receptors and correlate with metabolic syndrome.
Water mass, expressed as total body water (TBW), is recommended to be between 45% and 60% for normal limits (marked as 1 in our study). These values are modified according to age and sex. The ranges are as follows:
Ages < 65: Males 52–66% (average: 59%), Females 49–63% (average: 56%).
Ages 65–70: Males 43–73% (average: 59%), Females 41–60% (average: 50%).
Ages > 70: Males 47–67% (average: 56%), Females 39–57% (average: 47%).
Changes in total water volume are not directly related to water consumption but can be influenced by various factors such as inflammation, kidney disease, and unhealthy levels of fat mass (obesity).
In order to carry out the research included in this study, the approval (with the reference number CEFMF/19 dated 26 February 2021) of the Research Ethics Committee of the Faculty of Medicine and Pharmacy, University of Oradea, was obtained.
2.4. Statistical Analyses
The collected data were analyzed using the statistical software SPSS 20 (New York, NY, USA) through statistics such as ANOVA, Post-Hoc analysis, chi-square test, and inferential statistics (Student t-test). The three research groups were compared using the Bonferroni test.
4. Discussion
Plants have served multiple purposes over time, not only as a source of food but also as medicinal remedies [
37]. Traditionally, people commonly utilized the fruits and roots of plants, often overlooking other parts of the plants.
The liver, acknowledged as a vital organ, performs a variety of essential functions [
38]. These functions encompass the production of proteins, cholesterol, and bile, as well as the storage of vitamins, minerals, and carbohydrates [
39]. Furthermore, the liver plays a critical role in detoxifying the body by metabolizing substances like alcohol, medications, and metabolic by-products to maintain overall metabolic equilibrium within an organism [
40,
41]. Consequently, it is particularly vulnerable to oxidative stress [
42]. Both the hepatoprotective and antioxidant effects are commonly found together in most hepatoprotective supplements (HP).
A study conducted in 2022 [
43] examined the efficacy of hepatoprotective measures in preventing the development of liver lesions resulting from drug treatments. The study revealed a notable effectiveness of these measures with no significant associated health risks. This demonstrates that the current research focuses on addressing a pressing health issue, specifically liver damage, for which the utilization of natural HP may serve as a potential solution.
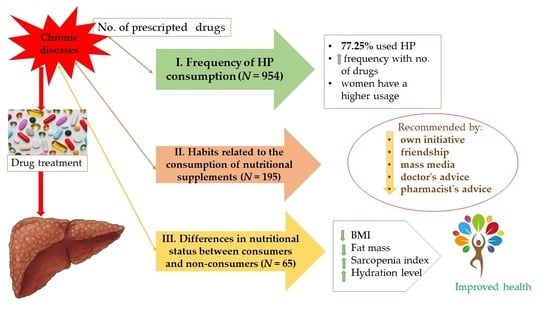
The results show that more than half of the subjects (76.93%) with chronic diseases who participated in this study use hepatoprotective supplements. A high percentage of patients choose to use supplements based on Silybum marianum L. Gaertn., which shows that this is the most known, recommended, and used HP.
Certainly, while the majority of research indicates that individuals with chronic liver conditions often incorporate nutritional supplements into their routines [
44], our study specifically examined the utilization of hepatoprotective supplements (HP) in patients who had non-hepatic chronic diseases. The treatment of chronic conditions typically involves a prolonged use of medication. While these drugs are effective in managing the illness, their complete advantages are frequently not fully achieved due to the fact that roughly 50% of patients do not adhere to their prescribed medication regimens [
45]. Patients who are prescribed numerous drugs for chronic diseases tend to have lower adherence to preventive hepatoprotective treatments [
46].
In this study, the number of prescribed medicines had an impact on how frequently HP was consumed. Group 7 had the highest consumption rates (83.33%, N = 40/48), followed by Groups 3 (82.84%, N = 180/217) and 4 (80.44%, N = 144/179), while Group 1 had the lowest usage rates (58%, N = 36/62). Most of the participants (59.5%) used extracts of Silybum marianum.
Even if nutritional supplements have many beneficial effects for health, they should be consumed on the recommendation of a specialist, following a carefully studied scheme, in suitable doses and in such a way as to avoid interactions with the drugs used at the same time. To see the supplement consumption habits among the patients with chronic diseases included in the study, a simple and reliable questionnaire was developed. This monitored whether the patient’s used supplements and, if so, the reasons for use (hepatoprotection and prevention, among others). The study also tracked where the supplements were purchased (pharmacy, naturists, internet, or others), who recommended them (own initiative, friends, mass media, doctor’s advice, and pharmacist’s advice, among others), whether the recommended dose was respected, and the duration of supplement usage.
It was found that most patients use nutritional supplement on their own initiative or obtain information from the mass media. Only a small proportion of them (32.54%) use HP on the recommendation of the doctor or pharmacist, which corresponds to the tendency of self-medication of patients, which is observed worldwide. A percentage of 54% of patients have been using supplements for two months or longer.
Regarding nutritional status, a percentage of 88% of patients who use supplements showed minor or major improvements regarding their health status, compared to the percentage of 7.92% of patients who did not notice improvements in their health status.
The clinical evaluations to which the patients underwent were based on the measurement of BMI, fat mass, muscle mass, sarcopenic index, and hydration measurement. It was found that patients using HP have a lower mean BMI value, both compared to patients who use other types of nutritional supplements and compared to patients who do not use supplements. High values of the sarcopenic index were observed in the studied patients, which is associated with the infiltration of fats between actin and myosin, which can block insulin receptors and correlate with metabolic syndrome and other metabolic diseases. As the fat mass increases, the degree of hydration of the body is lower, triggering a pro-inflammatory process with water retention in the intercellular spaces. Among the patients who use HP, it can be observed that only 21.7% have a level above normal and only 4.3% have a high level of fat mass. Regarding hydration status, low levels can be observed in patients who consume different types of supplements. However, in patients who consume HP supplements, this level is much lower.
The likely mechanisms of action for these herbs may involve immunomodulation and a stimulation of hepatic DNA synthesis, as well as the stimulation of superoxide dismutase and glutathione reductase to inhibit oxidative stress in hepatocytes. Additionally, they can help reduce intracellular reactive oxygen species by enhancing antioxidant levels [
47]. In our study, individuals who integrate nutritional supplements into their daily routines typically observe enhanced well-being. This improvement in health is associated with the sustained use of hepatoprotective supplements (HP) for a period exceeding 2 months. Most important outcomes of our research can be schematized, as is depicted in the
Figure 6.
Due to the limitations of the sample (particularly, sampling methodology, sociodemographic characteristics, and sample size), it may not be viable to generalize our findings to the Romanian population.
It would be necessary to recruit more participants, to create a more age and gender-heterogeneous cohort for which to evaluate more precisely the chronic disease and the type/duration of the treatment, for providing more accurate results.
However, it is worth emphasizing that for the population with chronic diseases in Romania, until now, no similar study has been conducted regarding the use of HP. Therefore, this study represents a reference, and it allows similar application to other age groups, other disease categories, or to the general population.