3.1. Characterization of Fire-Born Particles
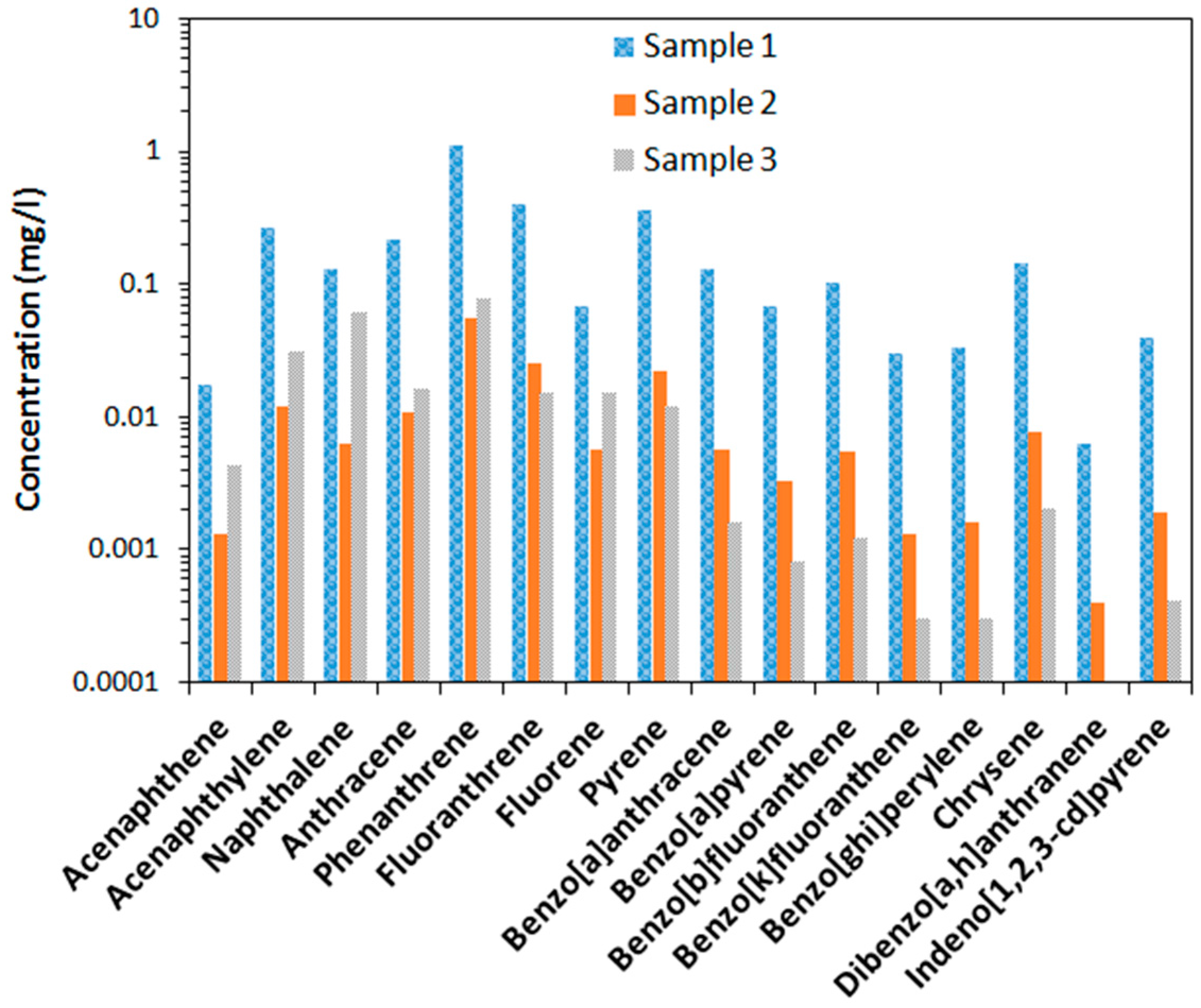
The concentrations of the 16 measured polyaromatic hydrocarbons from the three different sampling locations are shown in logarithmic scale in
Figure 1. The figure indicates the highest concentration of all form of PAH in sample 1 and lowest in sample 3. The compounds from benso[a]anthracene and to the right in
Figure 1 are considered to be of high molecular weight [
17] and these, except benzo[ghi]perylene, are also considered carcinogenic (e.g., [
18,
19,
20,
21,
22]). Low molecular weight PAHs may be found in the gas phase whereas the high molecular weight PAHs, which are also of greater environmental concern, have very low volatility and will generally be found in particles. All measured PAHs were found at all three sampling locations, and the relative abundance of the compounds was in relative agreement with previous studies (e.g., [
23]) although, comparatively, somewhat lower concentrations of the low molecular weight PAHs were observed. This may be due to some volatilization of low molecular weight PAHs before sampling was performed. The differences in concentrations between the different sampling locations was likely related to the fact that the extinguishing water was found in different state of concentration at the different locations, and, particularly, large amounts of black soot was observed at location 1. However, the local thermo-physical characteristics of the fire and the burning materials should also have influenced the proportion of different compounds that were locally formed. The sum of carcinogenic PAHs was larger than 10 times the Swedish guideline value for groundwater at all sampling locations (>10 × 0.2 = 2 μg/L), which (undiluted) would be considered a “very serious” state of contamination [
24].
The concentrations of the 10 measured metals at the three different sampling locations are shown in logarithmic scale in
Figure 2. The relative difference in metal concentrations between the different sampling locations is small as compared to the PAHs (
Figure 1), which may indicate less influence of local factors on the amount of metal particles formed in the fire. Some of the metals (Cd, Cu, Zn,
etc.) were highest in sample 1, but they don’t follow any proper order. The metal concentrations are also in relative agreement with previous findings [
23]. The concentrations of Pb, Cd, Cu and Zn were more than 10 times the Swedish water quality guideline values [
24] for all samples. For As, Cr and Ni, the concentrations were above the guideline values, whereas for Ba, Co and V, Swedish guideline values have not been established.
Figure 1.
Concentration of PAHs in the collected fire extinguishing water samples from three different locations.
Figure 1.
Concentration of PAHs in the collected fire extinguishing water samples from three different locations.
Figure 2.
Concentration of metals in the collected fire extinguishing water samples from three different locations.
Figure 2.
Concentration of metals in the collected fire extinguishing water samples from three different locations.
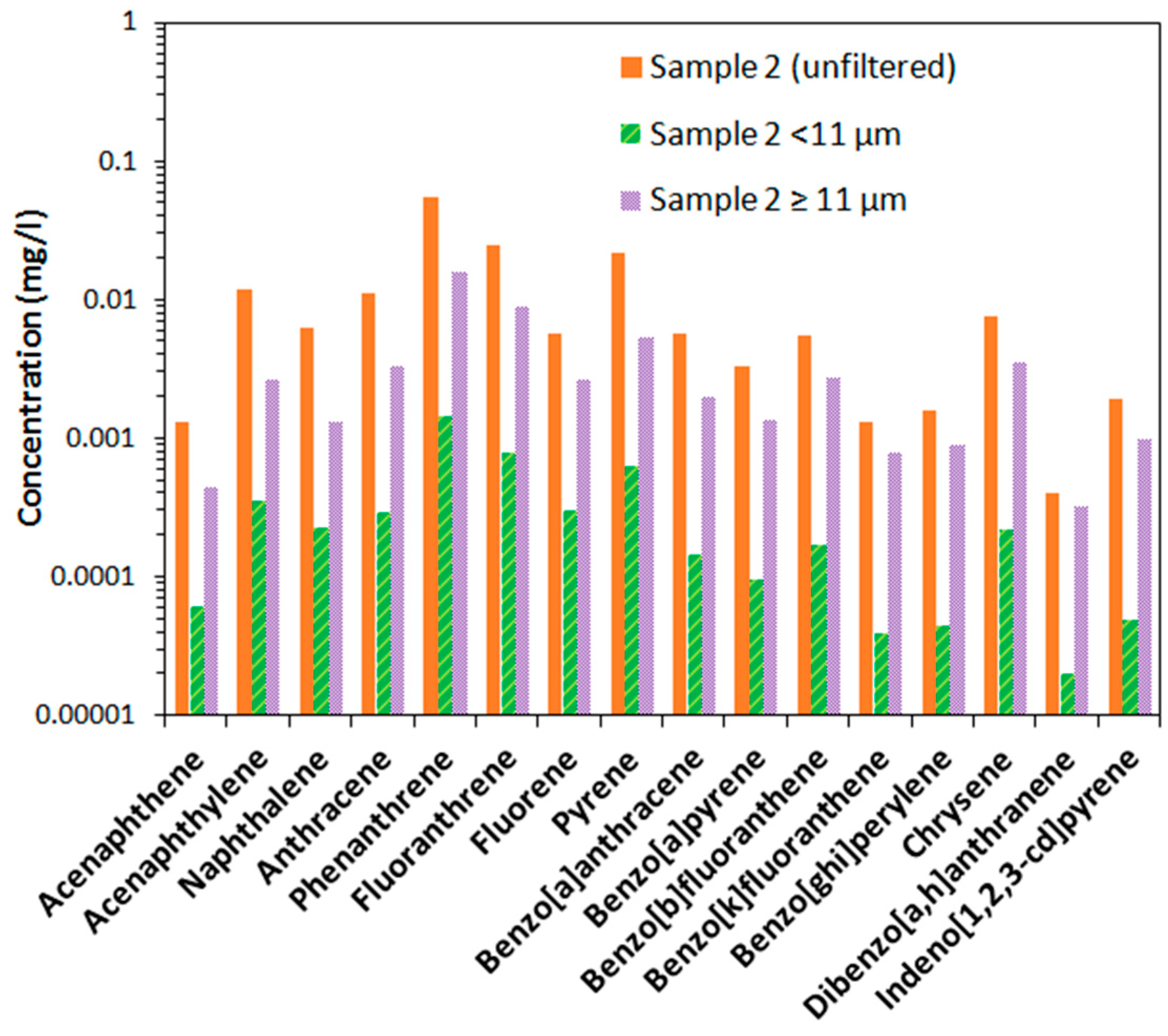
A critical factor for the mobility of particles in porous media is the size of the particles. It is therefore interesting to investigate how contaminants are distributed between different sizes of particles. This has also not previously been done for fire-generated particles in the extinguishing water. As shown in
Figure 3, the concentrations of PAHs were typically at least one order of magnitude lower in the particle solution filtered to sizes <11 μm compared to the solution of sizes greater than 11 μm and less than 100 μm. The difference was more pronounced for the high molecular weight (and carcinogenic) PAHs, which indicates that these may have a larger tendency to form larger particles or aggregates as compared to the lower molecular weight PAHs. The summed concentration of carcinogenic PAHs was, however, still slightly larger than the Swedish groundwater quality guideline value of 0.2 μg/L even for the particles of size <11 μm. Concentrations in the unfiltered sample were higher than in the <100 μm filtrate, indicating that particles or aggregates >100 μm existed in the original sample.
Figure 3.
Concentration of PAHs in filtered and unfiltered samples at location 2.
Figure 3.
Concentration of PAHs in filtered and unfiltered samples at location 2.
As shown in
Figure 4, six of the measured metals; As, Ba, Pb, Cd, Cr and Zn, had concentrations of at least one order of magnitude lower in the <11 μm filtrate compared to in particles between 11 μm and 100 μm. This indicates that these metals tend to be associated with larger particles, which may also be related to affinity of these metals to attach to larger soot particles. Four metals—Co, Cu, Ni and V—did not appear in significantly higher concentrations in the filtrate with larger particles. Co and Ni were even found in higher concentrations in the filtrate of smaller size particles (<11 μm). There appear to be a difference in characteristics between different metals, and the latter four may be less prone to aggregate. Contaminants found in smaller particles may also have larger mobility with water flowing through porous media, as will be further investigated in the following sections.
Figure 4.
Concentration of metals in filtered and unfiltered samples at location 2.
Figure 4.
Concentration of metals in filtered and unfiltered samples at location 2.
3.2. Physical Factors for Transport of Fire-Born Particles in Porous Media
A set of column experiments with FBPs filtered to sizes <100 μm and <11 μm was conducted. As can be seen in
Figure 5, the outflow concentrations from the column for samples filtered to less than 11 μm were close to their original (inflow) concentration. However, the outflow concentrations from the column for samples filtered to sizes less than 100 μm (note that this sample also contains particles <11 μm as opposed to in the chemical analyses) were much less than their original concentration. The steady state relative concentration of FBPs for finer sizes (<11 μm) was approximately 1, which means that the retention of these particles was negligible. It further indicates that attachment of FBPs did not occur at the natural chemical condition of the extinguishing water in combination with the selected physical condition (flow rate and porous media). However the results for FBPs <100 μm showed partial retention; at the same physical and chemical conditions, only 33% of the total particle concentration flowed through the column. The 77% retained particles in the column could not be mobilized even after changing the background solution to DI water (phase 3). It suggests that the particle larger than 11 μm were not deposited at the secondary energy minima locations. However, the result indicates that the selected porous media filtered out the larger particles in between 11 and 100 μm. Physical screening/straining was the likely retention mechanism in this scenario for FBPs, and other retention mechanisms should not have played any major role, as the remobilization of FBPs was negligible in phase 3 of the column experiment.
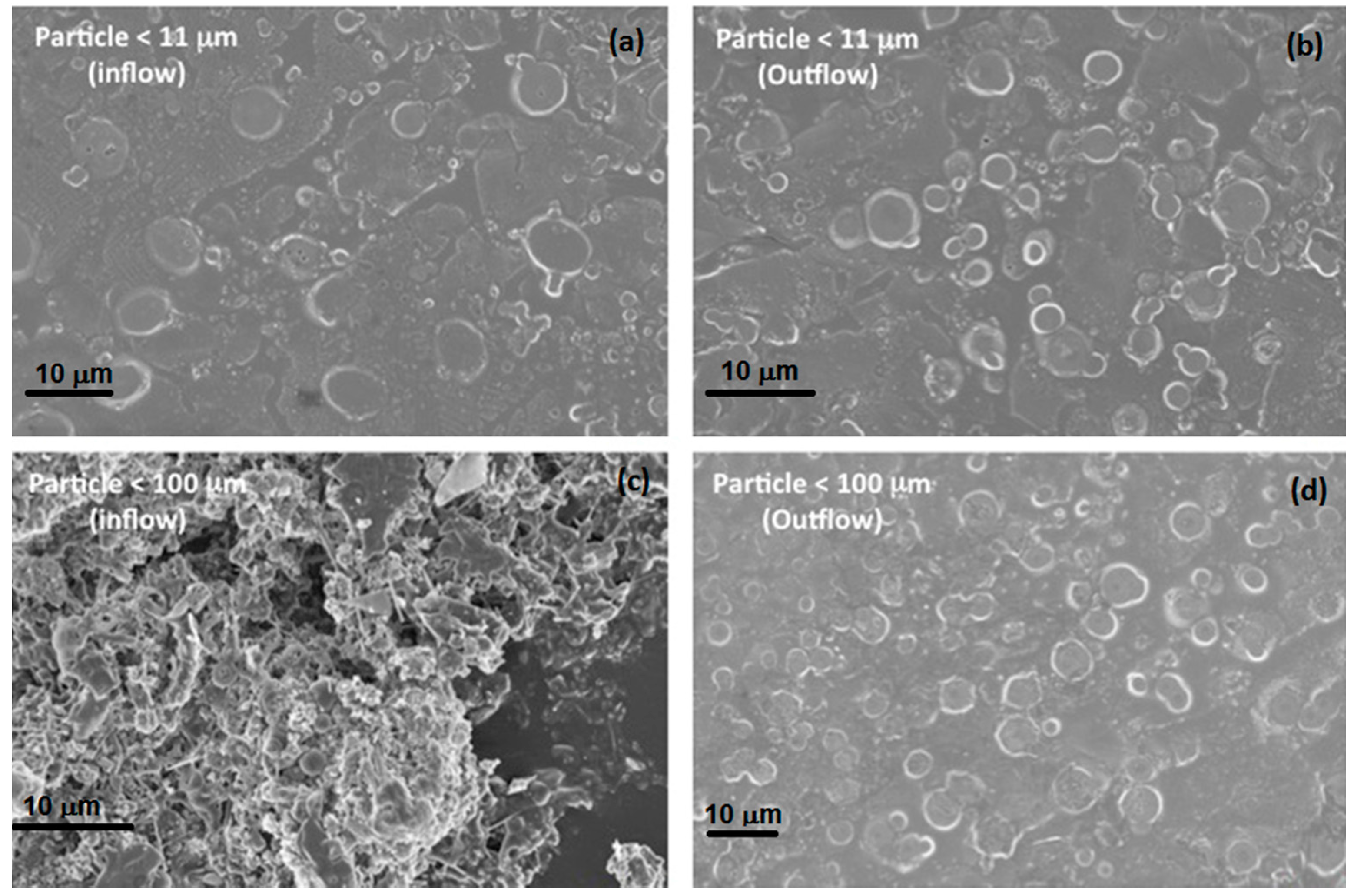
Figure 6 shows the SEM images of FBPs for inflow and outflow samples from the column for both size ranges (<11 μm and <100 μm). The particles <11 μm were nearly spherical in shape and show a similar distribution in images of inflow and outflow solution (
Figure 6a,b). This is in agreement with the column experiment results for this size range. However, the image of the inflow solution for particles <100 μm (
Figure 6c) was quite different (fibrous structure) from that of the outflow solution (
Figure 6d), where spherical shaped particles similar to the those in the sample filtered to sizes <11 μm can be observed. Thus, the larger fibrous kind of particles was filtered out during the flow through the porous media in the column. It can also be concluded that the FBPs <11 μm were highly mobile under the conditions tested here, posing a risk to reach the groundwater if they infiltrated into a subsurface system.
Figure 5.
Representative FBP breakthrough curves from column experiments using extinguishing water collected at site 2 after filtration using two different particle sizes.
Figure 5.
Representative FBP breakthrough curves from column experiments using extinguishing water collected at site 2 after filtration using two different particle sizes.
Column experiments for FBP transport through a wide range of porous media particle size distributions were conducted while keeping other physical and chemical conditions constant.
Figure 7 shows the column outflow breakthrough curves for four porous media sizes with average sizes of 450 μm, 325 μm, 213 μm, and 151 μm, respectively. The results indicate that the FBPs are completely mobile in the three largest porous media grain sizes, while in the smallest grain size a small portion of approximately 2%–3% of the FBPs were retained. A small peak of remobilization could be observed in phase 3 in these experiments (
Figure 7) but was not significant in terms of the total inflow mass of FBPs to the column. It can be concluded that even the average grain size of 151 μm (range 125–177 μm) was big enough to allow nearly full mobility of FBPs of sizes up to 11 μm. It can be noted that these FBPs were still more than one order of magnitude smaller than the sand grain size. This further confirms that the retention mechanism for larger FBPs is likely be straining, and no other retention mechanisms were operative for the present experimental scenarios.
Figure 6.
Scanning electron microscope images for fire-born particles from sampling site 2 for: (a) column inflow particle solution <11 μm; (b) column outflow particle solution <11 μm; (c) column inflow particle solution <100 μm; (d) column outflow particle solution <100 μm.
Figure 6.
Scanning electron microscope images for fire-born particles from sampling site 2 for: (a) column inflow particle solution <11 μm; (b) column outflow particle solution <11 μm; (c) column inflow particle solution <100 μm; (d) column outflow particle solution <100 μm.
Figure 7.
Representative FBP breakthrough curves from column experiments using fire extinguishing water collected at site 2 with four different porous media grain sizes.
Figure 7.
Representative FBP breakthrough curves from column experiments using fire extinguishing water collected at site 2 with four different porous media grain sizes.
3.3. Background Chemistry for Transport of Fire-Born Particles in Porous Media
The effect of background solution chemistry was also tested in this study. The ionic strength was not possible to vary in these fire extinguishing water samples because the salt concentration was very high (approximately 60 mM). The original pH of the fire extinguishing water was also high so the variation of solution chemistry is based on lowering the pH, as shown in
Table 1. The effects of pH change on FBP outflow concentrations are shown in
Figure 8. The results indicate that the relative concentration decreases with a decrease in solution pH. The relative concentration was close to 1.0, 0.92, and 0.88 for pH equal to 9.8, 6.5, and 3.0 respectively. An increase in retention of colloids/nanoparticles with a decrease in pH has been observed in several previous studies (such as, [
15,
16,
25]). The Derjaguin-Landau-Verwey-Overbeek (DLVO) interaction forces between FBPs and sand surfaces might change due to a decrease in pH. The measurements presented in
Table 1 show an increase in surface charges with a decrease in solution pH, which can favor particle retention at secondary energy minima. The surface charges are also in agreement with the absence of a secondary peak during phase 3 for pH 9.8 while small peaks were present for pH 6.5 and 3.0 (
Figure 8). These results indicate a partial retention of FBPs at very low pH, however, at pH representative of natural soil and groundwater (pH 6.5), the FBPs had nearly full mobility.
Figure 8.
Representative FBP breakthrough curves from column experiments using fire-extinguishing water collected at site 2 adjusted to three different pH.
Figure 8.
Representative FBP breakthrough curves from column experiments using fire-extinguishing water collected at site 2 adjusted to three different pH.
3.4. Mass Balance and Total Retention of Fire-Born Particles
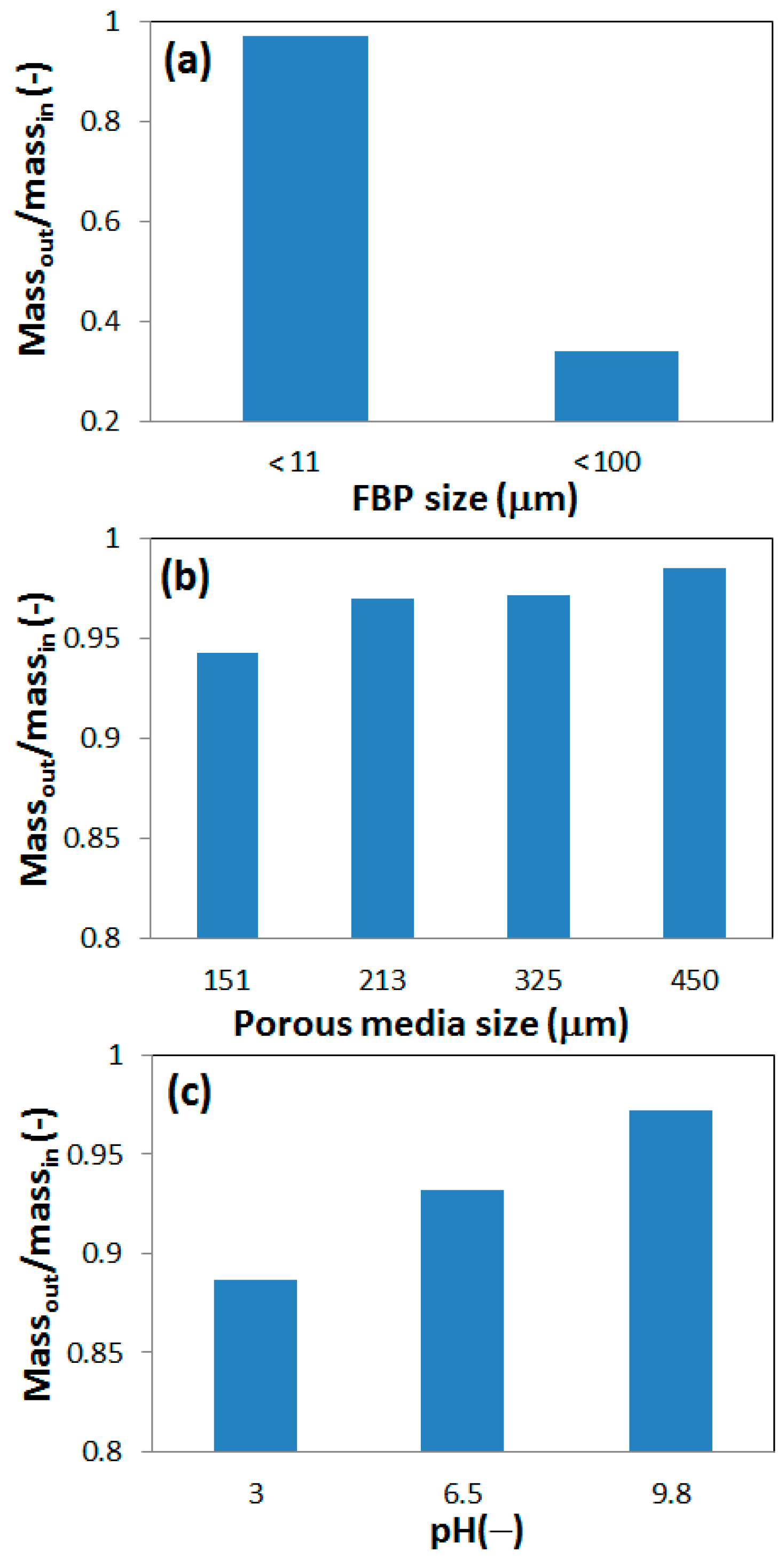
An integration of total particle mass was done to calculate the ratio of outflow mass to inflow mass.
Figure 9 shows the mass ratios for the different physical and chemical condition tested in this study. A high mass ratio indicates low retention of particles. The mass ratio was much higher for small size (<11 μm) FBPs than the larger (<100 μm) particles (
Figure 9a). It can also be noted that the actual outflow mass was in the same order for both cases (data not shown here), which indicates that most of the filtered/strained FBPs were in the range of 11 to 100 μm.
The mass ratio for phases 1 and 2 are plotted in
Figure 9b as a function of average grain size. Although the absolute differences in retention were relatively small, a clear trend of increase in outflow mass with increase in grain size could be observed (
Figure 9b). Physical straining was the likely retention mechanism.
Figure 9c shows the effect of pH on the outflow to inflow mass ratio. There is a clear trend of increase in outflow mass of FBPs with increase in pH. As discussed in the previous section, deposition at secondary energy minima is a possible retention mechanism at low pH, which was also supported by the observed remobilization of FBPs in phase 3.
From this study, we attempted to identify the presence of harmful contaminants (metals and PAH) in different sizes of fire burned particles from the selected location then we tried to represent the extreme pH condition for possible mobilization of these contaminant attached fire particles in porous media in order to warn the agencies involved in fire fighting operations (to save the ground- or surface-water pollution).
Figure 9.
Ratio of outflow to inflow mass of FBPs in column experiments for: (a) FBPs filtered to sizes <11 μm and <100 μm, respectively; (b) different porous media grain sizes; (c) different pH of solution.
Figure 9.
Ratio of outflow to inflow mass of FBPs in column experiments for: (a) FBPs filtered to sizes <11 μm and <100 μm, respectively; (b) different porous media grain sizes; (c) different pH of solution.