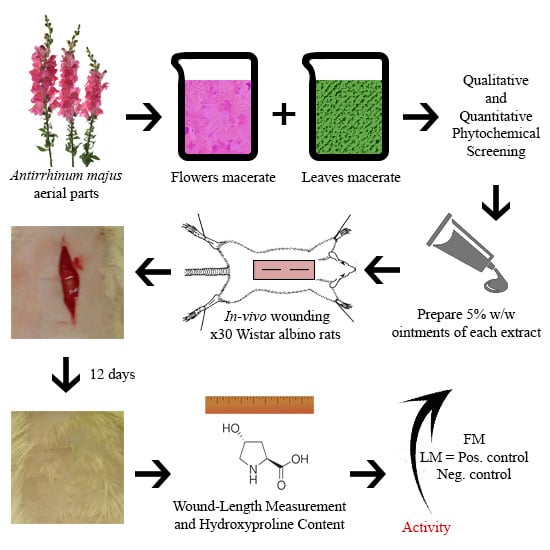
In Vivo Evaluation of Antirrhinum majus’ Wound-Healing Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection and Extraction
2.2. Fractionation
2.3. Phytochemical Screening
- a.
- Sodium hydroxide test: Treatment of 0.5 mL of the extract with aqueous sodium hydroxide forms a yellow-orange colour. Upon the addition of sulphuric acid, this color disappears, indicating flavonoids presence [18].
- b.
- Lead acetate test: A few drops of 10% lead acetate are to be mixed with alcoholic solution of the extract. Formation of a yellow precipitate points out the occurrence of flavonoids [19].
- c.
- Sodium hydroxide test: A small amount of alcoholic extract is dissolved in 1.0 mL water. Addition of sodium hydroxide solution produces a yellow color that specifies the presence of glycosides [19].
- d.
- Kellar Killani’s test: Extract is to be dissolved in water with the addition of glacial acetic acid and ferric chloride and concentrated sulphuric acid. The appearance of a reddish-brown ring at the junction points out the existence of glycosides [20].
- e.
- Froth test: Shake a slight amount of extract with 5.0 mL of water. Persistence of the foam produced for 10 min authorizes the presence of saponin [21].
- f.
- Hemolysis test: Add few drops of fresh-cut blood to 1.0 mL of extract in a test tube and mix gently. Allow the tube to stand for 15 min. Precipitation of red blood cells indicates hemolysis by saponin [22].
- g.
- Dragendroff’s test: Add a few drops of Dragendroff’s reagent to 1.0 mL of extract in a test tube. Appearance of orange colour indicates the presence of alkaloids [23].
- h.
- Picric acid test: Add a few drops of picric acid solution to 0.5 mL of extract. Formation of a yellow precipitate at the acid layer indicates the presence of alkaloids [24].
- i.
- Braemer’s test: Mix 0.5 mL of extract solution with 1% ferric chloride solution. Appearance of blue, green, or brownish green colour indicates tannins [24].
- j.
- Ferric chloride test: To 0.5 mL of extract solution add a few drops of 5% aqueous ferric chloride. Formation of deep blue to black color indicates the occurrence of phenols [25].
- k.
- Borntrager’s test: To 1.0 mL of chloroform extract add 0.5 mL of dilute (10%) ammonia. Formation of a pink-red colour in the ammonia (lower) layer directs the presence of anthraquinones [26].
- l.
- Liebermann-Burchardt test: Add 0.5 mL of chloroform, 1.0 mL of acetic anhydride, and 1 to 2 drops of concentrated sulphuric acid to 0.5 mL of methanolic extract in a test tube. Appearance of pink or red coloration points out terpenoids [18].
- m.
- Salkowski test: Add 0.5 mL of chloroform and 0.5 mL of concentrated sulphuric acid to 0.5 mL extract in a test tube. Formation of reddish brown colour of interface specifies terpenoids [25].
- n.
- Liebermann-Burchardt test: Add 0.5 mL of chloroform, 1.0 mL of acetic anhydride, and 1 to 2 drops of concentrated sulphuric acid to 0.5 mL of methanolic extract in a test tube. Appearance of dark green colouration points out steroids [18].
2.4. Free-Radical Scavenging Activity by 2,2′-Azino-Bis(3-Ethylbenzothiazoline-6-Sulphonic Acid Cation (ABTS+) Discoloration
2.5. Total Phenolic Content by Folin–Ciocalteu Reagent
2.6. Wound-Healing Activity
2.6.1. Ointments Preparation
2.6.2. Rats Wounding
2.6.3. Evaluation of Wound-Healing Activity
Visual Examination
Wound-Length Measurement
Estimation of Hydroxyproline Content
2.6.4. Statistical Analysis
3. Results
3.1. Fractionation
3.2. Phytochemical Screening
3.3. Free-Radical Scavenging Activity by ABTS+ Discolouration
3.4. Total Phenolic Content by Folin-Ciocalteu Reagent
3.5. Wound-Healing Activity
3.5.1. Visual Examination
3.5.2. Wound-Length Measurement
3.5.3. Estimation of Hydroxyproline Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Totelin, L. Hippocratic recipes; oral and written transmission of pharmacological knowledge in fifth- and fourth-century greece. In Studies in Ancient Medicine; Scaborough, J., Van der EiJk, P., Hanson, A., Siraisi, N., Eds.; Brill Academic Publishers: Leiden, The Netherlands; Boston, MA, USA, 2009; p. 34. [Google Scholar]
- Yaniv, Z.; Dudai, N. Medicinal and Aromatic Plants of the Middle-East; Springer: Dordrecht, The Netherlands, 2014; Volume 2, pp. 2–7. [Google Scholar]
- Mani, R.; Romanelli, M.; Shukla, V. Measurements in Wound Healing: Science and Practice; Springer: London, UK, 2012; pp. 73–78. [Google Scholar]
- Budovsky, A.; Yarmolinsky, L.; Ben-Shabat, S. Effect of medicinal plants on wound healing. Wound Repair Regen. 2015, 23, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Gaba, A. Phyto-extracts in wound healing. J. Pharm. Pharm. Sci. 2013, 16, 760–820. [Google Scholar] [CrossRef] [PubMed]
- Olmstead, R.; Reeves, P. Evidence for the polyphyly of the scrophulariaceae based on chloroplast rbcl and ndhf sequences. Ann. Mo. Bot. Gard. 1995, 82, 176–193. [Google Scholar] [CrossRef]
- Tank, D.; Beardsley, P.; Kelchner, S.; Olmstead, R. Review of the systematics of Scrophulariaceae s.l. and their current disposition. Aust. Syst. Bot. 2006, 19, 289–307. [Google Scholar] [CrossRef]
- Hudson, A.; Critchley, J.; Erasmus, Y. The genus antirrhinum (snapdragon): A flowering plant model for evolution and development. CSH Protoc. 2008, 2008, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lim, T. Antirrhinum majus. In Edible Medicinal and Non Medicinal Plants; Springer: Dordrecht, The Netherlands, 2014; Volume 8, pp. 633–639. [Google Scholar]
- Taifour, H.; El-Oqlah, A. Jordan Plant Red List; International Union for Conservation of Nature (IUCN): Gland, Switzerland, 2014; Volume 1, p. 95. [Google Scholar]
- Al-Snafi, A. The pharmacological importance of Antirrhinum majus—A review. Asian J. Pharm. Sci. Technol. 2015, 5, 313–320. [Google Scholar]
- Harkiss, K. Studies in the scrophulariaceae—Part III investigation of the occurrence of alkaloids in antirrhinum majus L. Planta Med. 1971, 20, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.; Marshall, G.; Bradley, J.; Schwinn, K.; Bloor, S.; Winefield, C.; Martin, C. Characterisation of aurone biosynthesis in Antirrhinum majus. Physiol. Plant. 2006, 128, 593–603. [Google Scholar] [CrossRef]
- Grotewold, E. The Science of Flavonoids; Springer: New York, NY, USA, 2006; pp. 1–5, 147–155, 213–217. [Google Scholar]
- Franzyk, H.; Frederiksen, S.; Jensen, S. Synthesis of antirrhinolide, a new lactone from Antirrhinum majus. Eur. J. Organ. Chem. 1998, 1998, 1665–1667. [Google Scholar] [CrossRef]
- Hogedal, B.; Molgaard, P. HPLC analysis of the seasonal and diurnal variation of iridoids in cultivars of Antirrhinum majus. Biochem. Syst. Ecol. 2000, 28, 949–962. [Google Scholar] [CrossRef]
- Ramadan, M.; El-Shamy, H. Snapdragon (Antirrhinum majus) seed oil: Characterization of fatty acids, bioactive lipids and radical scavenging potential. Ind. Crop. Prod. 2013, 42, 373–379. [Google Scholar] [CrossRef]
- Alabri, T.; Al Musalami, A.; Hossain, M.; Weli, A.; Al-Riyami, Q. Comparative study of phytochemical screening, antioxidant and antimicrobial capacities of fresh and dry leaves crude plant extracts of Datura metel L. J. King Saud Univ. Sci. 2014, 26, 237–243. [Google Scholar] [CrossRef]
- Geetha, T.; Geetha, N. Phytochemical screening, quantitative analysis of primary and secondary metabolites of Cymbopogan citratus (DC) stapf. leaves from kodaikanal hills, tamilnadu. Int. J. PharmTech Res. 2014, 6, 521–529. [Google Scholar]
- Akinyemi, K.; Oladapo, O.; Okwara, C.; Ibe, C.; Fasure, K. Screening of crude extracts of six medicinal plants used in south-west nigerian unorthodox medicine for anti-methicillin resistant Staphylococcus aureus activity. BMC Complement. Altern. Med. 2005, 5, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.; AL-Raqmi, K.; AL-Mijizy, Z.; Weli, A.; Al-Riyami, Q. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown thymus vulgaris. Asian Pac. J. Trop. Biomed. 2013, 3, 705–710. [Google Scholar] [CrossRef]
- Yusuf, A.; Zakir, A.; Shemau, Z.; Abdullahi, M.; Halima, S. Phytochemical analysis of the methanol leaves extract of Paullinia pinnata L. J. Pharm. Phytother. 2014, 6, 10–16. [Google Scholar]
- Abdul-Wahab, F.; Jalil, T. Study of iraqi spinach leaves (phytochemical and protective effects against methotrexate-induced hepatotoxicity in rats). Iraqi J. Pharm. Sci. 2017, 21, 8–17. [Google Scholar]
- Yadav, R.; Khare, R.; Singhal, A. Qualitative phytochemical screening of some selected medicinal plants of shivpuri district (mp). Int. J. Life Sci. Sci. Res. 2017, 3, 844–847. [Google Scholar]
- Khanam, Z.; Wen, C.; Bhat, I. Phytochemical screening and antimicrobial activity of root and stem extracts of wild Eurycoma longifolia jack (tongkat ali). J. King Saud Univ. Sci. 2015, 27, 23–30. [Google Scholar] [CrossRef]
- Yogeshwari, C.; Kalaichelvi, K. Comparitive phytochemical screening of acmella calva (dc.) rk jansen and crotalaria ovalifolia wall: Potential medicinal herbs. J. Med. Plant. 2017, 5, 277–279. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Lahouar, L.; El Arem, A.; Ghrairi, F.; Chahdoura, H.; Salem, H.; El Felah, M.; Achour, L. Phytochemical content and antioxidant properties of diverse varieties of whole barley (hordeum vulgare L.) grown in tunisia. Food Chem. 2014, 145, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Cercato, L.; de Santana Souza, M.; de Oliveira Melo, A.; dos Santos Lima, B.; Duarte, M.C.; de Souza Araujo, A.; e Silva, A.; Camargo, E. The ethanol extract of Leonurus sibiricus L. induces antioxidant, antinociceptive and topical anti-inflammatory effects. J. Ethnopharmacol. 2017, 206, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Nakagawa, T.; Kishikawa, A.; Ohnuki, K.; Shimizu, K. In vitro bioactivities and phytochemical profile of various parts of the strawberry (fragaria × ananassa var. Amaou). J. Funct. Foods 2015, 13, 38–49. [Google Scholar] [CrossRef]
- Wintola, O.; Afolayan, A. Chemical constituents and biological activities of essential oils of Hydnora africana thumb used to treat associated infections and diseases in south africa. Appl. Sci. 2017, 7, 443. [Google Scholar] [CrossRef]
- Molan, A.; Flanagan, J.; Wei, W.; Moughan, P. Selenium-containing green tea has higher antioxidant and prebiotic activities than regular green tea. Food Chem. 2009, 114, 829–835. [Google Scholar] [CrossRef]
- Molan, A.; Yousif, A.; Al-Bayati, N. Total phenolic contents and antiradical activities of pomaces and their ingredients of two iraqi date (Phoenix dactylifera L.) cultivars. World J. Pharm. Pharm. Sci. 2017, 6, 167–180. [Google Scholar]
- Ritskes-Hoitinga, J.; Jilge, B. Felasa—Quick Reference Paper on Laboratory Animal Feeding and Nutrition; AAALAC: Frederick, MD, USA, 2001; pp. 1–11. [Google Scholar]
- Li, H.; Deng, Y.; Zhang, Z.; Fu, Q.; Zheng, Y.; Cao, X.; Nie, J.; Fu, L.; Chen, L.; Xiong, Y.; et al. Evaluation of effectiveness in a novel wound healing ointment-crocodile oil burn ointment. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Ang, E.; Lee, S.; Gan, C.; See, P.; Chan, Y.; Ng, L.; Machin, D. The role of alternative therapy in the management of partial thickness burns of the face—Experience with the use of moist exposed burn ointment (mebo) compared with silver sulphadiazine. Ann. Acad. Med. Singap. 2000, 29, 7–10. [Google Scholar] [PubMed]
- Jewo, P.; Fadeyibi, I.; Babalola, O.; Saalu, L.; Benebo, A.; Izegbu, M.; Ashiru, O. A comparative study of the wound healing properties of moist exposed burn ointment (mebo) and silver sulphadiazine. Ann. Burn. Fire Disasters 2009, 22, 79–82. [Google Scholar]
- Lodhi, S.; Singhai, A. Preliminary pharmacological evaluation of Martynia annua linn leaves for wound healing. Asian Pac. J. Trop. Biomed. 2011, 1, 421–427. [Google Scholar] [CrossRef]
- Nowland, M. Guidelines on Anesthesia and Analgesia in Rats. Available online: https://az.research.umich.edu/animalcare/guidelines/guidelines-anesthesia-and-analgesia-rats (accessed on 6 August 2017).
- Asmis, R.; Qiao, M.; Zhao, Q. Low flow oxygenation of full-excisional skin wounds on diabetic mice improves wound healing by accelerating wound closure and reepithelialization. Int. Wound J. 2010, 7, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Torkaman, G. Electrical stimulation of wound healing: A review of animal experimental evidence. Adv. Wound Care 2014, 3, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Garg, A.; Sharma, P.; Pandey, P. Wound healing and antioxidant effect of Calliandra haematocephala leaves on incision and excision wound models. Asian J. Pharm. Pharmacol. 2016, 2, 34–39. [Google Scholar]
- Siavash, M.; Shokri, S.; Haghighi, S.; Shahtalebi, M.; Farajzadehgan, Z. The efficacy of topical royal jelly on healing of diabetic foot ulcers: A double-blind placebo-controlled clinical trial. Int. Wound J. 2015, 12, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Woessner, J. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch. Biochem. Biophys. 1961, 93, 440–447. [Google Scholar] [CrossRef]
- Dwivedi, D.; Dwivedi, M.; Malviya, S.; Singh, V. Evaluation of wound healing, anti-microbial and antioxidant potential of Pongamia pinnata in wistar rats. J. Tradit. Complement. Med. 2017, 7, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.; O’brien, W. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Int. J. Clin. Chem. Diagn. Lab. Med. 1980, 104, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Jorge, M.; Madjarof, C.; Ruiz, A.; Fernandes, A.; Rodrigues, R.; de Oliveira Sousa, I.; Foglio, M.; de Carvalho, J. Evaluation of wound healing properties of Arrabidaea chica verlot extract. J. Ethnopharmacol. 2008, 118, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Gawronska-Kozak, B.; Bogacki, M.; Rim, J.S.; Monroe, W.; Manuel, J. Scarless skin repair in immunodeficient mice. Wound Repair Regen. 2006, 14, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Ahadi, A.; Partoazar, A.; Abedi-Khorasgani, M.; Shetab-Boushehri, S. Comparison of liquid-liquid extraction-thin layer chromatography with solid-phase extraction-high-performance thin layer chromatography in detection of urinary morphine. J. Biomed. Res. 2011, 25, 362–367. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, E.; Gillespie, K. Estimation of total phenolic content and other oxidation substrates in plant tissues using folin-ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Ma, H.; Fan, P.; Gao, R.; Jia, Z. Antioxidant potential, total phenolic and total flavonoid contents of Rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in pc12 cells. BMC Complement. Altern. Med. 2015, 15, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, A.; Florim, J.; Rodrigues, H.; Andrade-Oliveira, V.; Teixeira, S.; Vitzel, K.; Curi, R.; Saraiva Câmara, N.; Muscará, M.; Lamers, M.; et al. Oral administration of antioxidants improves skin wound healing in diabetic mice. Wound Repair Regen. 2016, 24, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Bardaa, S.; Halima, N.; Aloui, F.; Mansour, R.; Jabeur, H.; Bouaziz, M.; Sahnoun, Z. Oil from pumpkin (Cucurbita pepo L.) seeds: Evaluation of its functional properties on wound healing in rats. Lipids Health Dis. 2016, 15, 73–84. [Google Scholar] [CrossRef] [PubMed]









| Ointment Type | Active Ingredient Type | Active Ingredient | Glycerol (44.47%) | White Petrolatum (55.53%) | Final Product |
|---|---|---|---|---|---|
| Leaves macerate (LM) | LM extract | 2.5 g | 21.13 g | 26.38 g | 50 g |
| Flowers macerate (FM) | FM extract | 2.5 g | 21.13 g | 26.38 g | 50 g |
| Positive | MEBO | 0.75 g | 21.90 g | 27.35 g | 50 g |
| Negative | Nothing | 0.0 g | 22.24 g | 27.77 g | 50 g |
| Extract | n-Hexane (mg/g) | Dichloromethane (mg/g) | Ethyl Acetate (mg/g) | n-Butanol (mg/g) |
|---|---|---|---|---|
| LM | 199.55 | 13.39 | 22.32 | 306.47 |
| FM | − | − | 27.40 | − |
| Test | LM | FM | |
|---|---|---|---|
| Flavonoids | Sodium hydroxide | + | + |
| Lead acetate | + | + | |
| Glycosides | Sodium hydroxide | + | + |
| Kellar killani | + | + | |
| Saponin | Froth | − | − |
| Haemolysis | − | − | |
| Alkaloids | Dragendroff’s | + | + |
| Hager’s | + | + | |
| Tannins | Braemer’s | + | + |
| Phenols | Ferric chloride | + | + |
| Anthraquinones | Borntrager’s | + | + |
| Terpenoids | Liebermann–Burchardt | + | + |
| Salkowski | + | + | |
| Steroids | Liebermann–Burchardt | + | + |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saqallah, F.G.; Hamed, W.M.; Talib, W.H. In Vivo Evaluation of Antirrhinum majus’ Wound-Healing Activity. Sci. Pharm. 2018, 86, 45. https://doi.org/10.3390/scipharm86040045
Saqallah FG, Hamed WM, Talib WH. In Vivo Evaluation of Antirrhinum majus’ Wound-Healing Activity. Scientia Pharmaceutica. 2018; 86(4):45. https://doi.org/10.3390/scipharm86040045
Chicago/Turabian StyleSaqallah, Fadi G., Wafaa M. Hamed, and Wamidh H. Talib. 2018. "In Vivo Evaluation of Antirrhinum majus’ Wound-Healing Activity" Scientia Pharmaceutica 86, no. 4: 45. https://doi.org/10.3390/scipharm86040045
APA StyleSaqallah, F. G., Hamed, W. M., & Talib, W. H. (2018). In Vivo Evaluation of Antirrhinum majus’ Wound-Healing Activity. Scientia Pharmaceutica, 86(4), 45. https://doi.org/10.3390/scipharm86040045