Thiopyrano[2,3-d]Thiazoles as New Efficient Scaffolds in Medicinal Chemistry
Abstract
:1. Introduction
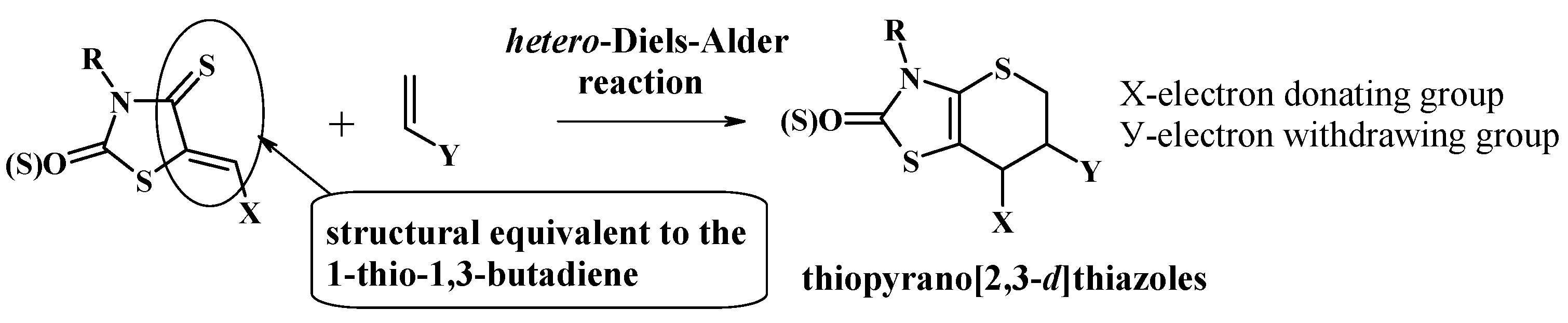
2. Hetero-Diels-Alder Reaction as a Key Approach for the Synthesis of Thiopyrano[2,3-d]Thiazole Derivatives
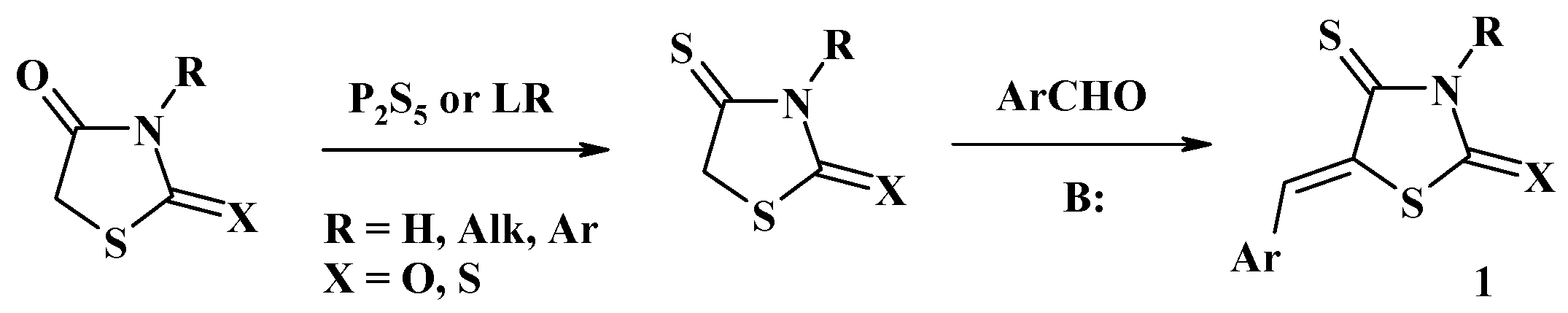
2.1. Utilization of 5-Arylidene-4-Thiazolidinethiones in the Hetero-Diels–Alder Reactions
2.2. Usage of 5-Alkylidene-4-Thiazolidinethiones in the Synthesis of Thiopyrano[2,3-d]Thiazole Core
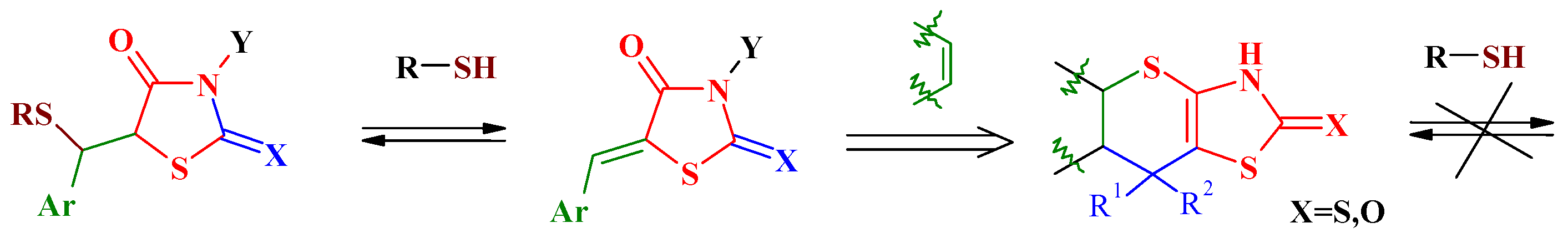
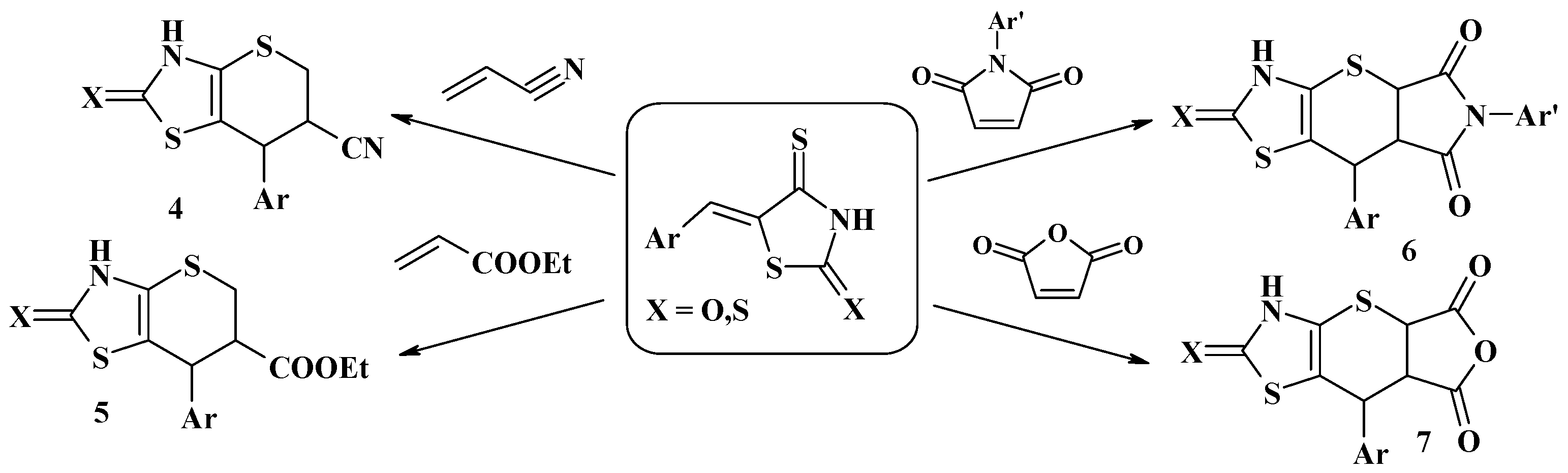
3. The Michael Reaction and Related Processes in the Synthesis of Thiopyrano[2,3-d]Thiazoles
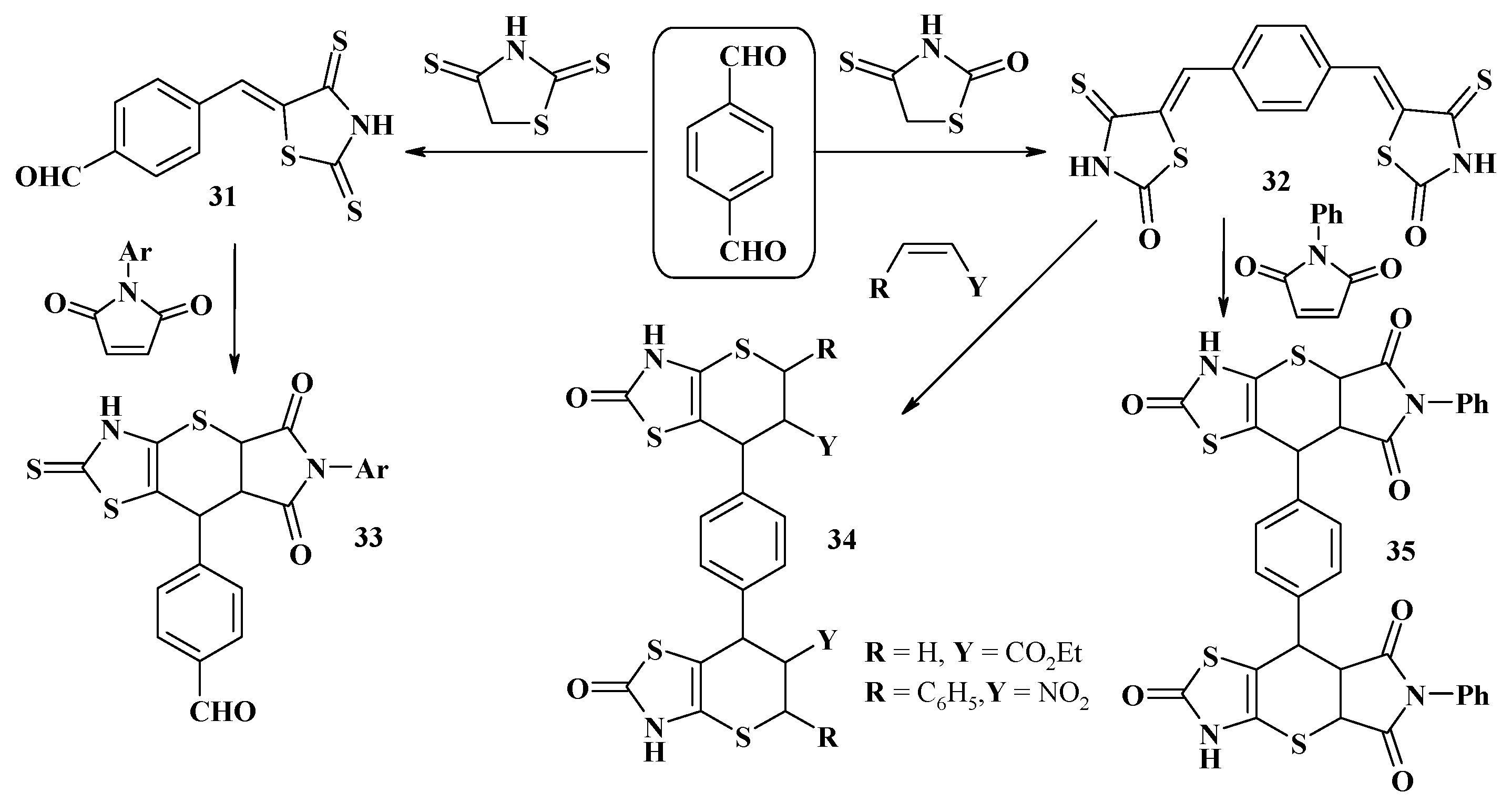
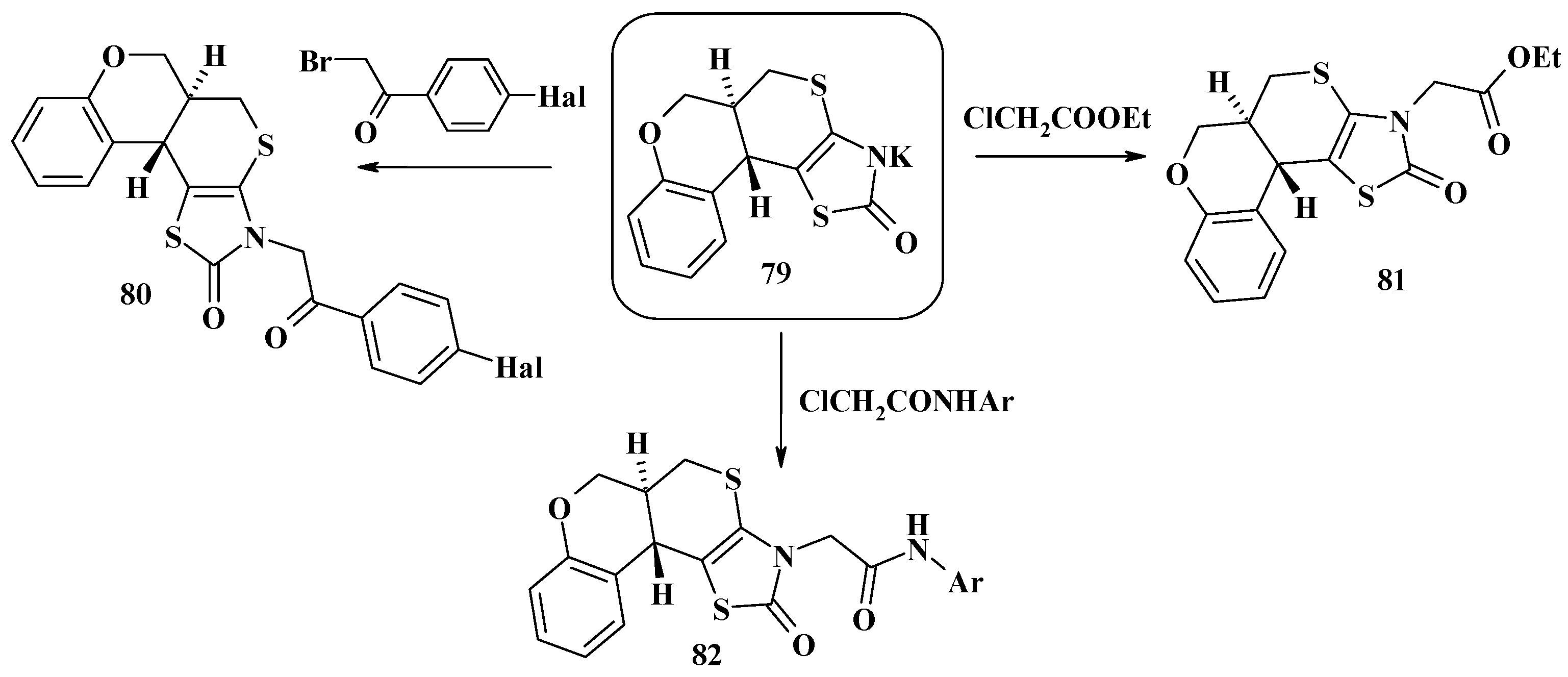
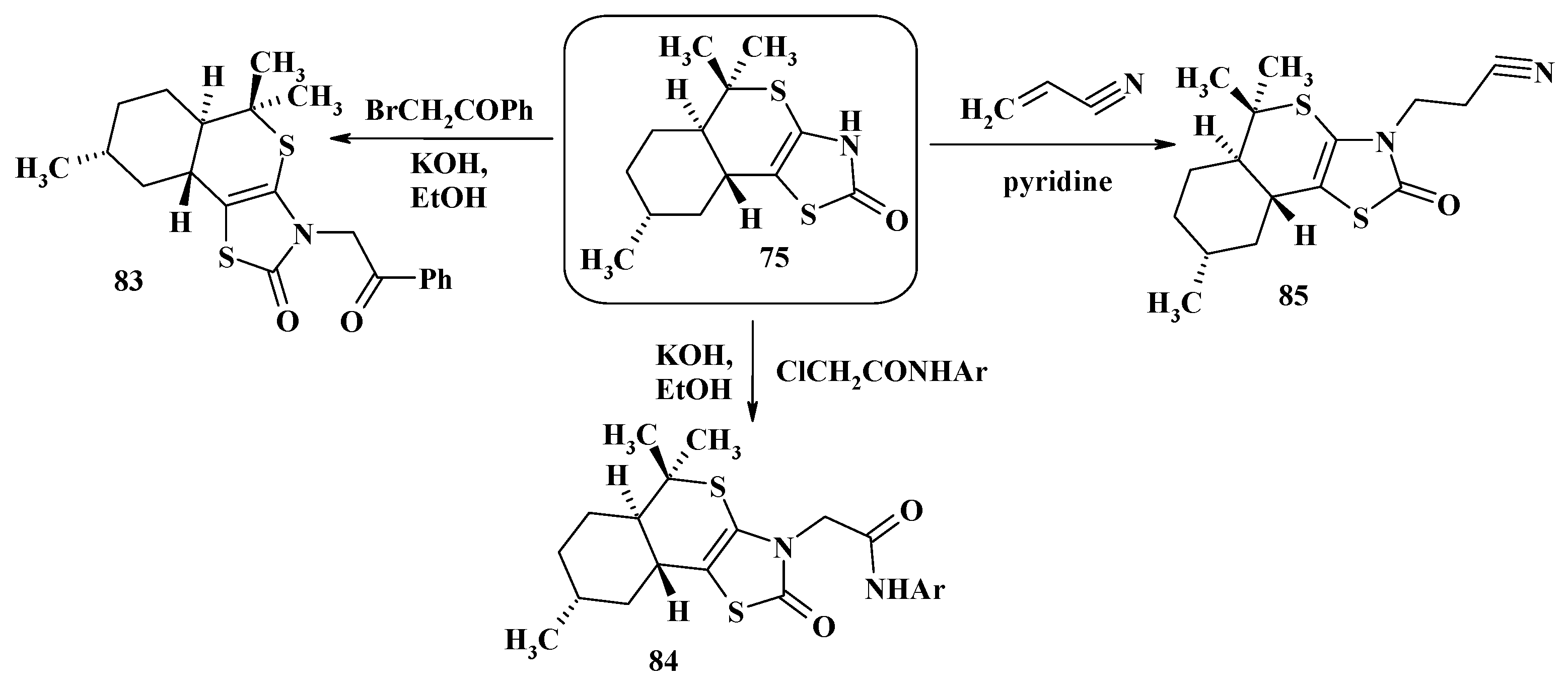
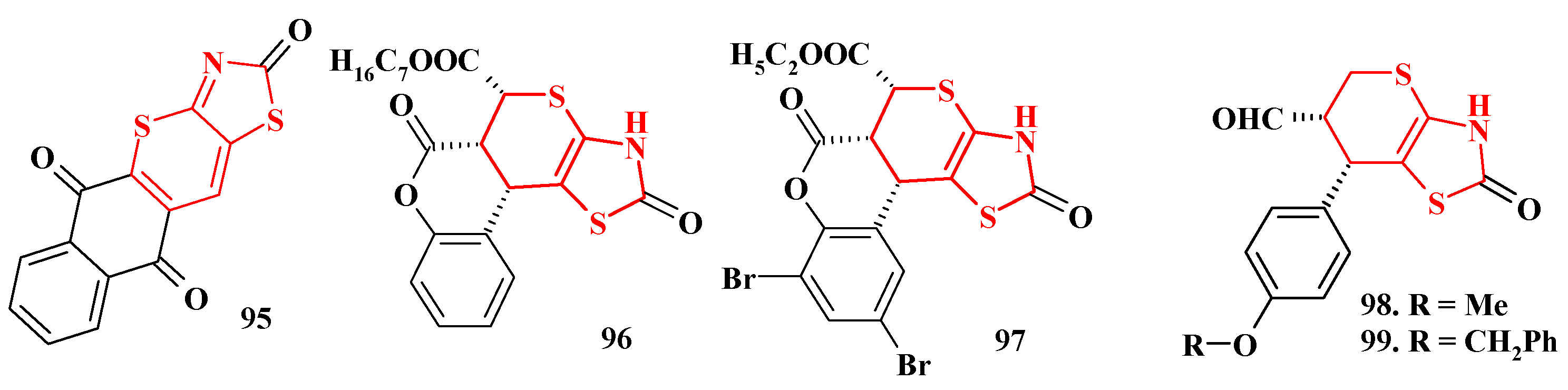
4. Synthesis of Polycondensed Thiopyrano[2,3-d]Thiazole Derivatives as Potentially Biological Active Compounds
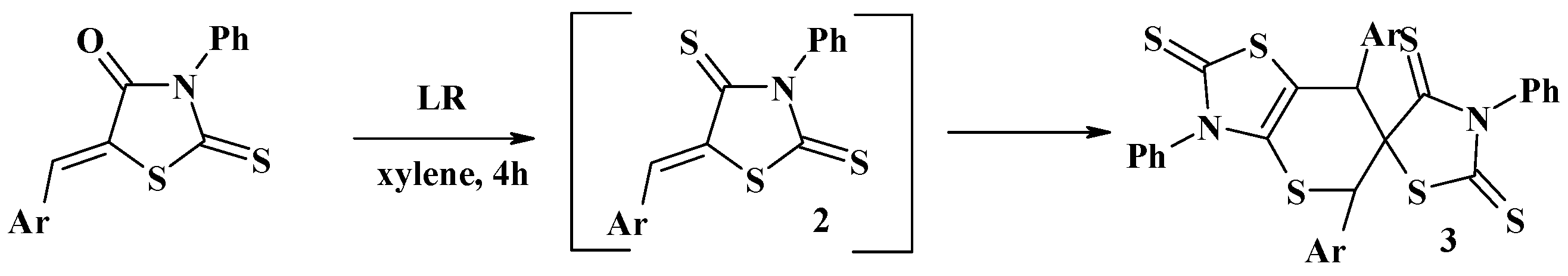
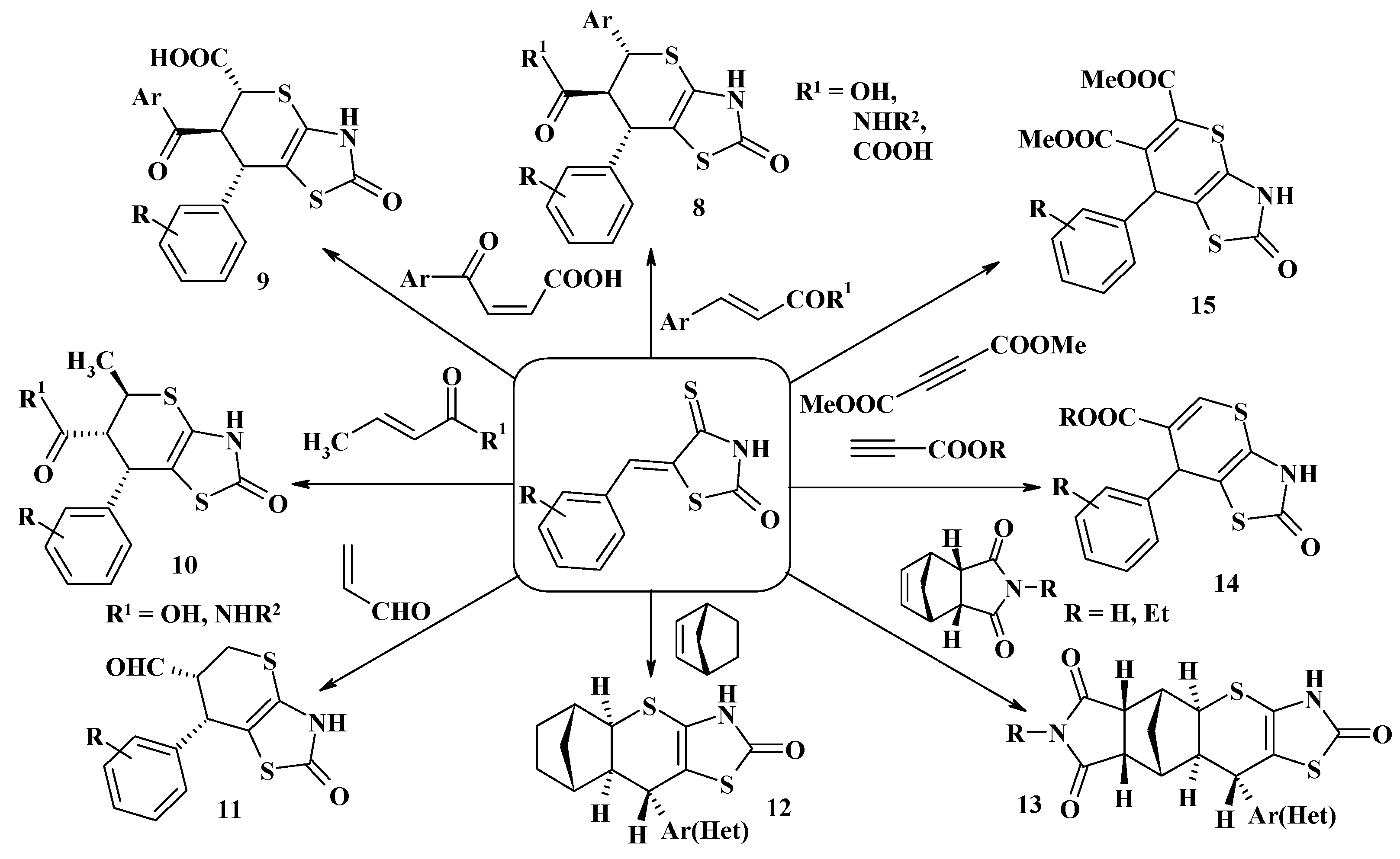
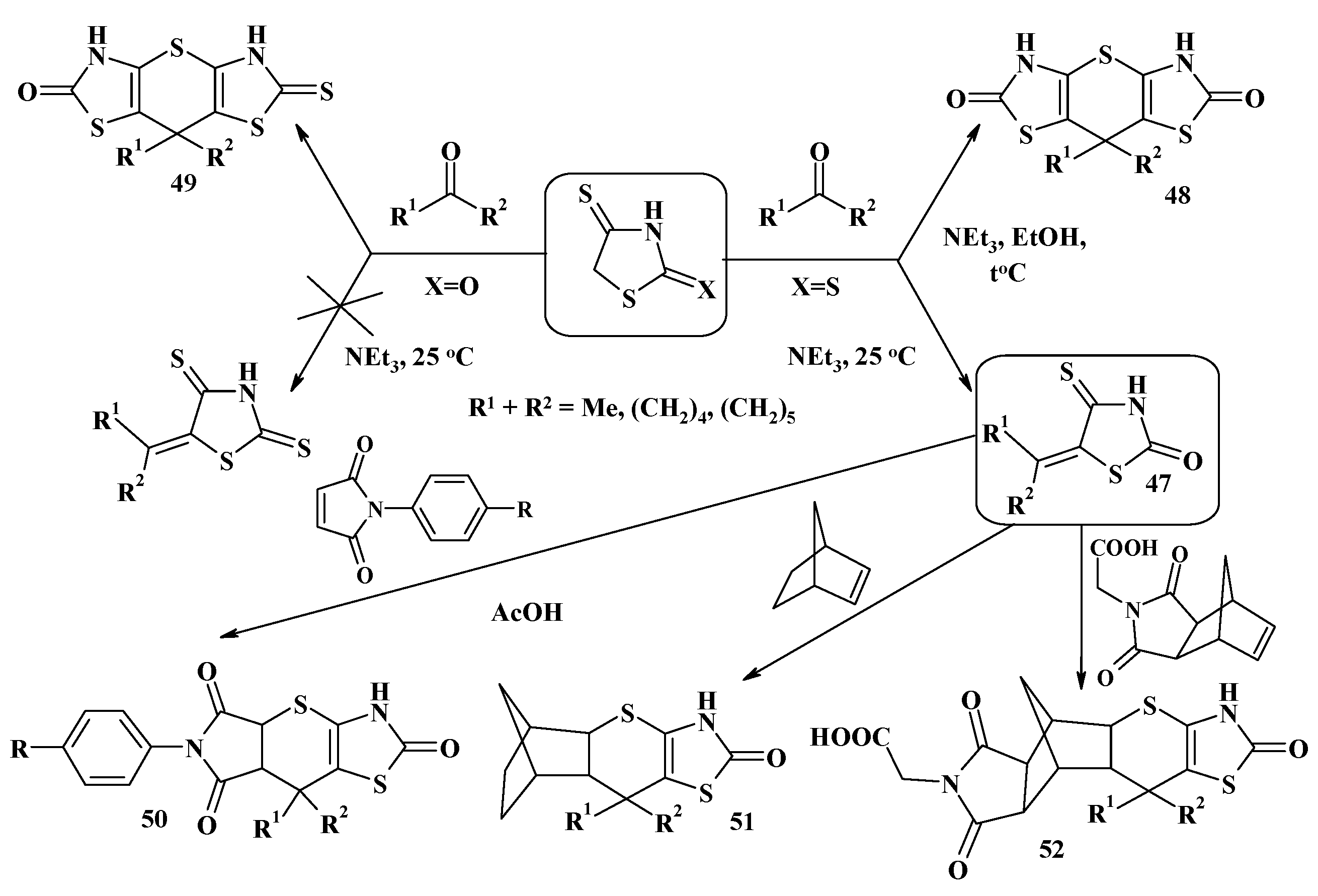
4.1. Peculiarities of the Tandem Reactions in the Synthesis of Polycondensed Thiopyrano[2,3-d]Thiazoles
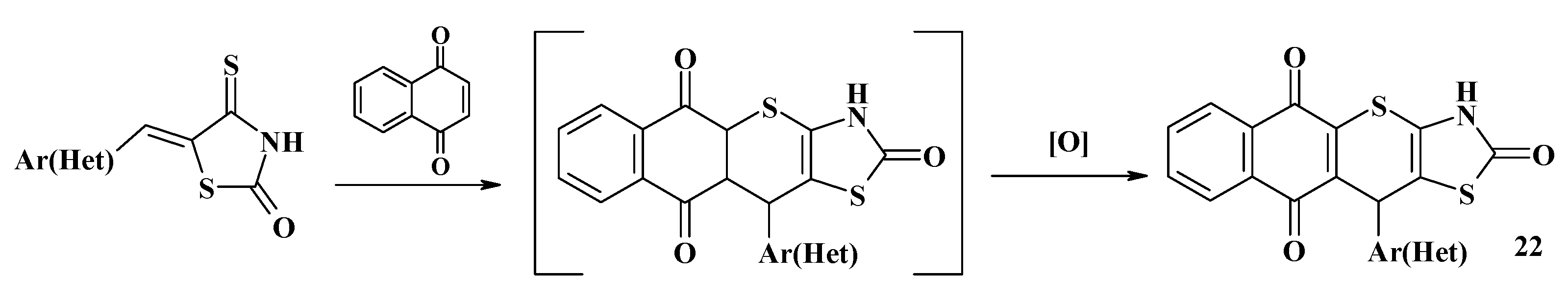
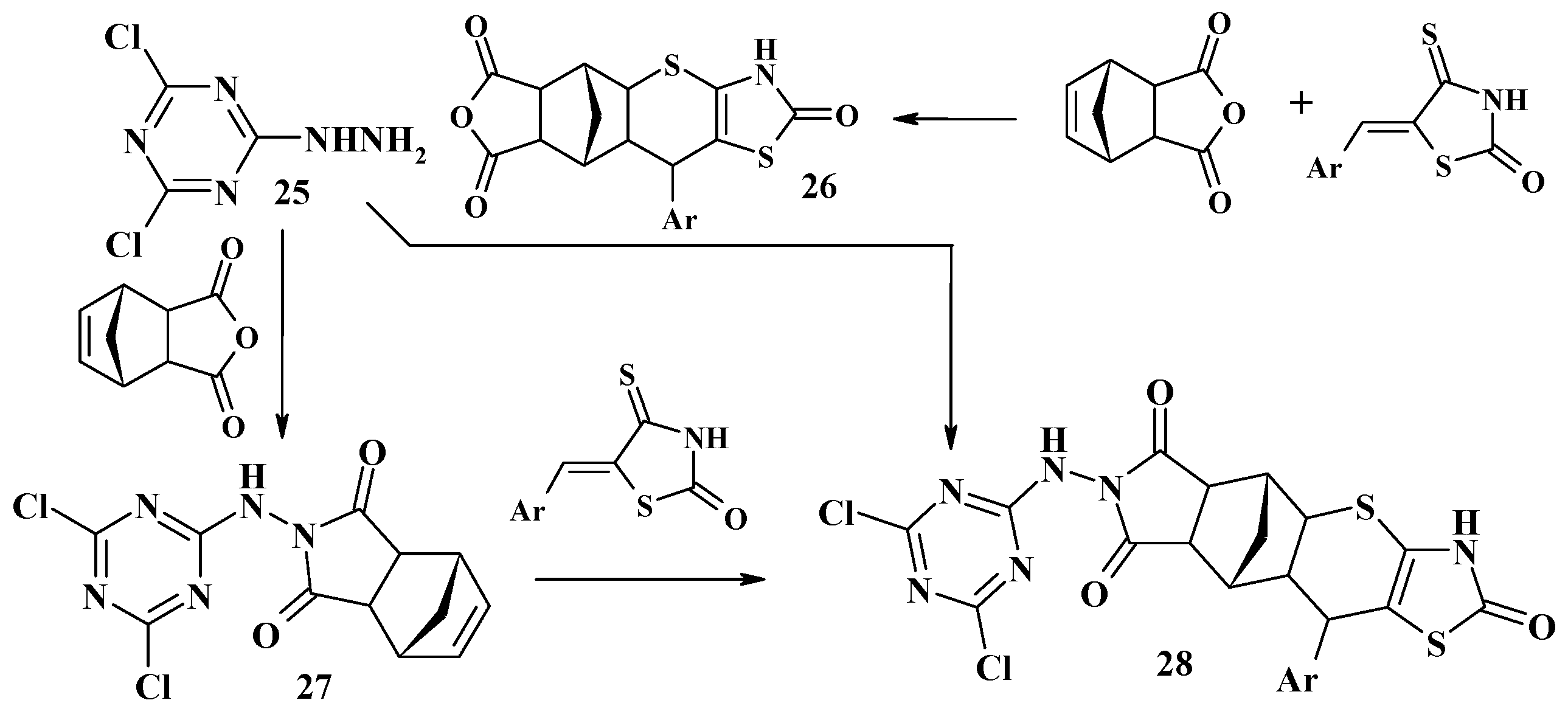
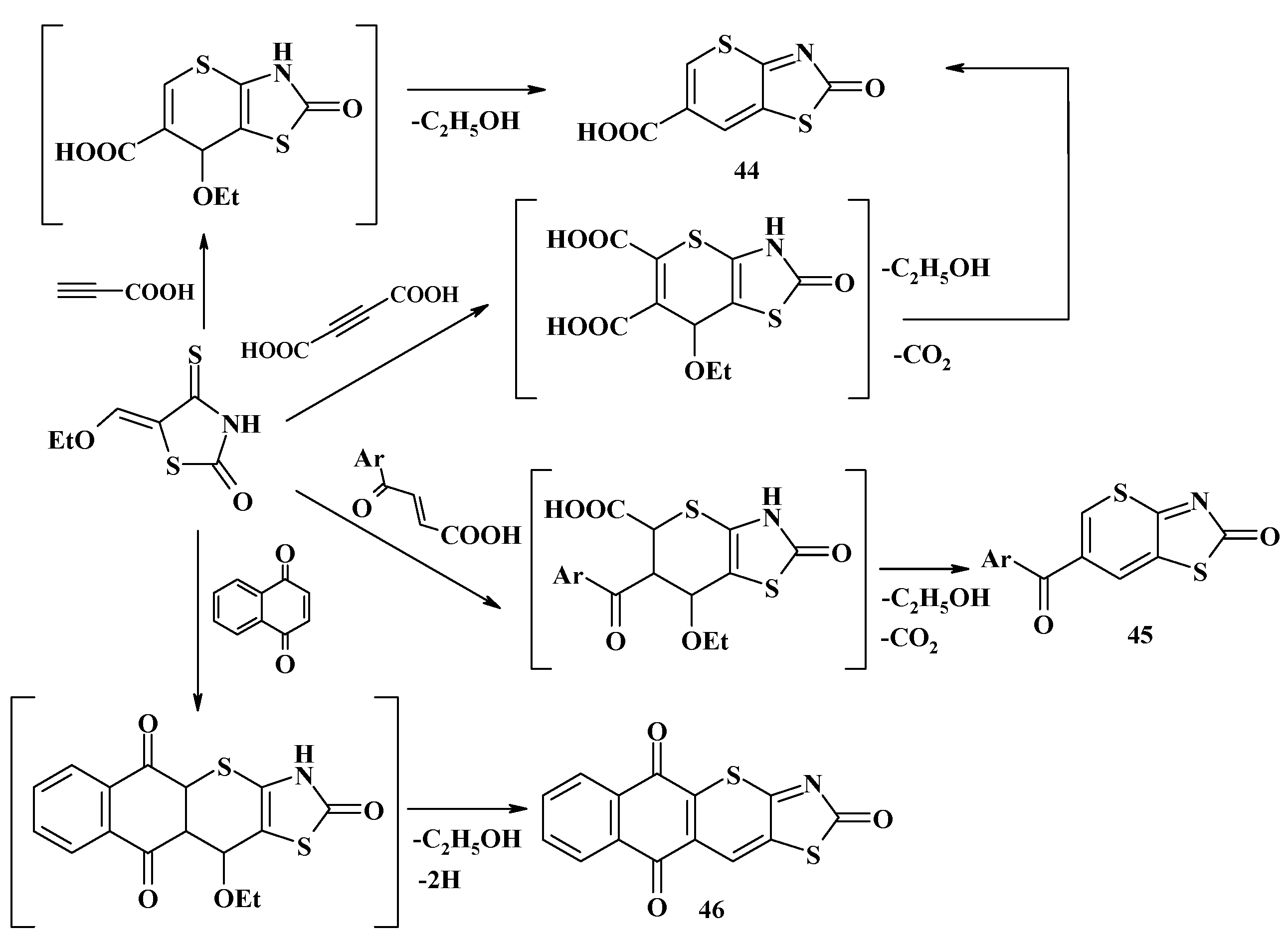
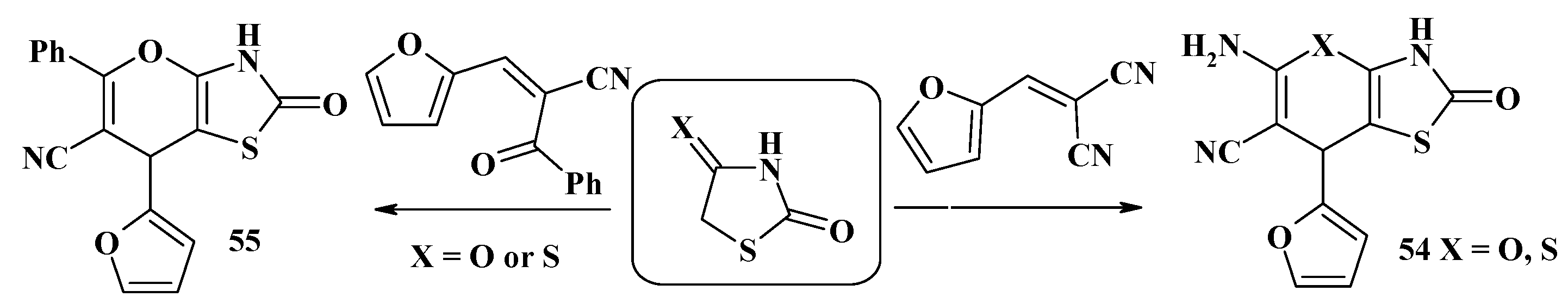
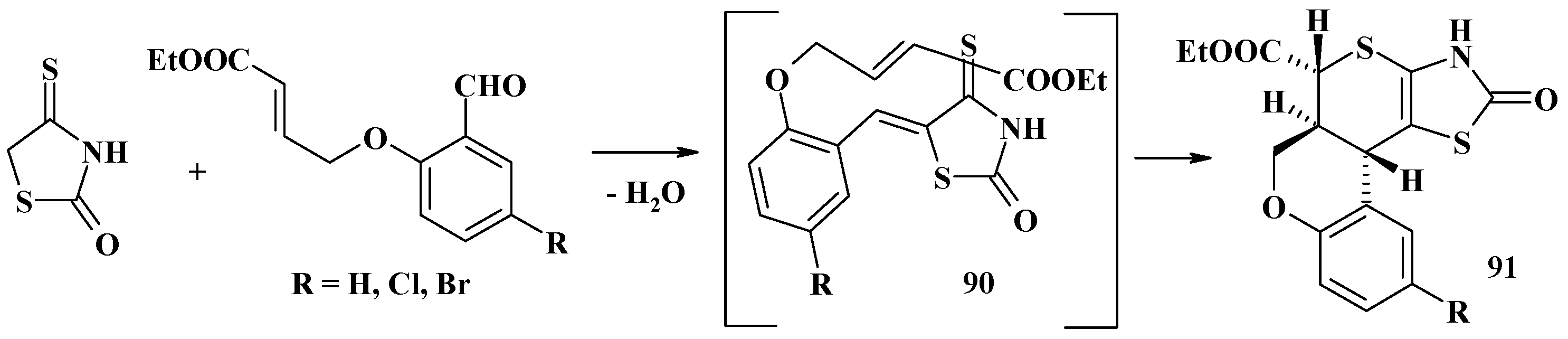
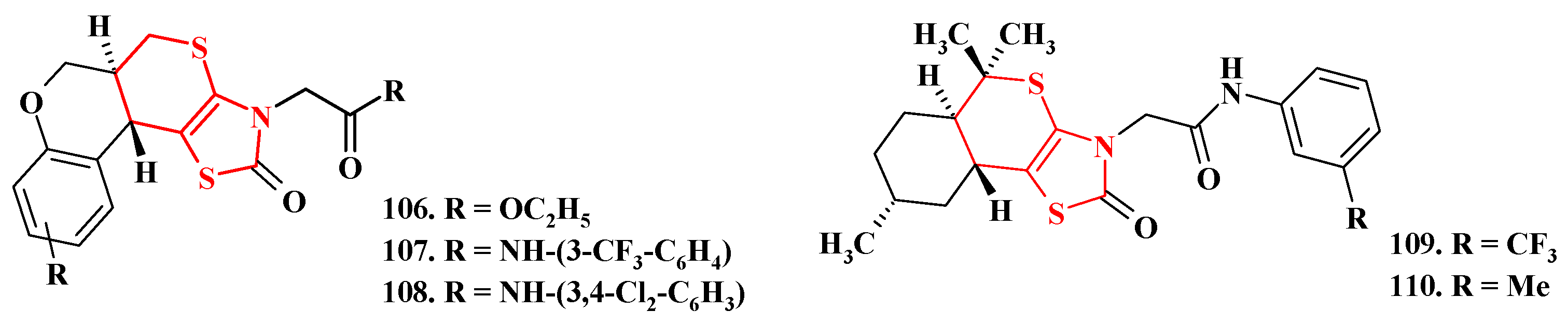
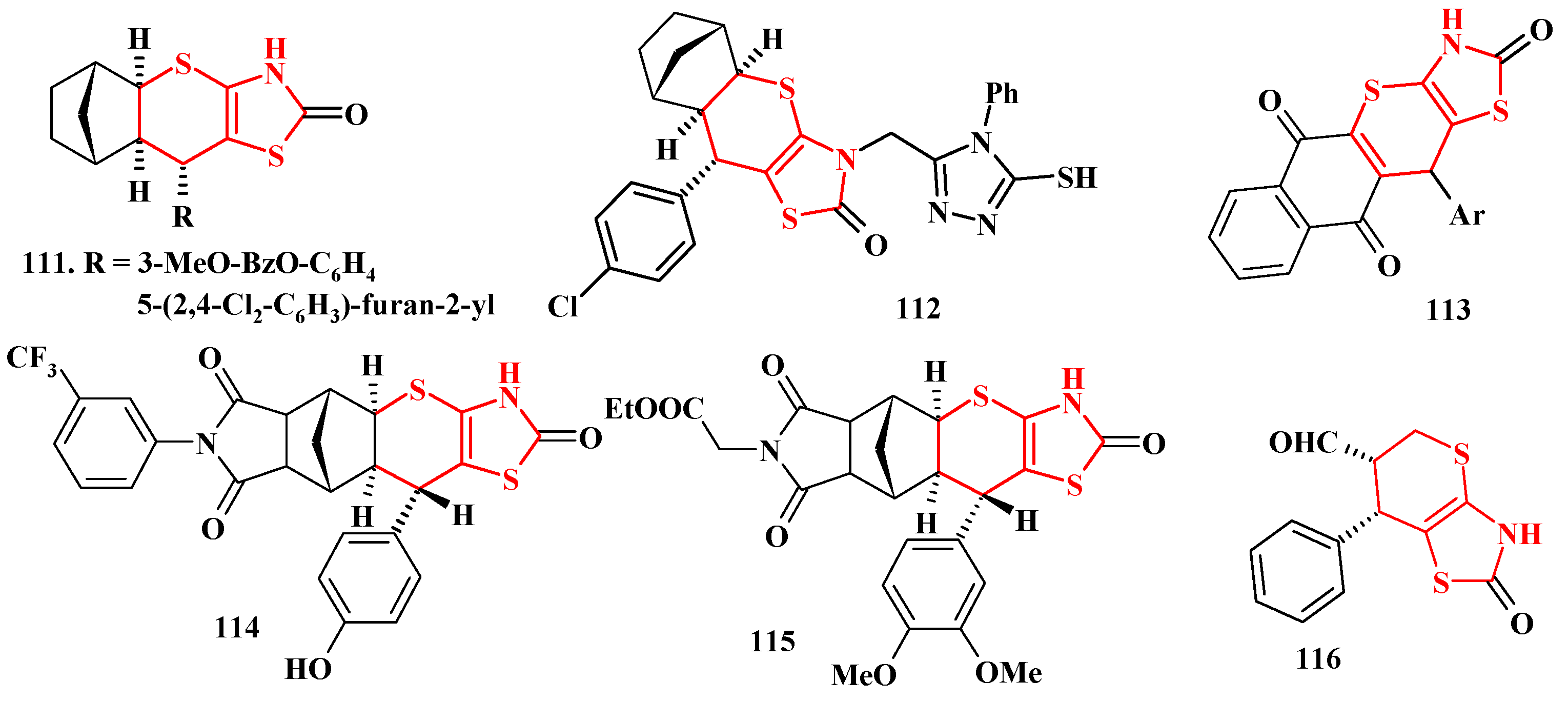
4.2. Domino Reactions as a Systematic Approach to the Synthesis of Fused Thiopyrano[2,3-d]Thiazoles
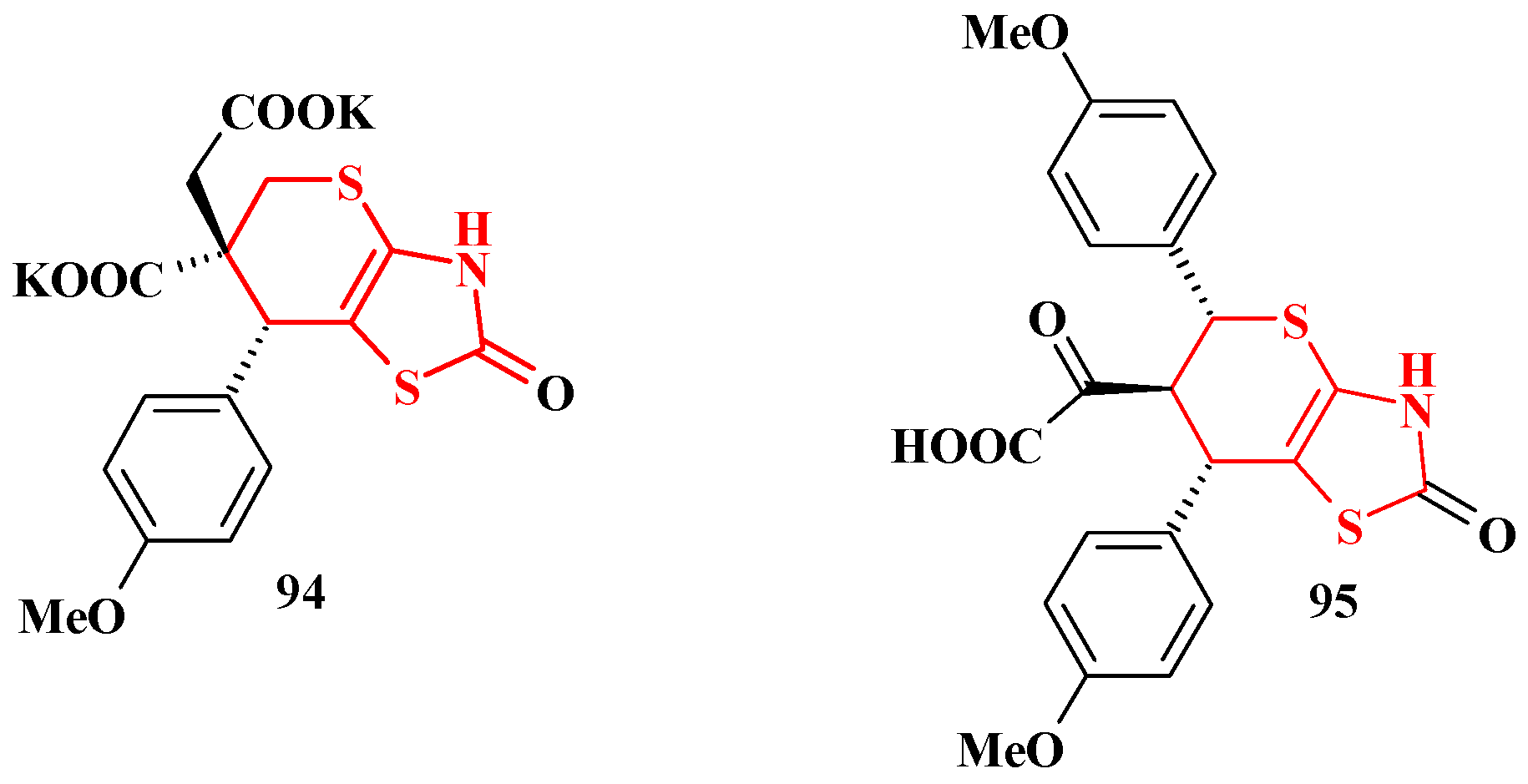
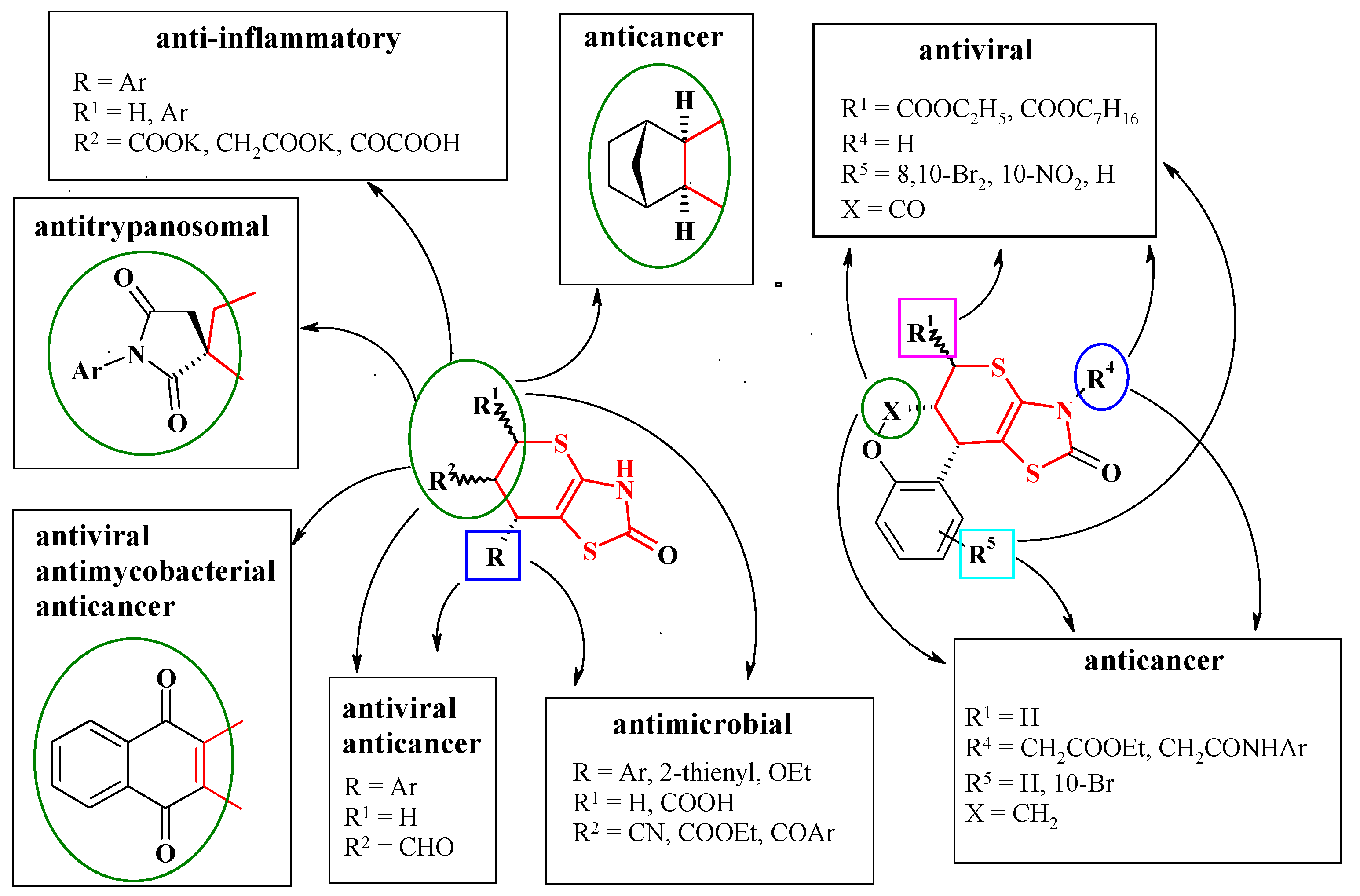
5. Biological Activity of Thiopyrano[2,3-d]Thiazole Derivatives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lesyk, R.B.; Zimenkovsky, B.S. 4-Thiazolidones: Centenarian history, current status and perspectives for modern organic and medicinal chemistry. Curr. Org. Chem. 2004, 8, 1547–1577. [Google Scholar] [CrossRef]
- Verma, A.; Saraf, S.K. 4-Thiazolidinone—A biologically active scaffold. Eur. J. Med. Chem. 2008, 43, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Lesyk, R.B.; Zimenkovsky, B.S.; Kaminskyy, D.V.; Kryshchyshyn, A.P.; Havrylyuk, D.Y.; Atamanyuk, D.V.; Subtel’na, I.Y.; Khylyuk, D.V. Thiazolidinone motif in anticancer drug discovery. Experience of DH LNMU medicinal chemistry scientific group. Biopolym. Cell 2011, 27, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, A.C.; Gupta, S.J.; Fatima, G.N.; Sonar, P.K.; Verma, A.; Saraf, S.K. 4-Thiazolidinones: The advances continue…. Eur. J. Med. Chem. 2014, 72, 52–77. [Google Scholar] [CrossRef] [PubMed]
- Reginato, M.J.; Bailey, S.T.; Krakow, S.L.; Minami, C.; Ishii, S.; Tanaka, H.; Lazar, M.A. A potent antidiabetic thiazolidinedione with unique peroxisome proliferator-activated receptor gamma activating properties. J. Biol. Chem. 1998, 273, 32679–32684. [Google Scholar] [CrossRef] [PubMed]
- Kador, P.F.; Kinoshita, J.H.; Sharpless, N.E. Aldose reductase inhibitors: A potential new class of agents for the pharmacological control of certain diabetic complications. J. Med. Chem. 1985, 28, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Mishaux, C. Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur. J. Med. Chem. 2003, 38, 645–659. [Google Scholar] [CrossRef]
- Palla, R.; Distratis, C.; Cominotto, R.; Panichi, V.; Pozzetti, G.; Bionda, A.; Neri, M.; Frattarelli, L. Renal Effects of Etozolin in Man. In Diuretics: Basic, Pharmacological, and Clinical Aspects. Developments in Nephrology; Andreucci, V.E., Dal Canton, A., Eds.; Springer: Boston, MA, USA, 1987; Volume 18, pp. 553–555. ISBN 978-1-4612-9227-2. [Google Scholar]
- Löscher, W.; von Hodenberg, A.; Nolting, B.; Fassbender, C.-P.; Taylor, C. Ralitoline: A reevaluation of anticonvulsant profile and determination of “active” plasma concentrations in comparison with prototype antiepileptic drugs in mice. Epilepsia 1991, 32, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.B.; Holloway, G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.B. Feeling nature’s PAINS: Natural products, natural product drugs, and pan assay interference compounds (PAINS). J. Nat. Prod. 2016, 79, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, C.; Bertozzi, C.; Georg, G.I.; Kiessling, L.; Lindsley, C.; Liotta, D.; Merz, K.M., Jr.; Schepartz, A.; Wang, S. The ecstasy and agony of assay interference compounds. ACS Cent. Sci. 2017, 3, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Kaminskyy, D.; Kryshchyshyn, A.; Lesyk, R. Recent developments with rhodanine as a scaffold for drug discovery. Expert Opin. Drug Discov. 2017, 12, 1233–1252. [Google Scholar] [CrossRef] [PubMed]
- Kaminskyy, D.; Kryshchyshyn, A.; Lesyk, R. 5-Ene-4-thiazolidinones—An efficient tool in medicinal chemistry. Eur. J. Med. Chem. 2017, 140, 542–594. [Google Scholar] [CrossRef] [PubMed]
- Lesyk, R.; Zimenkovsky, B.; Atamanyuk, D.; Jensen, F.; Kiec-Kononowicz, K.; Gzella, A. Anticancer thiopyrano[2,3-d][1,3]thiazol-2-ones with norbornane moiety. Synthesis, cytotoxicity, physico-chemical properties, and computational studies. Bioorg. Med. Chem. 2006, 14, 5230–5240. [Google Scholar] [CrossRef] [PubMed]
- Atamanyuk, D.; Zimenkovsky, B.; Lesyk, R. Synthesis and anticancer activity of novel thiopyrano[2,3-d]thiazole-based compounds containing norbornane moiety. J. Sulfur Chem. 2008, 29, 151–162. [Google Scholar] [CrossRef]
- Atamanyuk, D.; Zimenkovsky, B.; Atamanyuk, V.; Nektegayev, I.; Lesyk, R. Synthesis and biological activity of new thiopyrano[2,3-d]thiazoles containing a naphthoquinone moiety. Sci. Pharm. 2013, 81, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Komarista, I.D. Synthesis, Transformations and Biological Activity of Some Azolidones and Their Condensed Derivatives. Ph.D. Thesis, Sechenov First State Medical Institute, Moscow, Russia, 1989. [Google Scholar]
- Kassab, N.A.; Allah, S.O.A.; Messeha, N.A. Reactions with 5-Substituted 2-Thiazolidinone-4-thiones. Part IV. J. Prakt. Chem. 1974, 316, 209–214. [Google Scholar] [CrossRef]
- Kassab, N.A.; Abd-Allah, S.O.; Abd-El-Razik, F.M. Reactions of 5-substituted 2-thiazolidone-4-thiones with dienophiles, acrylonitrile, ethyl acrylate, styryl ethyl ketone, ω-nitrostyrene, N-arylmaleimides and maleic anhydride. Indian J. Chem. 1976, 14B, 864–867. [Google Scholar]
- Komaritsa, I.D.; Baranov, S.N.; Grischuk, A.P. 4-Thiazolidines, derivatives and analogs. Chem. Heterocycl. Comp. 1967, 3, 533–534. [Google Scholar] [CrossRef]
- Plevachuk, N.E.; Komaritsa, I.D. A study of azolidones and their derivatives. Chem. Heterocycl. Comp. 1970, 6, 144–145. [Google Scholar] [CrossRef]
- Grishchuk, A.P.; Komaritsa, I.D.; Baranov, S.N. 4-Thionazolidones, derivatives and analogs. Chem. Heterocycl. Comp. 1966, 2, 541–543. [Google Scholar] [CrossRef]
- Omar, M.T.; El-Khamry, A.; Youssef, A.M.; Ramadan, S. Synthesis and stereochemistry of thiapyranothiazoles as Diels-Alder adducts obtained from spirodimers of 1,3-Thiazolidines with cinnamic acid and its ester. Phosphorus Sulfur Silicon Relat. Elem. 2003, 178, 721–735. [Google Scholar] [CrossRef]
- Metwally, N.H. A simple green synthesis of (Z)-5-arylmethylene-4-thioxothiazolidines and thiopyrano[2,3-d]thiazolidine-2-thiones in PEG-400 under catalyst-free conditions. J. Sulfur Chem. 2014, 35, 528–537. [Google Scholar] [CrossRef]
- Zelisko, N.; Atamanyuk, D.; Vasylenko, O.; Bryhas, A.; Matiychuk, V.; Gzella, A.; Lesyk, R. Crotonic, cynnamic and propiolic acids motifs in the synthesis of thiopyrano[2,3-d][1,3]thiazoles via hetero-Diels-Alder reaction and related tandem processes. Tetrahedron 2014, 70, 720–729. [Google Scholar] [CrossRef]
- Lozynskyi, A.; Zimenkovsky, B.; Lesyk, R. Synthesis and Anticancer Activity of New Thiopyrano[2,3-d]thiazoles Based on Cinnamic Acid Amides. Sci. Pharm. 2014, 82, 723–733. [Google Scholar] [CrossRef] [PubMed]
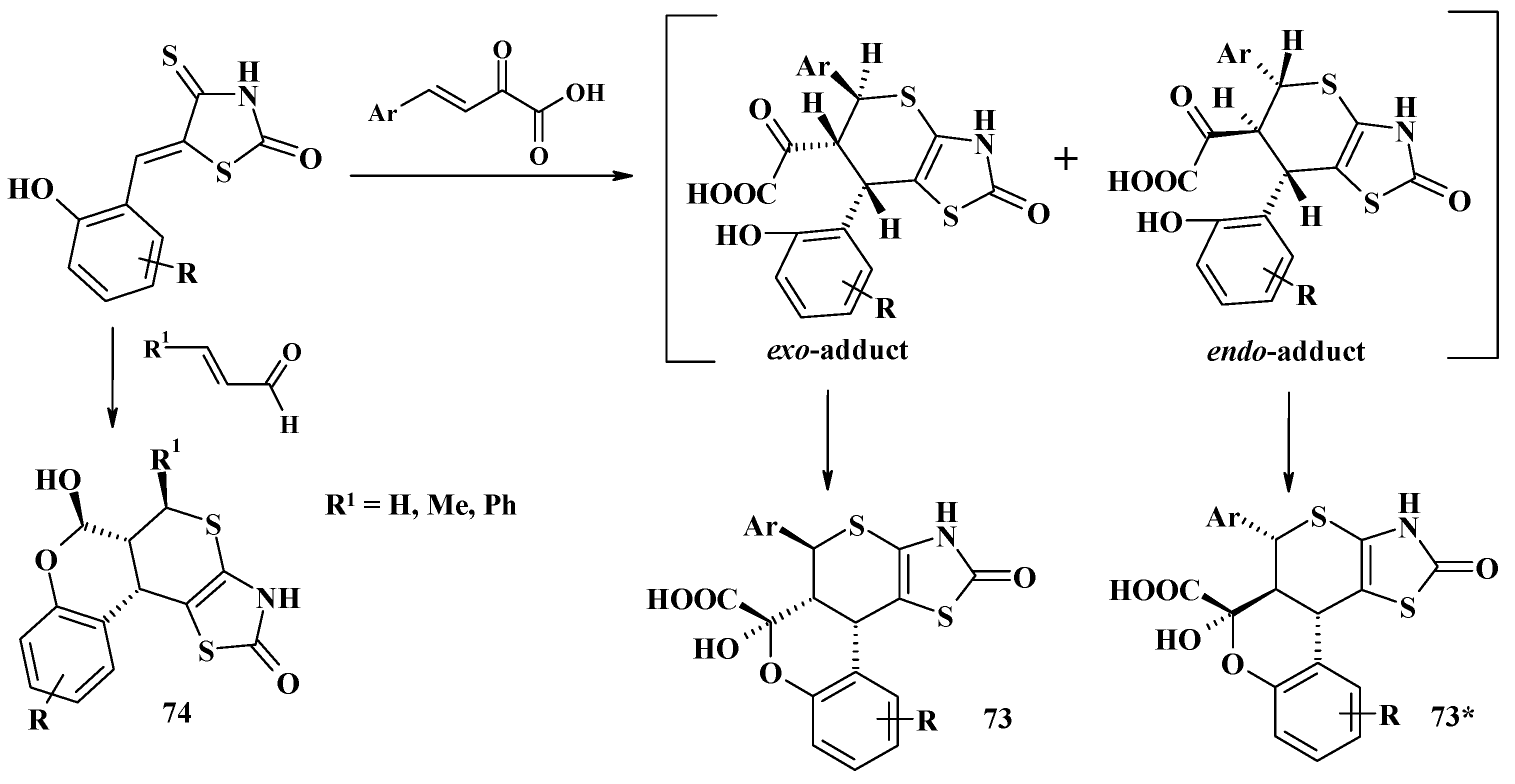
- Lozynskyi, A.; Zasidko, V.; Atamanyuk, D.; Kaminskyy, D.; Derkach, H.; Karpenko, O.; Ogurtsov, V.; Kutsyk, R.; Lesyk, R. Synthesis, antioxidant and antimicrobial activities of novel thiopyrano[2,3-d]thiazoles based on aroylacrylic acids. Mol. Divers. 2017, 21, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Lozynskyi, A.; Zimenkovsky, B.; Nektegayev, I.; Lesyk, R. Arylidene pyruvic acids motif in the synthesis of new thiopyrano[2,3-d]thiazoles as potential biologically active compounds. Heterocycl. Commun. 2015, 21, 55–59. [Google Scholar] [CrossRef]
- Atamanyuk, D.V. Synthesis, Transformation and Biological Activity of Polycyclic Fused Systems Based on 4-Thiazolidones. Ph.D. Thesis, Danylo Halytsky Lviv National Medical University, Lviv, Ukraine, 2008. [Google Scholar]
- Lozynskyi, A.; Golota, S.; Zimenkovsky, B.; Atamanyuk, D.; Gzella, A.; Lesyk, R. Synthesis, anticancer and antiviral activities of novel thiopyrano[2,3-d]thiazole-6-carbaldehydes. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1245–1249. [Google Scholar] [CrossRef]
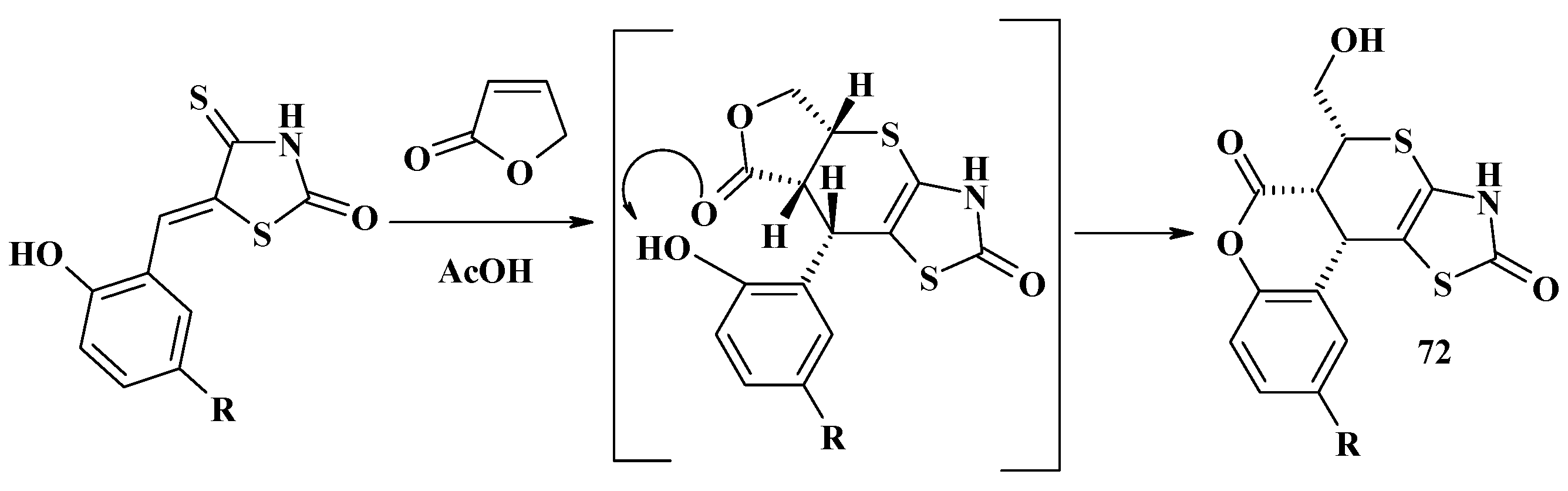
- Lozynskyi, A.; Zimenkovsky, B.; Karkhut, A.; Polovkovych, S.; Gzella, A.K.; Lesyk, R. Application of the 2(5H)furanone motif in the synthesis of new thiopyrano[2,3-d]thiazoles via the hetero-Diels–Alder reaction and related tandem processes. Tetrahedron Lett. 2016, 57, 3318–3321. [Google Scholar] [CrossRef]
- Zelisko, N.; Karpenko, O.; Muzychenko, V.; Gzella, A.; Grellier, P.; Lesyk, R. trans-Aconitic acid-based hetero-Diels-Alder reaction in the synthesis of thiopyrano[2,3-d][1,3]thiazole derivatives. Tetrahedron Lett. 2017, 58, 1751–1754. [Google Scholar] [CrossRef]
- Zelisko, N.I.; Finiuk, N.S.; Shvets, V.M.; Medvid, Y.O.; Stoika, R.S.; Lesyk, R.B. Screening of spiro-substituted thiopyrano[2,3-d]thiazoles for their cytotoxic action on tumor cells. Biopolym. Cell 2017, 33, 282–290. [Google Scholar] [CrossRef]
- Ead, H.A.; Metwalli, N.H. Heterodiene synthesis: Annulation of norbornenylogous systems with thiazolodine-4-thiones. Egypt. J. Chem. 1991, 32, 691–696. [Google Scholar]
- Polovkovych, S.V.; Karkhut, A.I.; Marintsova, N.G.; Lesyk, R.B.; Zimenkovsky, B.S.; Novikov, V.P. Synthesis of New Schiff Bases and Polycyclic Fused Thiopyranothiazoles Containing 4,6-Dichloro-1,3,5-Triazine Moiety. J. Heterocycl. Chem. 2013, 50, 1419–1424. [Google Scholar] [CrossRef]
- Metwally, N.H. A convenient synthesis of some new 5-substituted-4-thioxo-thiazolidines and fused thiopyrano[2,3-d]thiazole derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 2073–2085. [Google Scholar] [CrossRef]
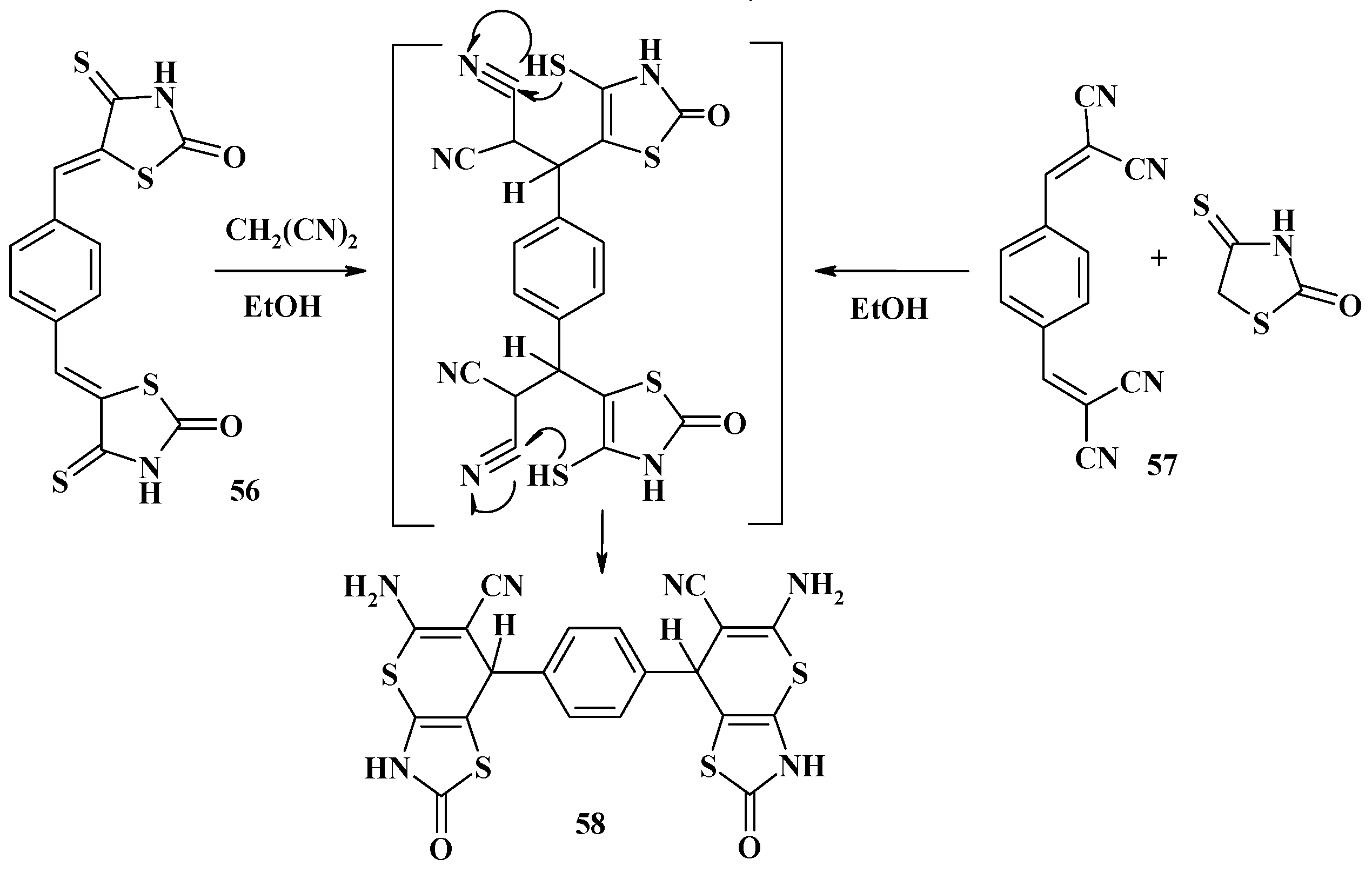
- Metwally, N.H. A novel synthesis of 1,4-bis(thiopyrano[2,3-d]thiazolyl)benzene derivatives. Heterocycles 2008, 75, 319–329. [Google Scholar] [CrossRef]
- Ead, H.A.; Abdallah, S.O.; Kassab, N.A.; Metwalli, N.H.; Saleh, Y.E. 5-(Ethoxymethylene)thiazolidine-2,4-dione derivatives: Reactions and biological activities. Arch. Pharm. 1987, 320, 1227–1232. [Google Scholar] [CrossRef]
- Atamanyuk, D.; Zimenkovsky, B.; Atamanyuk, V.; Lesyk, R. 5-Ethoxymethylidene-4-thioxo-2-thiazolidinone as Versatile Building Block for Novel Biorelevant Small Molecules with Thiopyrano[2,3-d][1,3]thiazole Core. Synth. Commun. 2014, 44, 237–244. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Vasylenko, O.; Atamanyuk, D.; Gzella, A.; Lesyk, R. Isorhodanine and thiorhodanine motifs in the synthesis of fused thiopyrano[2,3-d][1,3]thiazoles. Synlett 2011, 10, 1385–1388. [Google Scholar] [CrossRef]
- Allah, S.O.A.; Ead, H.A.; Kassab, N.A.; Metwally, N.H. Activated nitriles in heterocyclic synthesis: Novel synthesis of thiopyrano[2,3-d]thiazoles. Heterocycles 1983, 20, 637–639. [Google Scholar] [CrossRef]
- Abdelrazek, F.M.; El-Sh Kandeel, Z.; Himly, K.M.H.; Elnagdi, M.H. Substituted acrylonitriles in heterocyclic synthesis. The reaction of α-substituted β-(2-furyl)-acrylonitriles with some active-methylene heterocycles. Synthesis 1985, 4, 432–434. [Google Scholar] [CrossRef]
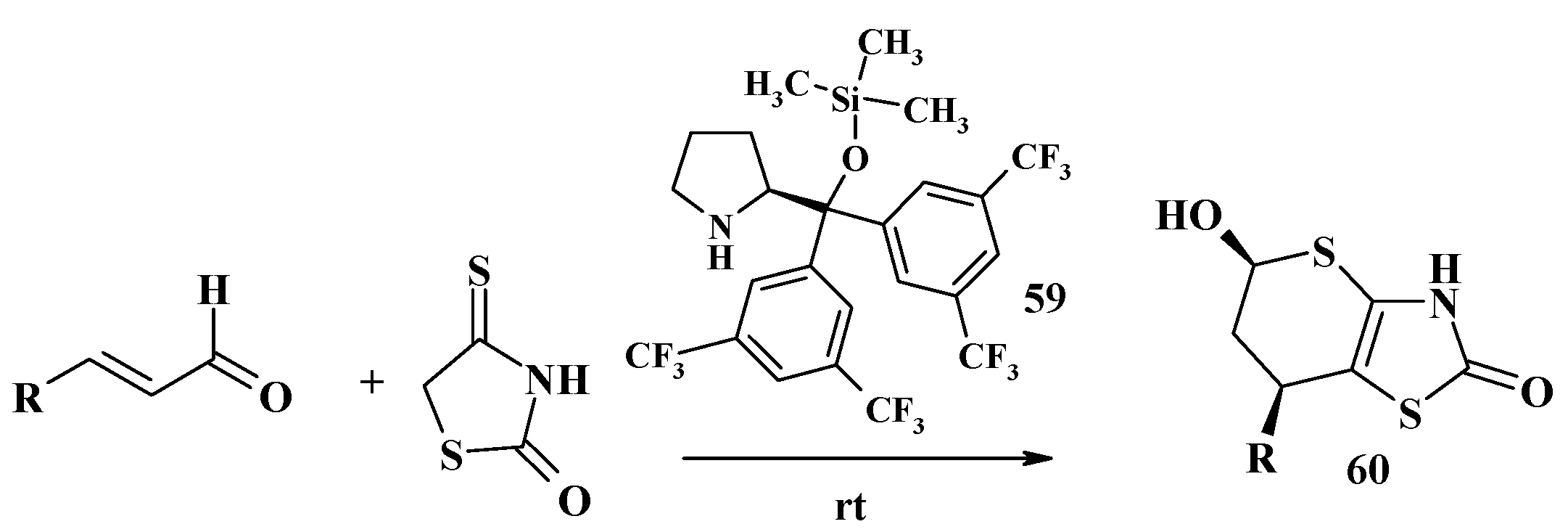
- Zhang, Y.; Wang, S.; Wu, S.; Zhu, S.; Dong, G.; Miao, Z.; Yao, J.; Zhang, W.; Sheng, C.; Wang, W. Facile construction of structurally diverse thiazolidinedione-derived compounds via divergent stereoselective cascade organocatalysis and their biological exploratory studies. ACS Comb. Sci. 2013, 15, 298–308. [Google Scholar] [CrossRef] [PubMed]
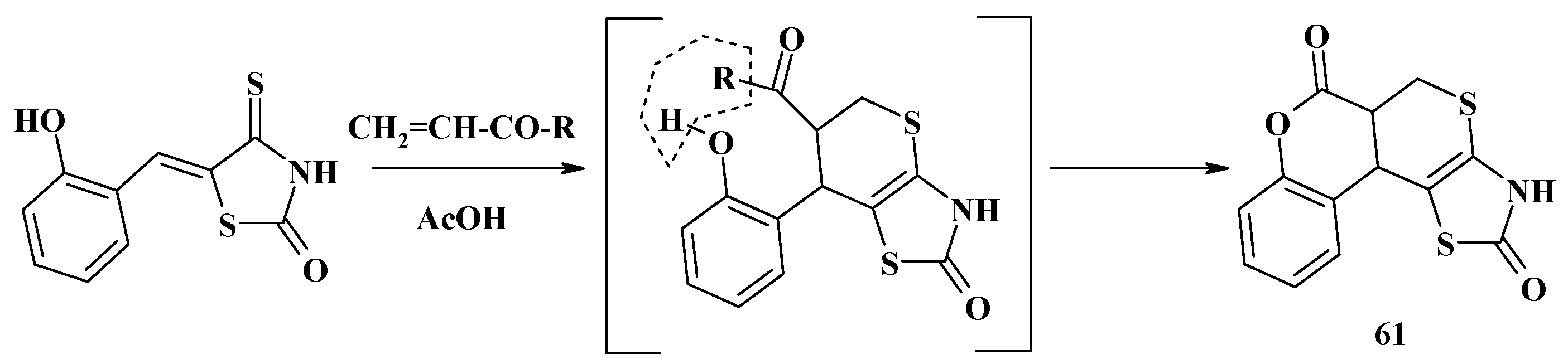
- Metwally, N.H. Synthesis of some new fused thiopyrano[2,3-d]thiazoles and their derivatives. J. Sulfur Chem. 2007, 28, 275–284. [Google Scholar] [CrossRef]
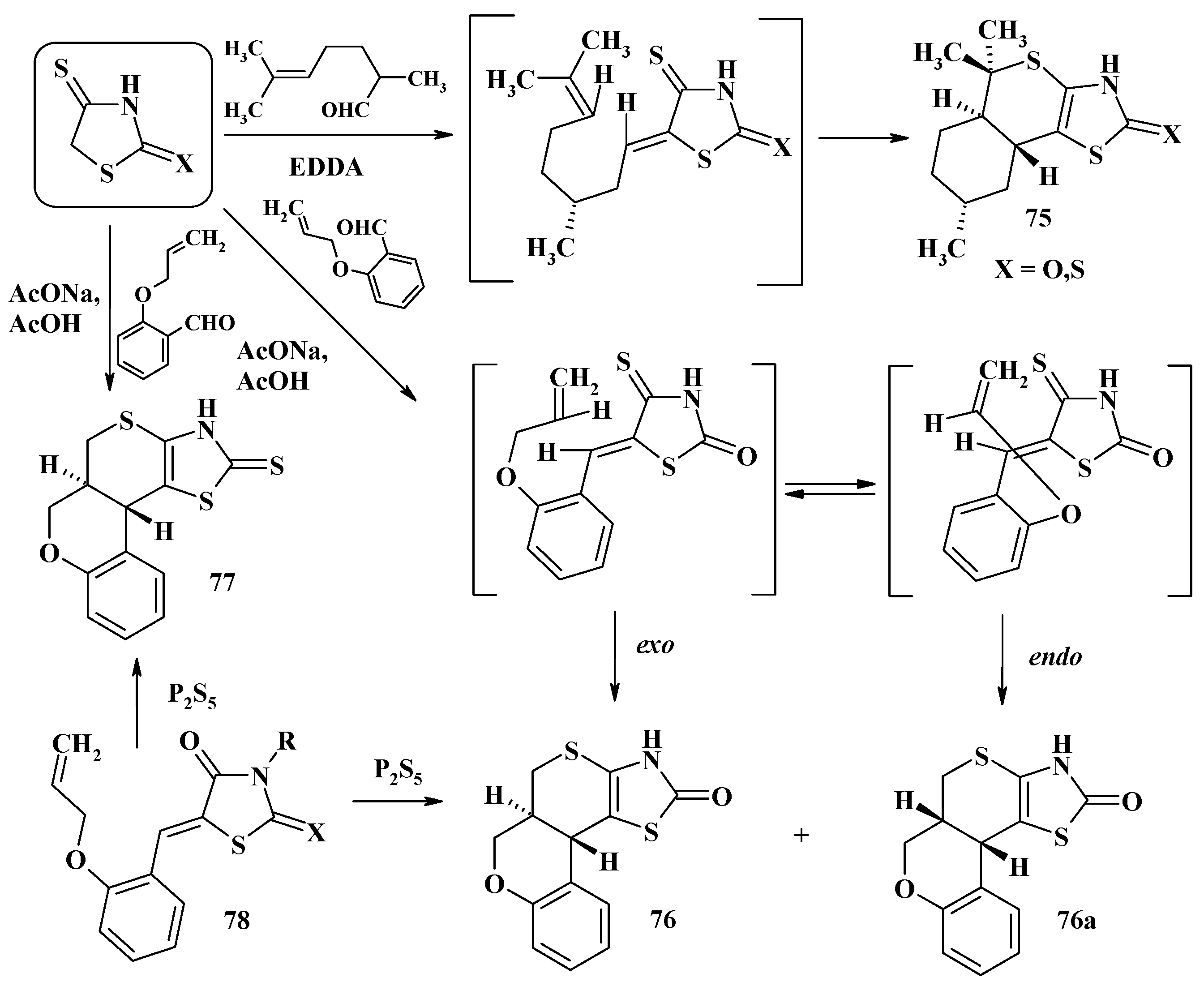
- Zelisko, N.; Atamanyuk, D.; Ostapiuk, Y.; Bryhas, A.; Matiychuk, V.; Gzella, A.; Lesyk, R. Synthesis of fused thiopyrano[2,3-d][1,3]thiazoles via hetero-Diels–Alder reaction related tandem and domino processes. Tetrahedron 2015, 71, 9501–9508. [Google Scholar] [CrossRef]
- Zelisko, N.I.; Demchuk, I.L.; Lesyk, R.B. New thiopyrano[2,3-d]thiazoles as potential antiviral agents. Ukr. Biochem. J. 2016, 88, 105–112. [Google Scholar] [CrossRef]
- Zelisko, N.; Atamanyuk, D.; Vasylenko, O.; Grellier, P.; Lesyk, R. Synthesis and antitrypanosomal activity of new 6,6,7-trisubstituted thiopyrano[2,3-d][1,3]thiazoles. Bioorg. Med. Chem. Lett. 2012, 22, 7071–7074. [Google Scholar] [CrossRef] [PubMed]
- Kowiel, M.; Zelisko, N.; Atamanyuk, D.; Lesyk, R.; Gzella, A.K. 2-[7-(3,5-Dibromo-2-hydroxyphenyl)-6-ethoxycarbonyl-2-oxo-5H-2,3,6,7-tetrahydrothiopyrano[2,3-d][1,3]thiazol-6-yl]acetic acid ethanol monosolvate. Acta Crystalogr. E 2012, E68, o2721–o2722. [Google Scholar] [CrossRef] [PubMed]
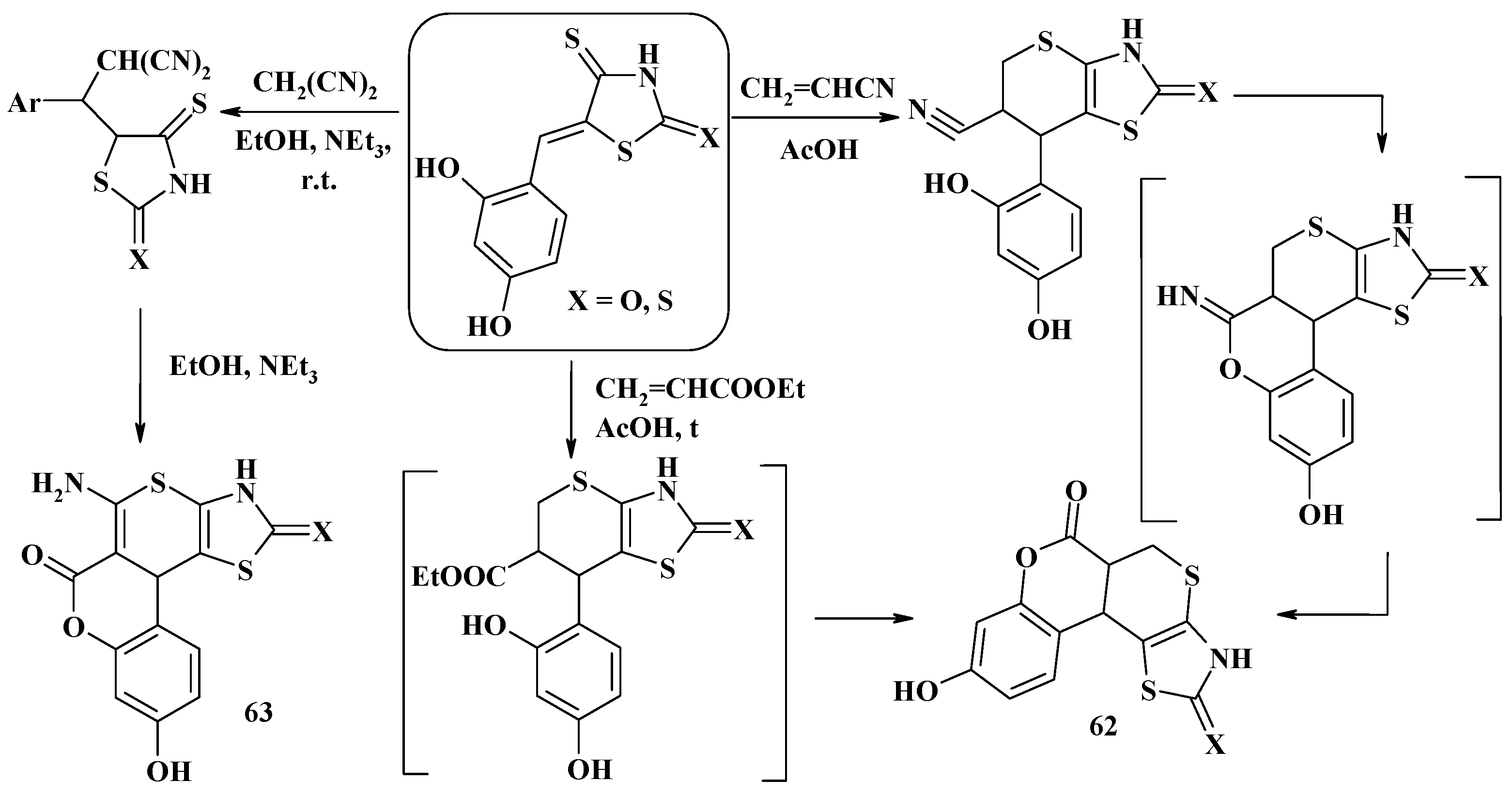
- Lozynskyi, A.; Zimenkovsky, B.; Gzella, A.K.; Lesyk, R. Arylidene pyruvic acids motif in the synthesis of new 2H,5H-chromeno[4′,3′:4,5]thiopyrano[2,3-d]thiazoles via tandem hetero-Diels-Alder-hemiacetal reaction. Synth. Commun. 2015, 45, 2266–2270. [Google Scholar] [CrossRef]
- Lozynskyi, A.; Matiychuk, V.; Karpenko, O.; Gzella, A.K.; Lesyk, R. Tandem hetero-Diels–Alder-hemiacetal reaction in the synthesis of new chromeno[4′,3′:4,5]thiopyrano[2,3-d]thiazoles. Heterocycl. Commun. 2017, 23, 1–5. [Google Scholar] [CrossRef]
- Tietze, L.F. Domino reactions in organic synthesis. Chem. Rev. 1996, 96, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Matiychuk, V.; Lesyk, R.; Obushak, M.; Gzella, A.; Atamanyuk, D.; Ostapiuk, Y.; Kryshchyshyn, A. A new domino-Knoevenagel-hetero-Diels-Alder reaction. Tetrahedron Lett. 2008, 49, 4648–4651. [Google Scholar] [CrossRef]
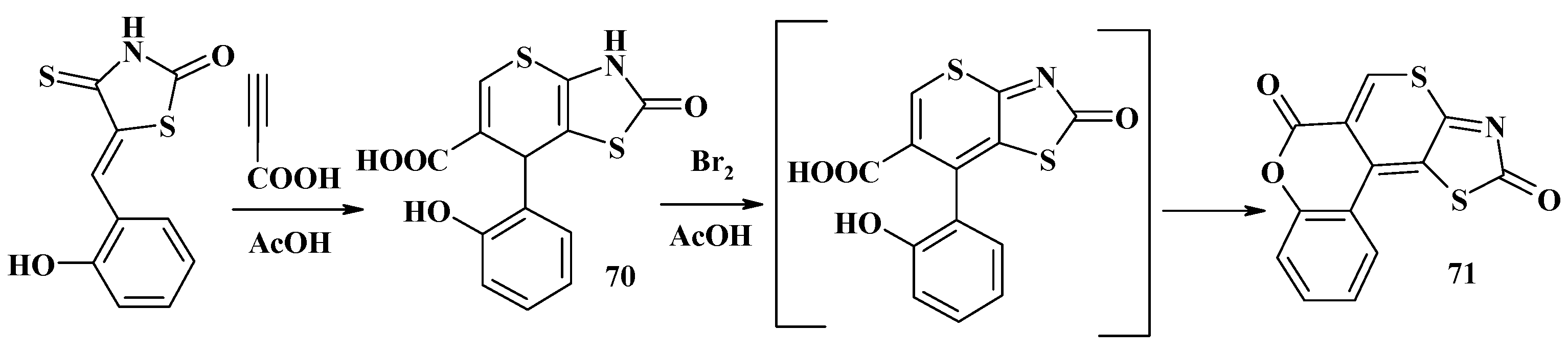
- Kryshchyshyn, A.P. Synthesis and Biological Activity of Chromeno[4′,3′:4,5]thiopyrano[2,3-d]thiazole and Isothiochromeno[4a,4-d]thiazole Derivatives. Ph.D. Thesis, Danylo Halytsky Lviv National Medical University, Lviv, Ukraine, 2011. [Google Scholar]
- Kryshchyshyn, A.; Atamanyuk, D.; Lesyk, R. Fused thiopyrano[2,3-d]thiazole derivatives as potential anticancer agents. Sci. Pharm. 2012, 80, 509–529. [Google Scholar] [CrossRef] [PubMed]
- Kryshchyshyn, A.; Zimenkovsky, B.; Lesyk, R. Synthesis and anticancer activity of isothiochromeno[3,4-d]thiazole derivatives. Ann. Univ. Mariae Curie Sklodowska DDD Pharm. 2008, 21, 247–251. [Google Scholar] [CrossRef]
- Bryhas, A.O.; Horak, Y.I.; Ostapiuk, Y.I.; Obushak, M.D.; Matiychuk, V.S. A new three-step domino Knoevenagel–hetero-Diels–Alder oxidation reaction. Tetrahedron Lett. 2011, 52, 2324–2326. [Google Scholar] [CrossRef]
- Zelisko, N.I. Synthesis, Transformation and Biological Activity of Thiopyrano[2,3-d]thiazole-Carboxylic Acids. Ph.D. Thesis, Danylo Halytsky Lviv National Medical University, Lviv, Ukraine, 2012. [Google Scholar]
- Biswas, S.; Jennens, L.; Field, H.J. The helicase primase inhibitor, BAY 57-1293 shows potent therapeutic antiviral activity superior to famciclovir in BALB/c mice infected with herpes simplex virus type 1. Antivir. Res. 2007, 75, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Maga, G.; Falchi, F.; Garbelli, A.; Belfiore, A.; Witvrouw, M.; Manetti, F.; Botta, M. Pharmacophore modeling and molecular docking led to the discovery of inhibitors of human immunodeficiency virus-1 replication targeting the human cellular aspartic acid−glutamic acid−alanine−aspartic acid box polypeptide 3. J. Med. Chem. 2008, 51, 6635–6638. [Google Scholar] [CrossRef] [PubMed]
- Ead, H.A.; Metwalli, N.H.; Morsi, N.M. Cycloaddition reactions of 5-(2-thienyl)methylene derivatives of thiazolidinone-4-thiones and their antimicrobial activities. Arch. Pharm. Res. 1990, 13, 5–8. [Google Scholar] [CrossRef]
- Ead, H.A.; Abdallah, S.O.; Kassab, N.A.; Metwalli, N.H. Synthesis of the novel thiazolo[5,4-e][1,2,3]-thiazinees and their biological activities. Sulfur Lett. 1989, 9, 23–32. [Google Scholar]
- Kryshchyshyn, A.P.; Atamanyuk, D.V.; Kaminskyy, D.V.; Grellier, P.; Lesyk, R.B. Investigation of anticancer and anti-parasitic activity of thiopyrano[2,3-d]thiazoles bearing norbornane moiety. Biopolym. Cell 2017, 33, 183–205. [Google Scholar] [CrossRef]
- Kryshchyshyn, A.; Kaminskyy, D.; Grellier, P.; Lesyk, R. Trends in research of antitrypanosomal agents among synthetic heterocycles. Eur. J. Med. Chem. 2014, 85, 51–64. [Google Scholar] [CrossRef] [PubMed]












© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kryshchyshyn, A.; Roman, O.; Lozynskyi, A.; Lesyk, R. Thiopyrano[2,3-d]Thiazoles as New Efficient Scaffolds in Medicinal Chemistry. Sci. Pharm. 2018, 86, 26. https://doi.org/10.3390/scipharm86020026
Kryshchyshyn A, Roman O, Lozynskyi A, Lesyk R. Thiopyrano[2,3-d]Thiazoles as New Efficient Scaffolds in Medicinal Chemistry. Scientia Pharmaceutica. 2018; 86(2):26. https://doi.org/10.3390/scipharm86020026
Chicago/Turabian StyleKryshchyshyn, Anna, Olexandra Roman, Andrii Lozynskyi, and Roman Lesyk. 2018. "Thiopyrano[2,3-d]Thiazoles as New Efficient Scaffolds in Medicinal Chemistry" Scientia Pharmaceutica 86, no. 2: 26. https://doi.org/10.3390/scipharm86020026





