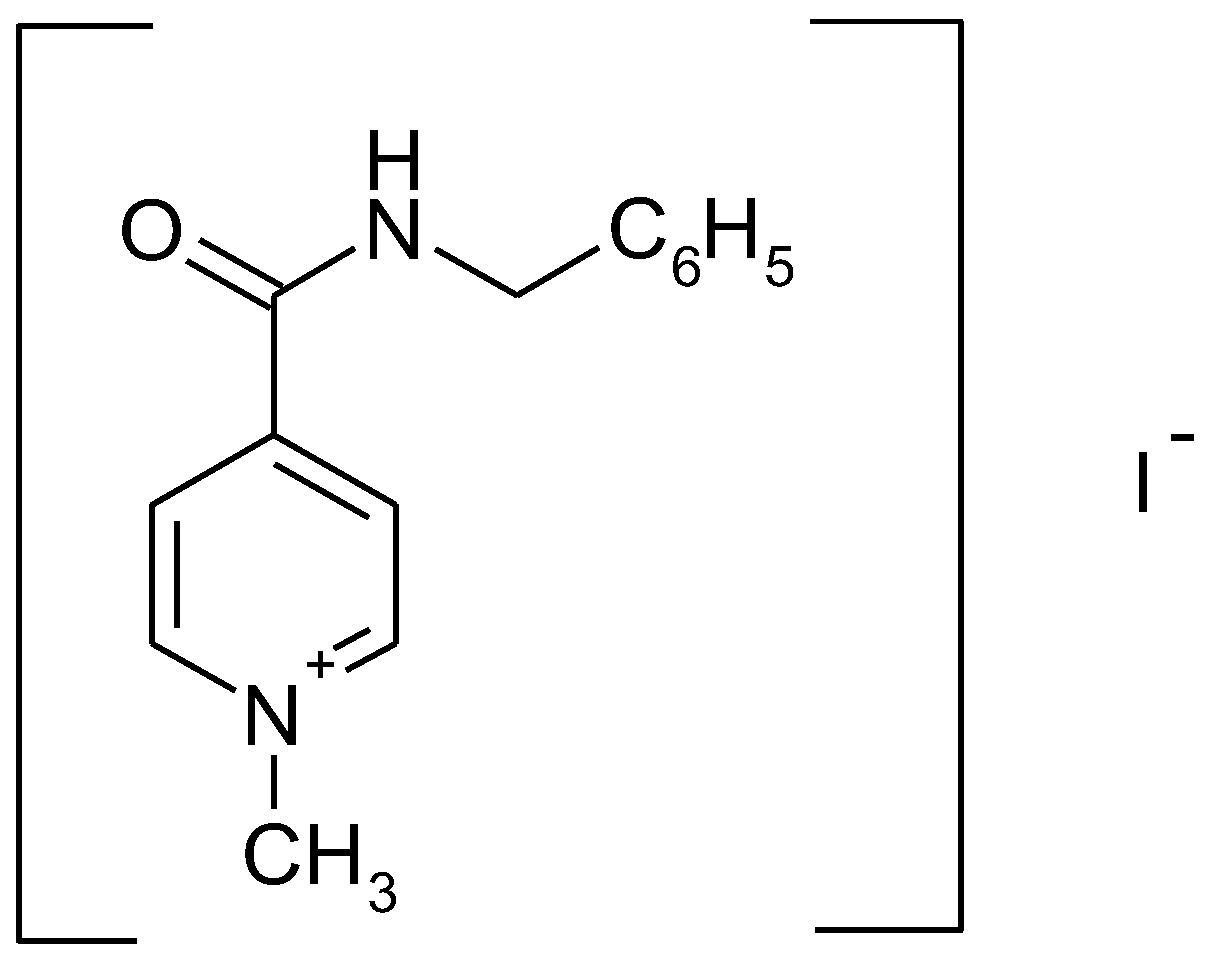
In Vitro Bioavailability Study of an Antiviral Compound Enisamium Iodide
Abstract
:1. Introduction
- BCS class I
- “high” solubility–“high” permeability
- BCS class II
- “low” solubility–“high” permeability
- BCS class III
- “high” solubility–“low” permeability
- BCS class IV
- “low” solubility–“low” permeability
2. Materials and Methods
2.1. Materials
2.1.1. Solubility Study
2.1.2. Permeability Study
2.2. Methods
2.2.1. Solubility Study
2.2.2. Permeability Study
3. Results
3.1. Solubility Study
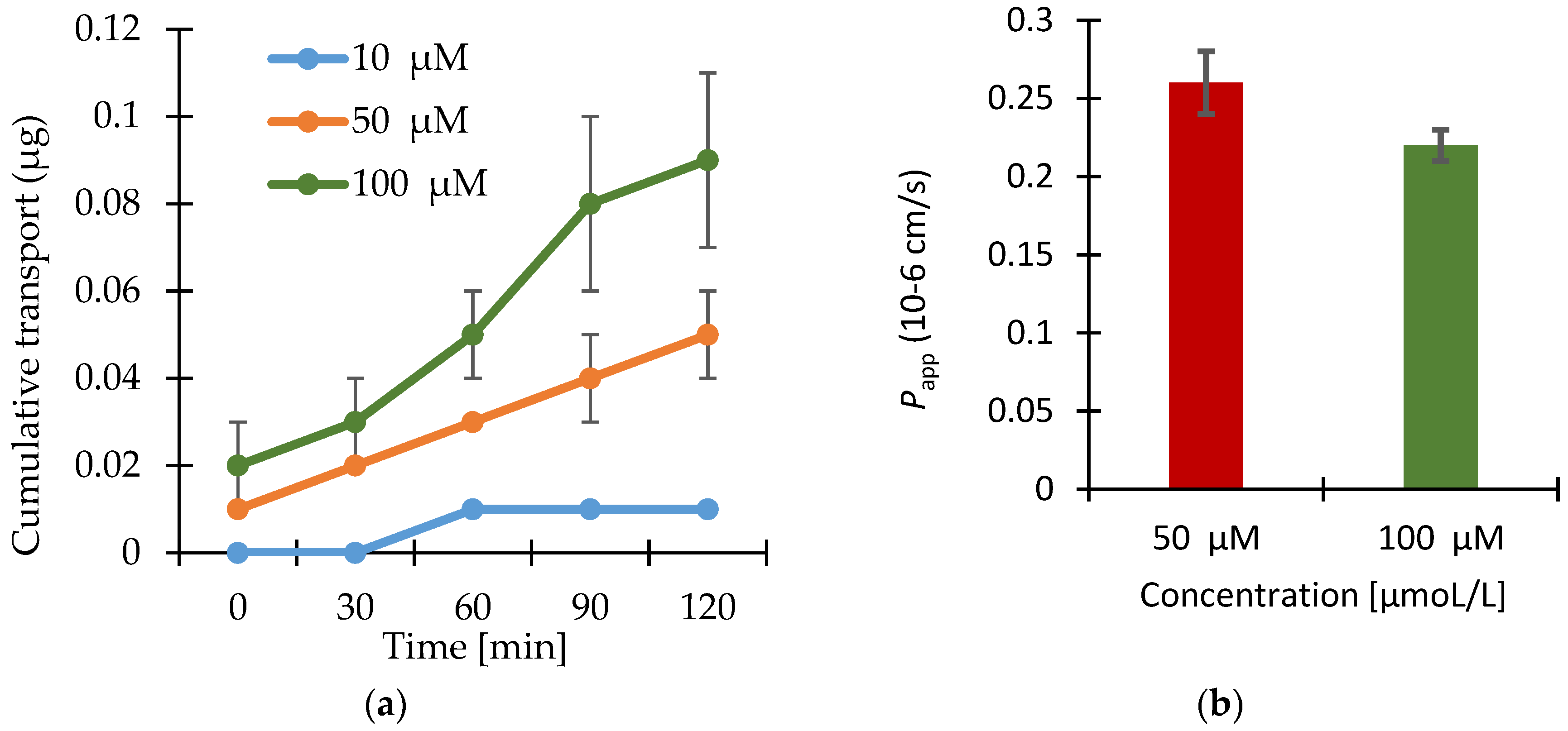
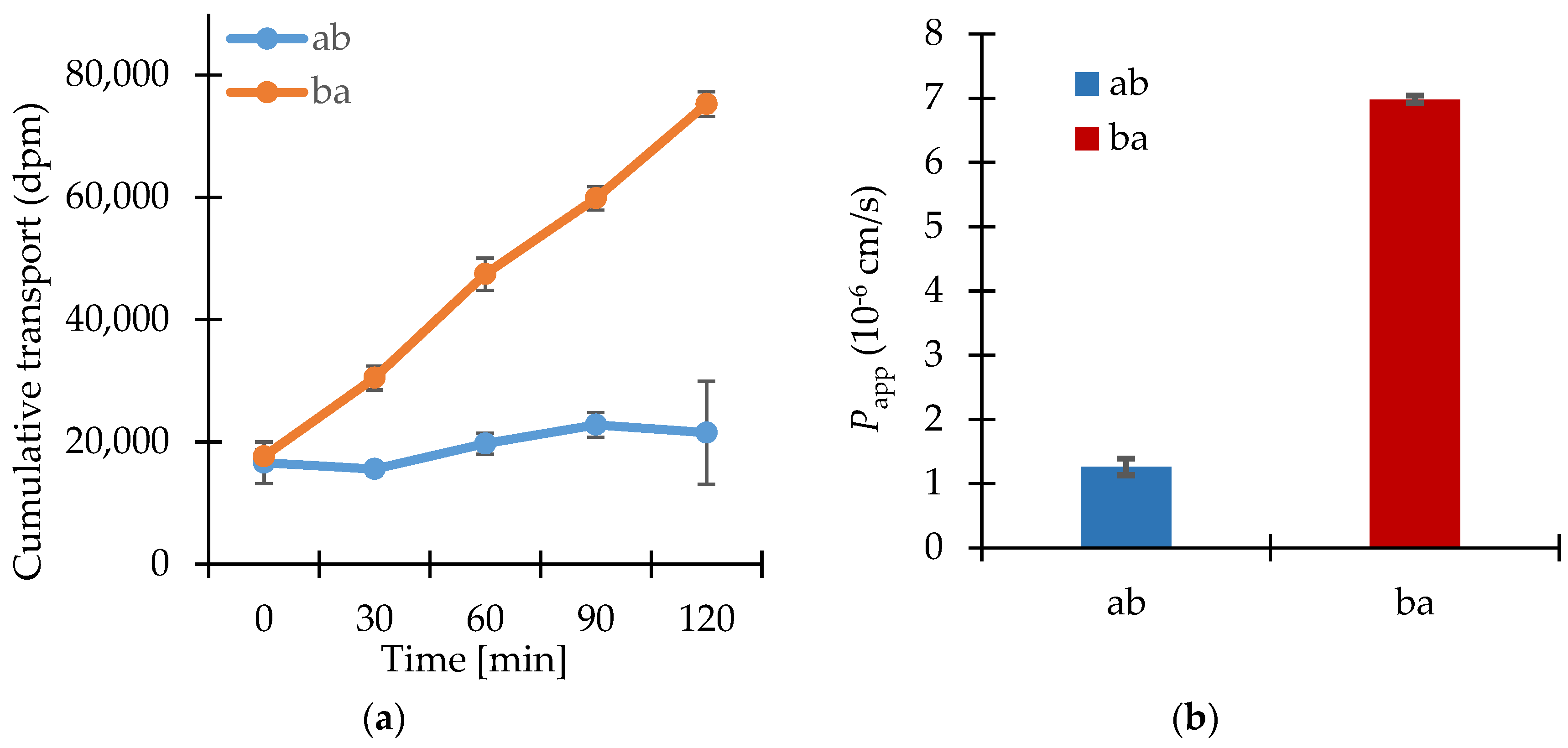
3.2. Permeability Study
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Boltz, D.; Peng, X.; Muzzio, M.; Dash, P.; Thomas, P.; Mehta, R.; Margitich, V. Antiviral activity of enisamium against influenza viruses in differentiated normal human bronchial epithelial cells. In Proceedings of the 3rd Antivirals Conference, Amsterdam, The Netherlands, 12–14 October 2014. [Google Scholar]
- Cocking, D. A Study to Investigate the Efficacy of a Test Article in Preventing Infection of Influenza Virus in the Ferret Model; The Final Report; Retroscreen Virology Ltd.: London, UK, 2009; p. 85. [Google Scholar]
- Amidon, G.L.; Lennernas, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System, Guidance for Industry; CDER: Rockville, MD, USA, May 2015.
- Volpe, D.A.; Patrick, J.; Faustino, P.J.; Anthony, B.; Ciavarella, A.B.; Ebenezer, B.; Asafu-Adjaye, E.B.; Christopher, D.; Ellison, C.D.; Lawrence, X. Classification of Drug Permeability with a Caco-2 Cell Monolayer Assay. Clin. Res. Regul. Aff. 2007, 24, 39–47. [Google Scholar] [CrossRef]
- Brouwers, J.; Mols, R.; Annaert, P.; Augustijns, P. Validation of a differential in situ perfusion method with mesenteric blood sampling in rats for intestinal drug interaction profiling. Biopharm. Drug Dispos. 2010, 31, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.M.; Dressman, J.B. The developability classification system: Application of biopharmaceutics concepts to formulation development. J. Pharm. Sci. 2010, 99, 4940–4954. [Google Scholar] [CrossRef] [PubMed]
- Kansy, M.; Senner, F.; Gubernator, K. Physicochemical high throughput screening: Parallel artificial membrane permeation assay in the description of passive absorption processes. J. Med. Chem. 1998, 41, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Committee for Medicinal Products for Human Use (CHMP). Guideline on the Investigation of Bioequivalence; (CPMP/EWP/QWP/1401/98 Rev. 1/ Corr. **): London, UK, 20 January 2010. [Google Scholar]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef]
- Panchagnula, R.; Thomas, N.S. Biopharmaceutics and pharmacokinetics in drug research. Int. J. Pharm. 2000, 201, 131–150. [Google Scholar] [CrossRef]
- Löbenberg, R.; Amidon, G.L. Modern bioavailability, bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards. Eur. J. Pharm. Biopharm. 2000, 50, 3–12. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Andrade, F.; Silva, S.B.; Rodrigues, F.; Neves, J.; Ferreira, D. Cell-based in vitro models for predicting drug permeability. Expert Opin. Drug Metab. Toxicol. 2012, 8, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Ferruzza, S.; Rossi, C.; Scarino, M.L.; Sambuy, Y. A Protocol for in situ enzyme assays to assess the differentiation of human intestinal Caco-2 cells. Toxicol. In Vitro 2012, 26, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Béduneau, A.; Tempesta, C.; Fimbel, S.; Pellequer, Y.; Jannin, V.; Demarne, F.; Lamprecht, A. A tunable Caco-2/HT29-MTX co-culture model mimicking variable permeabilities of the human intestine obtained by an original seeding procedure. Eur. J. Pharm. Biopharm. 2014, 87, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Leoni, B.D.; Natoli, M.; Nardella, M.; Bucci, B.; Zucco, F.; D’Agnano, I.; Felsani, A. Differentiation of Caco-2 cells requires both transcriptional and posttranslational down-regulation of Myc. Differentiation 2012, 83, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Des Rieux, A.; Fievez, V.; Théate, I.; Mast, J.; Préat, V.; Schneider, Y.J. An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur. J. Pharm. Sci. 2007, 30, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Araújo, F.; Sarmento, B. Towards the characterization of an in vitro triple co-culture intestine cell model for permeability studies. Int. J. Pharm. 2013, 458, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.V.; Khandavilli, S.; Ashokraj, Y.; Jain, A.; Dhanikula, A.; Sood, A.; Thomas, N.S.; Pillai, O.; Sharma, P.; Gandhi, R.; et al. Biopharmaceutic classification system: A scientific framework for pharmacokinetic optimization in drug research. Curr. Drug Metab. 2004, 5, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Lindenberg, M.; Kopp, S.; Dressman, J.B. Classification of orally administered drugs on the World Health Organization model list of essential medicines according to the biopharmaceutics classification system. Eur. J. Pharm. Biopharm. 2004, 58, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Lennernäs, H.; Abrahamsson, B. The use of biopharmaceutic classification of drugs in drug discovery and development: Current status and future extension. J. Pharm. Pharmacol. 2005, 57, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Esch, M.B.; Smith, A.S.; Prot, J.M.; Oleaga, C.; Hickman, J.J.; Shuler, M.L. How multi-organ microdevices can help foster drug development. Adv. Drug Deliv. Rev. 2014, 69–70, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 Cell Line as a Model of the Intestinal Barrier: Influence of Cell and Culture-Related Factors on Caco-2 Cell Functional Characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Westerhout, J.; van de Steeg, E.; Grossouw, D.; Zeijdner, E.E.; Krul, C.A.; Verwei, M.; Wortelboer, H.M. A new approach to predict human intestinal absorption using porcine intestinal tissue and biorelevant matrices. Eur. J. Pharm. Sci. 2014, 63, 167–177. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER); U.S. Government Printing Office. Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System, Guidance for Industry; CDER; U.S. Government Printing Office: Washington, DC, USA, 2000.
- Anderberg, E.K.; Artursson, P. Epithelial transport of drugs in cell culture. VIII: Effects of sodium dodecyl sulfate on cell membrane and tight junction permeability in human intestinal epithelial (Caco-2) cells. J. Pharm. Sci. 1993, 82, 392–398. [Google Scholar] [CrossRef] [PubMed]

| Batch No. | pH 1.2 | pH 4.5 | pH 6.8 | pH 7.5 | ||||
|---|---|---|---|---|---|---|---|---|
| 25 °C | 37 °C | 25 °C | 37 °C | 25 °C | 37 °C | 25 °C | 37 °C | |
| 170215 | 63.2 | 151.5 | 59.7 | 132.2 | 60.8 | 131.3 | 58.9 | 126.7 |
| 180215 | 63.5 | 155.0 | 59.3 | 132.7 | 59.5 | 128.0 | 59.2 | 129.9 |
| 190315 | 63.2 | 154.7 | 60.9 | 135.5 | 60.2 | 131.4 | 59.1 | 125.8 |
| Mean | 63.3 | 153.7 | 60.0 | 133.5 | 60.2 | 130.3 | 59.1 | 127.5 |
| RSD % | 0.22 | 1.26 | 1.34 | 1.32 | 1.07 | 1.47 | 0.27 | 1.70 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haltner-Ukomadu, E.; Gureyeva, S.; Burmaka, O.; Goy, A.; Mueller, L.; Kostyuk, G.; Margitich, V. In Vitro Bioavailability Study of an Antiviral Compound Enisamium Iodide. Sci. Pharm. 2018, 86, 3. https://doi.org/10.3390/scipharm86010003
Haltner-Ukomadu E, Gureyeva S, Burmaka O, Goy A, Mueller L, Kostyuk G, Margitich V. In Vitro Bioavailability Study of an Antiviral Compound Enisamium Iodide. Scientia Pharmaceutica. 2018; 86(1):3. https://doi.org/10.3390/scipharm86010003
Chicago/Turabian StyleHaltner-Ukomadu, Eleonore, Svitlana Gureyeva, Oleksii Burmaka, Andriy Goy, Lutz Mueller, Grygorii Kostyuk, and Victor Margitich. 2018. "In Vitro Bioavailability Study of an Antiviral Compound Enisamium Iodide" Scientia Pharmaceutica 86, no. 1: 3. https://doi.org/10.3390/scipharm86010003
APA StyleHaltner-Ukomadu, E., Gureyeva, S., Burmaka, O., Goy, A., Mueller, L., Kostyuk, G., & Margitich, V. (2018). In Vitro Bioavailability Study of an Antiviral Compound Enisamium Iodide. Scientia Pharmaceutica, 86(1), 3. https://doi.org/10.3390/scipharm86010003




