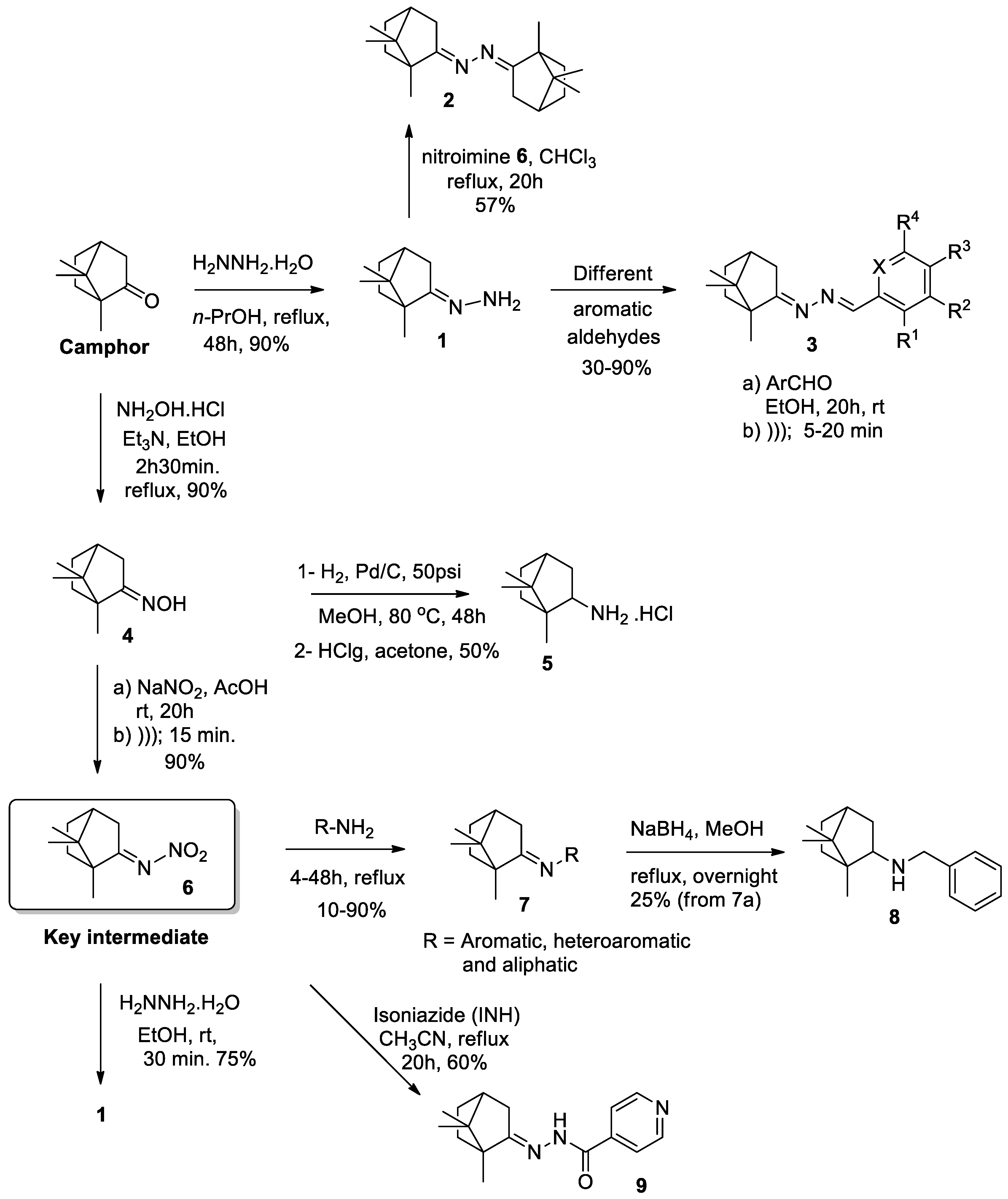
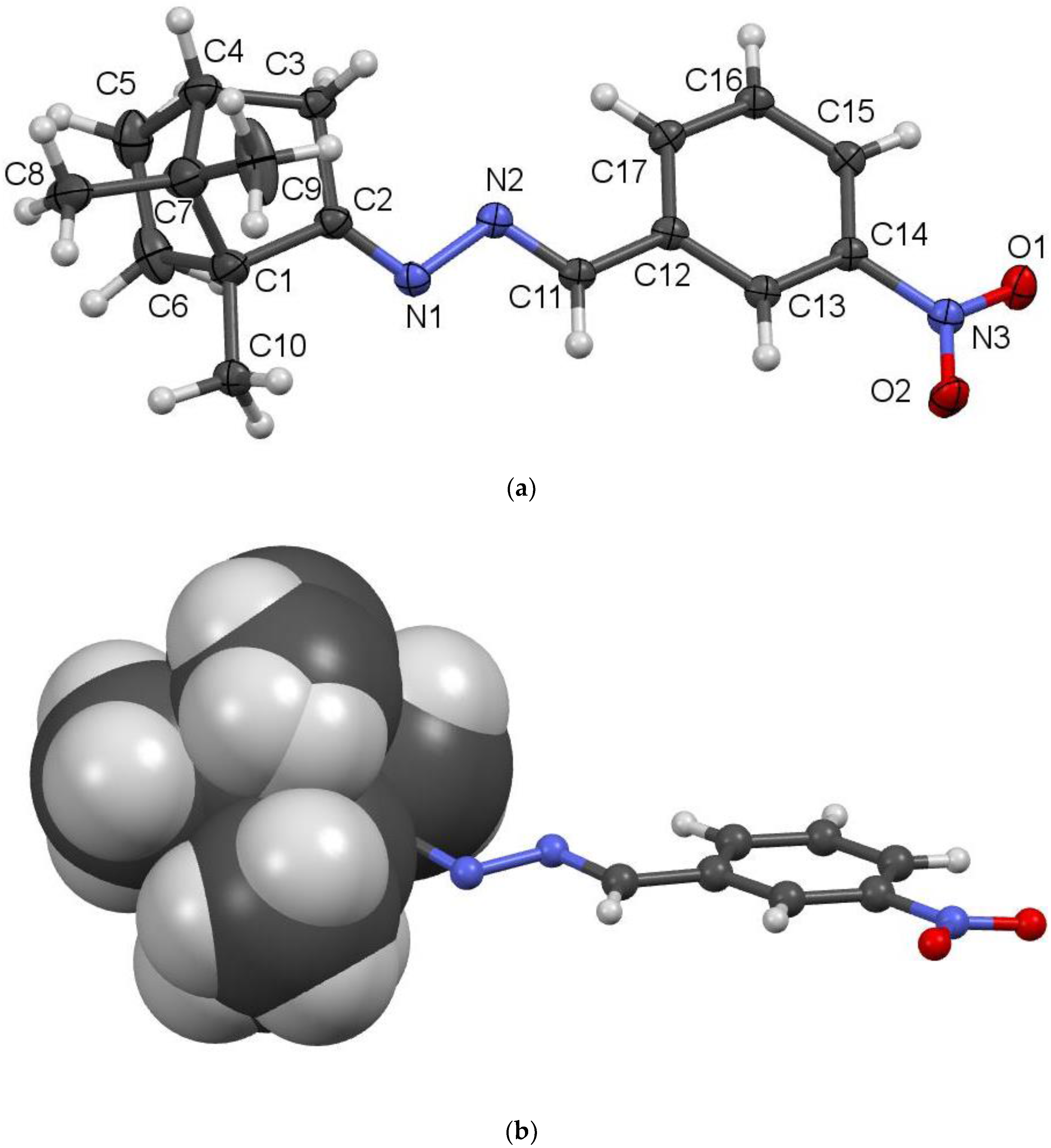
3.4. General Procedures for the Synthesis of Camphor Hydrazone Derivatives (3)
(a) Without Sonication
To a solution of hydrazine 1 (0.25 g; 1.5 mmol) in ethanol (10 mL) was added the appropriate arenealdehyde (1 or 0.8 eq.). The reaction mixture was stirred for 20 h when TLC indicated the consumption of the aldehyde. The hydrazone product was normally obtained pure after filtering and washing with cold ethanol or diethyl ether (5 to 10 mL). However, in some cases trituration or column chromatography was necessary, as indicated below.
(b) With Sonication
Similar amounts of reagents and solvents were used as in (a). The reaction mixture was irradiated with an ultrasonic probe until TLC indicated the reaction was complete. With the exception of the reaction producing 7e, all other reactions were complete in 5–18 min.
(1E,2E)-1-Benzylidene-2-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazine (3a). Purified by column chromatography on silica gel (70–230 mesh, AcOEt/n-Hex 5%). Yield: 64%, oil. 1H-NMR (CDCl3, 400 MHz) δ: 8.33 (1H, s, CH=N), 7.30–7.80 (5H, m, phenyl), 2.10–2.75 (2H, m, N=C-CH2), 1.94 (1H, t, CH, J = 4.28 Hz), 1.25–1.92 (4H, m, CH2-CH2), 1.10 (3H, s, CH3), 0.96 (3H, s, CH3), 0.84 (3H, s, CH3). 13C-NMR (CDCl3, 100 MHz) δ: 182.77, 157.70, 134.96, 130.68, 128.81, 128.29, 52.88, 48.13, 44.12, 36.21, 32.87, 27.52, 19.85, 19.02, 11.50. IR (KBr) v, cm−1: 1650 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C17H22N2 + H]: 255.1861; Found: 255.1856.
(1E,2E)-1-(4-Fluorobenzylidene)-2-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazine (3b). Purified by column chromatography on silica gel (70–230 mesh, AcOEt/n-Hex 10%). Yield: 80%, mp: 123–125 °C. 1H-NMR (CDCl3, 400 MHz) δ: 8.31 (1H, s, CH=N), 7.00–7.76 (4H, m, phenyl), 2.15–2.67 (2H, m, N=C-CH2), 1.94 (1H, t, CH, J = 4.28 Hz), 1.25–1.95 (4H, m, CH2-CH2), 1.10 (3H, s, CH3), 0.97 (3H, s, CH3), 0.84 (3H, s, CH3). 13C-NMR (CDCl3, 100 MHz) δ: 180.76, 164.66, 162.19, 155.86, 130.98, 129.99, 115.84, 52.21, 47.33, 43.18, 35.34, 32.25, 26.73, 19.25, 18.84, 11.23. IR (KBr) v, cm−1: 1624 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C17H21FN2 +H]: 273.1767; Found: 273.1765.
(1E,2E)-1-(4-Chlorobenzylidene)-2-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazine (3c). Purified by column chromatography on silica gel (70–230 mesh, AcOEt/n-Hex 10%). Yield: 71%, oil. 1H-NMR (CDCl3, 400 MHz) δ: 8.30 (1H, s, CH=N), 7.35–7.75 (4H, m, phenyl), 2.15-2.71 (2H, m, N=C-CH2), 1.94 (1H, t, CH, J =4.24 Hz), 1.20–1.95 (4H, m, CH2-CH2), 1.10 (3H, s, CH3), 0.97 (3H, s, CH3), 0.84 (3H, s, CH3). 13C-NMR (CDCl3, 100 MHz) δ: 181.03, 155.89, 135.10, 133.27, 129.44, 128.80, 52.27, 47.35, 43.18, 40.14, 35.35, 32.24, 26.71, 19.25, 18.50, 11.22. IR (KBr) v, cm−1: 1650 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C17H21ClN2 + H]: 289.1472; Found: 289.1456.
(1E,2E)-1-(4-Bromobenzylidene)-2-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazine (3d). Purified by column chromatography on silica gel (70–230 mesh, AcOEt/n-Hex 10%). Yield: 57%, mp: 60–62 °C. 1H-NMR (CDCl3, 400 MHz) δ: 8.28 (1H, s, CH=N), 7.50–7.65 (4H, m, phenyl), 2.15–2.70 (2H, m, N=C-CH2), 1.94 (1H, t, CH, J = 4.28 Hz), 1.20–1.90 (4H, m, CH2-CH2), 1.09 (3H, s, CH3), 0.97 (3H, s, CH3), 0.84 (3H, s, CH3). 13C-NMR (CDCl3, 100 MHz) δ: 183.38, 156.61, 133.91, 132.09, 129.67, 125.01, 52.97, 48.17, 44.11, 36.26, 32.85, 27.51, 19.86, 19.02, 11.47. IR (KBr) v, cm−1: 1650, (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C17H21BrN2 + H]: 333.0966; Found: 333.0957.
4-((E)-((E)-(1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazono)methyl)phenol (3e). Purified by column chromatography on silica gel (70–230 mesh, AcOEt/n-Hex 20%). Yield: 70%, mp: 157–160 °C. 1H-NMR (DMSO, 400 MHz) δ: 9.95 (1H, s, OH), 8.24 (1H, s, CH=N), 6.75–7.65 (4H, m, phenyl), 2.05–2.60 (2H, m, N=C-CH2), 1.90 (1H, t, CH, J = 4.20 Hz), 1.20–1.80 (4H, m, CH2-CH2), 0.99 (3H, s, CH3), 0.92 (3H, s, CH3), 0.76 (3H, s, CH3). 13C-NMR (DMSO, 100 MHz) δ: 179.88, 159.74, 157.18, 129.61, 125.39, 115.45, 52.01, 47.21, 43.13, 35.33, 32.26, 26.72, 19.22, 18.47, 11.28. IR (KBr) v, cm−1: 1655 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C17H22N2O + H]: 271.1810; Found: 271.1797.
(1E,2E)-1-(4-Methoxybenzylidene)-2-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazine (3f). Purified by column chromatography on silica gel (70–230 mesh, AcOEt/n-Hex 20%). Yield: 75%, mp: 67–69 °C. 1H-NMR (DMSO, 400 MHz) δ: 8.29 (1H, s, CH=N), 6.95–7.75 (4H, m, phenyl), 2.00–2.55 (2H, m, N=C-CH2), 1.91 (1H, t, CH, J = 4.20 Hz), 1.20–1.80 (4H, m, CH2-CH2), 1.00 (3H, s, CH3), 0.93 (3H, s, CH3), 0.76 (3H, s, CH3). 13C-NMR (DMSO, 100 MHz) δ: 179.88, 159.74, 157.18, 129.61, 125.39, 115.45, 52.01, 47.21, 43.13, 35.33, 32.26, 26.72, 19.22, 18.47, 11.28. IR (KBr) v, cm−1: 1575 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C18H24N2O + H]: 285.1967; Found: 285.1952.
(1E,2E)-1-(4-Nitrobenzylidene)-2-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazine (3g). Purified by filtration and washing with cold ethyl ether. Yield: 36%, mp: 100–102 °C. 1H-NMR (DMSO, 400 MHz) δ: 8.48 (1H, s, CH=N), 8.00–8.35 (4H, m, phenyl), 2.05–2.65 (2H, m, N=C-CH2), 1.94 (1H, t, CH, J =3.20 Hz), 1.20–1.90 (4H, m, CH2-CH2), 1.02 (3H, s, CH3), 0.94 (3H, s, CH3), 0.78 (3H, s, CH3). 13C-NMR (DMSO, 100 MHz) δ: 181.89, 155.20, 148.37, 140.42, 128.86, 123.98, 52.52, 47.53, 43.20, 35.43, 32.25, 26.72, 19.32, 18.55, 11.26. IR (KBr), v, cm−1: 1612, 1651 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C17H21N3O2 + H]: 300.1712; Found: 300.1710.
(1E,2E)-1-(3-Chlorobenzylidene)-2-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazine (3h). Purified by column chromatography on silica gel (70–230 mesh, AcOEt/n-Hex 10%). Yield: 70%, mp: 58–60 °C. 1H-NMR (DMSO, 400 MHz) δ: 8.33 (1H, s, CH=N), 7.48–7.82 (4H, m, phenyl), 2.09–2.61 (2H, m, N=C-CH2), 1.92 (1H, t, CH, J = 4.04 Hz), 1.22–1.84 (4H, m, CH2-CH2), 1.00 (3H, s, CH3), 0.93 (3H, s, CH3), 0.80 (3H, s, CH3). 13C-NMR (DMSO, 100 MHz) δ: 181.34, 155.66, 136.57, 133.51, 130.65, 130.27, 127.18, 126.47, 52.35, 47.43, 35.39, 32.25, 26.72, 19.29, 18.53, 11.27. IR (KBr) v, cm−1: 1709, 1655 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C17H21ClN2 + H]: 289.1472; Found: 289.1477.
3-((E)-((E)-(1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazono)methyl)benzonitrile (3i). Purified by column chromatography on silica gel (70–230 mesh, AcOEt/n-Hex 10%). Yield: 85%, mp: 93–94 °C. 1H-NMR (DMSO, 400 MHz) δ: 8.39 (1H, s, CH=N), 7.65–8.18 (4H, m, phenyl), 2.49–2.52 (2H, m, N=C-CH2), 2.10 (1H, t, CH, J = 3.92 Hz), 1.03–2.00 (4H, m, CH2-CH2), 1.01 (3H, s, CH3), 0.94 (3H, s, CH3), 0.77 (3H, s, CH3). 13C-NMR (DMSO, 100 MHz) δ: 181.59, 155.05, 135.52, 133.74, 131.33, 129.95, 118.27, 111.83, 52.31, 47.37, 43.09, 35.33, 32.15, 26.63, 19.20, 18.44, 11.16. IR (KBr) v, cm−1: 1647 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C18H21N3 + H]: 280.1814; Found: 280.1822.
(1E,2E)-1-(3-Methoxybenzylidene)-2-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazine (3j). Purified by column chromatography on silica gel (70–230 mesh, AcOEt/n-Hex 10%). Yield: 61%, oil. 1H-NMR (DMSO, 400 MHz) δ: 8.29 (1H, s, CH=N), 7.00–7.37 (4H, m, phenyl), 3.78 (3H, s, -OCH3), 2.09–2.51 (2H, m, N=C-CH2), 1.91 (1H, t, CH, J = 4.04 Hz), 1.25–1.38 (4H, m, CH2-CH2), 1.00 (3H, s, CH3), 0.93 (3H, s, CH3), 0.77 (3H, s, CH3). 13C-NMR (DMSO, 100 MHz) δ: 182.46, 159.77, 157.26, 136.12, 129.60, 121.31, 116.87, 111.87, 55.31, 52.66, 47.92, 43.89, 35.97, 32.63, 27.29, 19.63, 18.81, 11.28. IR (KBr) v, cm−1: 1653 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C18H24N2O + H]: 285.1967; Found: 285.1964.
(1E,2E)-1-(3-Nitrobenzylidene)-2-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazine (3k). Purified by filtration and washing with cold ethyl ether. Yield: 50%, mp: 110–113 °C. 1H-NMR (DMSO, 400 MHz) δ: 8.59 (1H, s, phenyl), 8.49 (1H, s, CH=N), 7.70–8.35 (3H, m, phenyl), 2.10–2.65 (2H, m, N=C-CH2), 1.94 (1H, t, CH, J = 4.08 Hz), 1.20–1.85 (4H, m, CH2-CH2), 1.02 (3H, s, CH3), 0.94 (3H, s, CH3), 0.78 (3H, s, CH3). 13C-NMR (DMSO, 100MHz) δ: 184.41, 155.04, 148.66, 136.58, 133.66, 129.64, 124.80, 122.55, 52.97, 48.07, 43.88, 36.19, 32.63, 27.28, 19.64, 18.80, 11.22; IR (KBr) v, cm−1: 1648 (C=N); HRMS (ESI, +H): Theoretical mass calculated for C17H23N3O2 + H: 300.1712; Found: 300.1705.
(1E,2E)-1-(2-Fluorobenzylidene)-2-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazine (3l). Purified by column chromatography on silica gel (70–230 mesh, AcOEt/n-Hex 5%). Yield: 68%, oil. 1H-NMR (DMSO, 400 MHz) δ: 8.44 (1H, s, CH=N), 7.25–7.90 (4H, m, phenyl), 2.10–2.65 (2H, m, N=C-CH2), 1.92 (1H, t, CH, J = 4.15 Hz), 1.20–1.85 (4H, m, CH2-CH2), 1.01 (3H, s, CH3), 0.94 (3H, s, CH3), 0.78 (3H, s, CH3). 13C-NMR (DMSO,100 MHz) δ: 181.44, 162.29, 159.79, 149.88, 149.85, 132.67, 132.58, 127.41, 127.39, 124.72, 124.69, 121.68, 121.58, 116.09, 115.88, 52.25, 47.35, 43.07, 35.32, 32.13, 26.62, 19.20, 18.44, 11.15. IR (KBr) v, cm−1: 1640 (C=N). MS (ESI, +H): 278.
(1E,2E)-1-(2-Chlorobenzylidene)-2-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazine (3m). Purified by column chromatography on silica gel (70–230 mesh, AcOEt/n-Hex 5%). Yield: 51%, mp: 66–69 °C. 1H-NMR (DMSO, 400 MHz) δ: 8.58 (1H, s, CH=N), 7.40–8.05 (3H, m, phenyl), 2.05–2.65 (2H, m, N=C-CH2), 1.93 (1H, t, CH, J = 4.04 Hz), 1.20–1.85 (4H, m, CH2-CH2), 1.01 (3H, s, CH3), 0.94 (3H, s, CH3), 0.78 (3H, s, CH3). 13C-NMR (DMSO, 100 MHz) δ: 181.93, 152.98, 133.80, 132.10, 131.20, 129.86, 127.79, 127.44, 52.31, 47.38, 43.08, 35.38, 32.12, 26.62, 19.20, 18.44, 11.15. IR (KBr) v, cm−1: 1651 (C=N); HRMS (ESI, +H): Theoretical mass calculated for [C17H21ClN2 + H]: 289.1472; Found: 289.1471.
(1E,2E)-1-(2-Bromobenzylidene)-2-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazine (3n). Purified by column chromatography on silica gel (70–230 mesh, AcOEt/n-Hex 5%). Yield: 55%, mp: 88–90 °C. 1H-NMR (DMSO, 400 MHz) δ: 8.52 (1H, s, CH=N), 7.39–8.00 (4H, m, phenyl), 1.94–2.51 (2H, m, N=C-CH2), 1.93 (1H, t, CH, J = 4.02 Hz), 1.23–1.80 (4H, m, CH2-CH2), 1.02 (3H, s, CH3), 0.94 (3H, s, CH3), 0.78 (3H, s, CH3). 13C-NMR (DMSO, 100 MHz) δ: 181.99, 155.28, 133.08, 132.64, 132.34, 128.15, 127.95, 124.12, 52.32, 47.38, 43.07, 43.07, 35.40, 32.11, 26.61, 19.20, 18.43, 11.15. IR (KBr) v, cm−1: 1657 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C17H21BrN2 + H]: 333.0966; Found: 333.0978.
2-((E)-((E)-(1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazono)methyl)phenol (3o). Purified by column chromatography on silica gel (70–230 mesh, AcOEt/n-Hex 10%). Yield: 64%, mp: 54–57 °C. 1H-NMR (CDCl3, 400 MHz) δ: 12.00 (1H, s, OH), 8.61 (1H, s, CH=N), 6.85–7.40 (4H, m, phenyl), 2.10–2.75 (2H, m, N=C-CH2), 2.00 (1H, t, CH, J =4.28 Hz), 1.20-1.95 (4H, m, CH2-CH2), 1.12 (3H, s, CH3), 0.98 (3H, s, CH3), 0.83 (3H, s, CH3). 13C-NMR (CDCl3, 100 MHz) δ: 185.26, 162.27, 160.05, 132.48, 132.12, 119.46, 118.18, 117.00, 53.23, 48.44, 44.02, 36.49, 32.78, 27.43, 19.82, 18.96, 11.40. IR (KBr) v, cm−1: 1650, 1624 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C17H22N2O + H]: 271.1810; Found: 271.1797.
(1E,2E)-1-(2-Nitrobenzylidene)-2-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazine (3p). Purified by column chromatography on silica gel (70–230 mesh, AcOEt/n-Hex 10%). Yield: 77%, mp: 72–75 °C. 1H-NMR (CDCl3, 400 MHz) δ: 8.53 (1H, s, CH=N), 7.65–8.05 (4H, m, phenyl), 2.05–2.55 (2H, m, N=C-CH2), 1.93 (1H, t, CH, J = 4.28 Hz), 1.20–1.95 (4H, m, CH2-CH2-), 1.01 (3H, s, CH3), 0.94 (3H, s, CH3), 0.78 (3H, s, CH3). 13C-NMR (CDCl3, 100 MHz) δ: 181.48, 152.76, 148.73, 133.36, 131.13, 129.38, 128.34, 124.32, 52.30, 47.43, 43.04, 35.31, 32.08, 26.64, 19.18, 18.42, 11.14. IR (KBr) v, cm−1: 1647 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C17H21N3O2 + H]: 300.1712; Found: 300.1707.
(1E,2E)-1-(2,3-Dichlorobenzylidene)-2-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazine (3q). Purified by column chromatography on silica gel (70–230 mesh, AcOEt/n-Hex 10%). Yield: 78%, mp: 69–72 °C. 1H-NMR (DMSO, 400 MHz) δ: 8.60 (1H, s, CH=N), 7.42–8.00 (3H, m, phenyl), 2.10–2.60 (2H, m, N=C-CH2-), 1.92 (1H, t, CH, J = 4.24 Hz), 1.03–1.70 (4H, m, CH2-CH2), 1.02 (3H, s, CH3), 0.94 (3H, s, CH3), 0.78 (3H, s, CH3). 13C-NMR (DMSO, 100 MHz) δ: 182.43, 153.04, 133.74, 132.39, 132.28, 131.75, 128.43, 126.55, 52.49, 47.52, 43.17, 35.49, 32.20, 26.70, 19.30, 18.53, 11.23. IR (KBr) v, cm−1: 1650 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C17H20Cl2N2 + H]: 323.1082; Found: 323.1082.
3-((E)-((E)-(1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazono)methyl)benzene-1,2-diol (3r). Purified by column chromatography on silica gel (70–230 mesh, AcOEt/n-Hex 10%). Yield: 73%, mp: 104–106 °C. 1H-NMR (CDCl3, 400 MHz) δ: 12.36 (1H, s, OH), 8.59 (1H, s, CH=N), 6.75–7.01 (3H, m, phenyl), 2.17–2.75 (2H, m, N=C-CH2), 2.01 (1H, t, CH, J = 4.28 Hz), 1.25–1.95 (4H, m, CH2-CH2), 1.12 (3H, s, CH3), 0.98 (3H, s, CH3), 0.83 (3H, s, CH3). 13C-NMR (CDCl3, 100 MHz) δ: 185.29, 162.20, 147.08, 144.95, 122.99, 119.77, 117.84, 117.39, 53.30, 48.47, 44.04, 36.43, 32.76, 27.42, 19.83, 18.95, 11.38. IR (KBr) v, cm−1: 1643 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C17H22N2O2 + H]: 287.1760; Found: 287.1785.
4-((E)-((E)-(1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazono)methyl)benzene-1,3-diol (3s). Purified by filtration and washing with cold ethyl ether. Yield: 25%, mp: 183–185 °C. 1H-NMR (DMSO, 500 MHz) δ: 12.01 (1H, s, OH), 10.15 (1H, s, OH), 8.59 (1H, s, CH=N), 6.25–7.35 (3H, m, phenyl), 2.08–2.65 (2H, m, N=C-CH2), 1.94 (1H, t, CH, J = 4.00 Hz), 1.30–1.90 (4H, m, CH2-CH2), 1.00 (3H, s, CH3), 0.94 (3H, s, CH3), 0.74 (3H, s, CH3). 13C-NMR (DMSO, 120 MHz) δ: 181.71, 161.66, 161.63, 161.12, 110.22, 107.84, 102.33, 52.39, 47.62, 43.18, 35.41, 32.25, 26.68, 19.28, 18.51, 11.26. IR (KBr) v, cm−1: 1661 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C17H22N2O2 + H]: 287.1760; Found: 287.1756.
4-((E)-((E)-(1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazono)methyl)benzene-1,2-diol (3t). Purified by filtration and washing with cold ethyl ether. Yield: 38%, mp: 186–190 °C. 1H-NMR (DMSO, 400 MHz) δ: 8.16 (1H, s, OH), 7.24 (1H, s, OH), 8.16 (1H, s, CH=N), 6.76–7.24 (3H, m, phenyl), 2.08–2.15 (2H, m, N=C-CH2), 1.91 (1H, t, CH, J = 4.04 Hz), 1.21–1.85 (4H, m, CH2-CH2), 0.99 (3H, s, CH3), 0.92 (3H, s, CH3), 0.76 (3H, s, CH3). 13C-NMR (DMSO, 100 MHz) δ: 157.57, 146.84, 143.69, 127.53, 122.90, 115.17, 113.92, 52.90, 48.11, 43.84, 36.14, 32.64, 27.24, 19.64, 18.78, 11.29. IR (KBr) v, cm−1: 1663 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C17H22N2O2 + H]: 287.1760; Found: 287.1778.
2-((E)-((E)-(1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazono)methyl)benzene-1,4-diol (3u). Purified by filtration and washing with cold ethyl ether. Yield: 64%, mp: 218–220 °C. 1H-NMR (DMSO, 400 MHz) δ: 10.94 (1H, s, OH), 9.00 (1H, s, OH), 8.60 (1H, s, CH=N), 6.74–6.94 (3H, m, phenyl), 2.09–2.60 (2H, m, N=C-CH2), 1.96 (1H, t, CH, J = 3.92 Hz), 1.24–1.87 (4H, m, CH2-CH2), 1.02 (3H, s, CH3), 0.94 (3H, s, CH3), 0.76 (3H, s, -CH3). 13C-NMR (DMSO, 100 MHz) δ: 185.44, 161.54, 153.99, 148.00, 120.10, 117.89, 117.55, 117.16, 53.06, 48.24, 43.78, 36.30, 32.55, 27.20, 19.61, 18.74, 11.18. IR (KBr) v, cm−1: 1651 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C17H22N2O2 + H]: 287.1760; Found: 287.1773.
4-Methoxy-2-((E)-((E)-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazono)methyl)phenol (3v). Purified by column chromatography on silica gel (70–230 mesh, AcOEt/n-Hex 10%). Yield: 78%, mp: 77–80 °C. 1H-NMR (DMSO, 400 MHz) δ: 12.07 (1H, 1s, OH), 8.64 (1H, s, CH=N), 6.47–7.47 (3H, m, phenyl), 3.78 (3H, s, OCH3), 2.00–2.70 (2H, m, N=C-CH2), 1.96 (1H, t, CH, J = 4.04 Hz), 1.23–1.87 (4H, m, CH2-CH2), 1.01 (3H, s, CH3), 0.93 (3H, s, CH3), 0.76 (3H, s, CH3). 13C-NMR (DMSO, 100 MHz) δ: 182.32, 163.37, 162.91, 162.06, 161.46, 161.04, 160.66, 133.31, 132.70, 111.40, 111.37, 107.10, 106.68, 100.95, 100.83. IR (KBr) v, cm−1: 1651, 1633 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C18H24N2O2 + H]: 301.1916; Found: 301.1915.
5-Nitro-2-((E)-((E)-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazono)methyl)phenol (3w). Purified by filtration and washing with cold ethanol. Yield: 54%, mp: 138–141 °C. 1H-NMR (DMSO, 400 MHz) δ: 12.77 (1H, s, OH), 8.80 (1H, s, CH=N), 7.12–8.61 (3H, m, phenyl), 2.14–2.65 (2H, m, N=C-CH2), 1.98 (1H, t, CH, J = 3.76 Hz), 1.41–1.97 (4H, m, CH2-CH2), 1.38 (3H, s, CH3), 1.36 (3H, s, CH3), 1.35 (3H, s, CH3). 13C-NMR (DMSO, 100 MHz) δ: 184.41, 164.08, 158.45, 139.80, 127.51, 126.75, 118.49, 117.34, 52.73, 47.70, 43.15, 35.44, 32.14, 26.54, 19.25, 18.46, 11.10. IR (KBr) v, cm−1: 1628 (C=N). HRMS (ES, +H): Theoretical mass calculated for [C17H21N3O3 + H]: 316.1661; Found: 316.1651.
2-((E)-((E)-(1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazono)methyl)pyridine (3x). Purified by column chromatography on silica gel (70–230 mesh, AcOEt/n-Hex 30%). Yield: 38%, oil. 1H-NMR (DMSO, 400 MHz) δ: 8.64 (1H, m, phenyl), 8.19 (1H, s, CH=N), 7.80–8.00 (3H, m, phenyl), 2.05–2.55 (2H, m, N=C-CH2), 1.92 (1H, t, CH, J = 4.12 Hz), 1.05–1.97 (4H, m, CH2-CH2), 1.01 (3H, s, CH3), 0.93 (3H, s, CH3), 0.77 (3H, s, CH3). 13C-NMR (DMSO, 100 MHz) δ: 181.05, 156.64, 152.95, 149.58, 136.79, 124.91, 120.86, 52.37, 47.45, 43.18, 35.33, 32.20, 26.70, 19.25, 18.51, 11.20. IR (KBr) v, cm−1: 1641 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C16H21N3 + H]: 256.1814; Found: 256.1800.
1,7,7-Trimethylbicyclo[2.2.1]heptan-2-amine hydrochloride 5 [
26]. A stirred solution of the oxime
4 (2.0 g, 12.40 mmol) in MeOH (120 mL) was hydrogenated using 5 bar pressure of H
2 and 10% Pd/C (0.12 g) for 48 h at 80 °C. The reaction mixture was filtered, rotary evaporated, and the oily residue dissolved in acetone (20 mL). The solution was acidified (pH = 2) with gaseous HCl. The precipitate of the hydrochloride
5 was collected, washed with cold acetone, and dried under vacuum. Yield: 0.75 g, 50%.
1H-NMR (DMSO, 400 MHz) δ: 7.96 (3H, sbr, NH
3), 3.00 (1H, m, CH-NH
3), 1.70–1.89 (3H, m, N=C-CH
2, CH), 1.10–1.69 (4H, m, CH
2-CH
2), 0.94 (3H, s, CH
3), 0.90 (3H, s, CH
3), 0.81 (3H, s, CH
3).
13C-NMR (DMSO, 100 MHz) δ: 57.17, 47.48, 46.66, 44.15, 35.75, 35.71, 26.17, 20.02, 19.93, 11.42. IR (KBr) v, cm
−1: 3039 (NH). MS (
m/
z, %): 153 (M
+, 43), 108 (25), 95 (99), 82 (100), 70 (24).
3.5. General Synthetic Procedure for the Imine Derivatives 7
The appropriate amine (1.5–2 equivalents) was added with stirring to a solution of nitroimine 1 (1.0–3.0 mmol) in CHCl3 or CH3CN (15–20 mL). The reaction mixture was refluxed until TLC indicated the consumption of 1 or lack of further reaction (3–72 h). The products 7a–7d were obtained pure after adequate procedure: the reaction solution (in this case chloroform) was extracted with a saturated aqueous solution of NaHCO3 (3 × 5 mL). The organic layer was washed with a saturated aqueous solution of brine (5 mL) and dried over MgSO4. The solvents were removed by rotary evaporation to furnish the product pure in accordance with spectra analyses (in these reactions the use of recrystallized oxime was necessary).
The products 7e–7n, after evaporation of the solvent, were purified by column chromatography on silica gel (70–230 mesh, AcEOt/n-Hex 10%–50%).
(E)-1-Phenyl-N-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)methanamine (
7a) [
27]. Yield: 55%, oil.
1H-NMR (MeOD, 400 MHz) δ 7.21–7.35 (5H, m, phenyl), 4.47 (2H, dd,
J =14.3 Hz), 1.95–2.50 (3H, m, N=C-CH
2, CH), 1.10–1.70 (4H, m, CH
2-CH
2), 1.00 (3H, s, CH
3), 0.96 (3H, s, CH
3), 0.79 (3H, s, CH
3).
13C-NMR (MeOD, 100 MHz) δ: 57.17, 47.48, 46.66, 44.15, 35.75, 35.71, 26.17, 20.02, 19.93, 11.42. IR (KBr) v, cm
−1: 3039. MS (ESI, +H): 242.
(E)-N-(1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ylidene)butan-1-amine (
7b) [
28]. Yield: 70%, oil.
1H-NMR (MeOD, 400 MHz) δ: 3.15–3.27 (m, 2H, N-CH
2), 1.15–2.40 (11H, m, N=C-CH
2, CH, CH
2-CH
2-camphor, CH
2-CH
2-side chain), 0.96 (s, 3H, CH
3), 0.93 (3H, s, CH
3 side chain), 0.92 (3H, s, CH
3), 0.75 (s, 3H, CH
3).
13C-NMR (MeOD, 100 MHz) δ: 53.67, 52.34, 47.09, 44.08, 35.62, 32.94, 32.45, 27.75, 20.93, 19.79, 19.22, 14.28, 11.71. IR (KBr) v, cm
−1: 1677 (C=N). MS (ESI, +H): 208.
(E)-N-(1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ylidene)cyclopropanamine (7c). Yield: 65%, oil. 1H-NMR (MeOD, 400 MHz) δ: 2.65–2.75 (m, 1H, N-CH), 1.95–2.50 (2H, m, N=C-CH2) 1.93 (t, 1H, CH, J = 7.2 Hz), 1.15–1.90 (4H, m, CH2-CH2), 0.93 (3H, s, CH3), 0.91 (3H, s, CH3), 0.76 (3H, s, CH3), 0.71–0.74 (m, 4H, cyclopropyl). 13C-NMR (MeOD, 100 MHz) δ: 52.82, 46.45, 43.31, 35.34, 33.52, 31.90, 26.99, 19.25, 18.75, 11.44, 7.61, 7.38. IR (KBr) v, cm−1: 1677 (C=N). HRMS (ESI, +H): Theoretical mass calculated for C13H21N + H: 192.1752; Found: 192.1744.
(E)-N-(1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ylidene)cyclohexanamine (
7d) [
29]. Yield: 73%, oil.
1H-NMR (MeOD, 400 MHz) δ: 3.07 (m, 1H, N-CH), 1.15–2.55 (17H, m, N=C-CH
2, CH, CH
2-CH
2-camphor, CH
2(CH
2)
3CH
2-cyclohexyl), 0.96 (3H, s, -CH
3), 0.91 (3H, s, -CH
3), 0.75 (3H, s, -CH
3).
13C-NMR (MeOD, 100 MHz) δ: 52.82, 46.45, 43.31, 35.34, 33.52, 31.90, 26.99, 19.25, 18.75, 11.44, 7.61, 7.38. IR (KBr) v, cm
−1: 1683 (C=N). GC: 100%, MS (
m/
z, %): 233 (M
+, 25), 205 (100), 136 (17), 109 (16), 95 (16).
(E)-4-Fluoro-N-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)aniline (7e). Yield: 79%, oil. 1H-NMR (MeOD, 400 MHz) δ: 7.07 (4H, m, phenyl), 2.15–2.25 (1H, m, -=C-CHa-, 1.75–1.97 (4H, m, -N=C-CHb, CH, CH2), 1.25–1.55 (2H, m, CH2), 1.07 (3H, s, CH3), 1.00 (3H, s, CH3), 0.87 (3H, s, CH3). 13C-NMR (MeOD, 100 MHz) δ: 189.73, 162.38, 159.99, 148.78, 148.76, 122.57, 122.49, 116.97, 116.75, 55.56, 45.30, 37.60, 33.21, 28.25, 20.06, 19.44, 11.71. IR (KBr) v, cm−1: 1682 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C16H20FN + H]: 246.1658; Found: 246.1658.
(E)-4-Chloro-N-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)aniline (7f). Yield: 52%, mp: 34–36 °C. 1H-NMR (MeOD, 400 MHz) δ: 6.60–7.27 (4H, m, phenyl); 2.14–2.22 (1H, m, -=N=C-CHa); 1.68–1.95 (4H, m, N=C-CHb, CH, CH2); 1.15–1.55 (2H, m, CH2); 1.07 (3H, s, CH3); 0.97 (3H, s, CH3); 0.85 (3H, s, CH3). 13C-NMR (MeOD, 100 MHz) δ: 185.74, 150.77, 128.76, 128.28, 120.85, 54.09, 47.20, 43.74, 36.23, 31.99, 27.38, 19.54, 18.99, 11.16. IR (KBr) v, cm−1: 1680 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C16H20ClN + H]: 262.1636; Found: 262.1635.
(E)-B-Bromo-N-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)aniline (7g). Yield: 73%, oil. 1H-NMR (MeOD, 400 MHz) δ: 6.61–7.39 (4H, m, phenyl), 2.10–2.20 (1H, m, N=C-CHa), 1.65–1.95 (4H, m, N=C-CHb, CH, -CH2-), 1.20–1.55 (2H, m, CH2), 1.07 (3H, s, CH3), 0.97 (3H, s, CH3), 0.85 (3H, s, CH3). 13C-NMR (MeOD, 100 MHz) δ: 184.22, 151.10, 131.73, 121.53, 114.96, 53.79, 46.73, 43.16, 35.48, 31.56, 26.77, 19.25, 18.77, 11.23. IR (KBr) v, cm−1: 1604 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C16H20BrN + H]: 306.0857; Found: 306.0856.
(E)-4-Hydroxy-N-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)aniline (
7h) [
30]. Yield: 81%, mp: 142–144 °C.
1H-NMR (DMSO, 400 MHz) δ: 9.02 (1H, s, OH); 6.50–6.60 (4H, m, phenyl), 2.11–2.20 (1H, m, N=C-CH
a), 1.70–1.90 (4H, m, N=C-CH
b, CH, CH
2), 1.20–1.45 (2H, m, CH
2), 0.96 (3H, s, CH
3), 0.92 (3H, s, CH
3), 0.77 (3H, s, CH
3).
13C-NMR (DMSO, 100 MHz) δ: 182.32, 153.14, 143.17, 120.47, 115.39, 53.56, 46.48, 43.24, 40.13, 35,64, 31.70, 26.90, 19.28, 18.82, 11.46. IR (KBr) v, cm
−1: 1665 (C=N). GC: 100%, MS (
m/
z, %): 243 (M
+, 100), 160 (40), 135 (46), 134 (20), 95 (32).
(E)-4-Methoxy-N-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)aniline (
7i) [
31]. Yield: 90%, mp: 41–43 °C.
1H-NMR (MeOD, 400 MHz) δ: 6.60–6.90 (4H, m, phenyl); 3.75 (3H, s, OCH
3), 2.15–2.26 (1H, m, N=C-CH
a), 1.75–1.95 (4H, m, N=C-CH
b, CH, CH
2), 1.25–1.55 (2H, m, CH
2), 0.96 (3H, s, CH
3), 0.92 (3H, s, CH
3), 0.77 (3H, s, CH
3).
13C-NMR (MeOD, 100 MHz) δ: 189.25, 158.06, 145.45, 122.13, 115.63, 56.01, 55.47, 45.32, 37.71, 33.26, 28.30, 19.45, 11.78. IR (KBr) v, cm
−1: 1675 (C=N). GC: 99%, MS (
m/
z, %): 257 (M
+, 100%), 174 (30), 149 (33), 95 (23).
(E)-2-Hydroxy-N-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)aniline (
7j) [
32]. Yield: 53%, mp: 94–96 °C.
1H-NMR (DMSO, 400 MHz) δ: 8.83(1H, s, OH); 6.50–6.85 (4H, m, phenyl), 2.10–2.25 (1H, m, N=C-CH
a), 1.65–1.90 (4H, m, N=C-CH
b, CH, CH
2), 1.10–1.25 (2H, m, CH
2), 0.99 (3H, s, CH
3), 0.92 (3H, s, CH
3), 0.83 (3H, s, CH
3).
13C-NMR (DMSO, 100 MHz) δ: 183.86, 146.58, 139.20, 123.47, 120.37, 119.18, 115.75, 53.75, 46.98, 43.24, 36.58, 31.76, 26.96, 19.39, 18.89, 11.34. IR (KBr) v, cm
−1: 1668 (C=N). GC: 96%, MS (
m/
z, %): 243 (88), 228 (60), 172 (62), 134 (68), 95 (100).
(E)-2,5-Dimethoxy-N-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)aniline (7k). Yield: 46%, oil. 1H-NMR (MeOD, 400 MHz) δ: 6.25–6.92 (3H, m, phenyl), 3.72 (3H, s, OCH3), 3.70 (3H, s, OCH3), 2.13–2.28 (1H, m, N=C-CHa), 1.60–1.90 (4H, m, N=C-CHb, CH, CH2), 1.20–1.55 (2H, m, CH2), 1.08 (3H, s, CH3), 0.99 (3H, s, CH3), 0.90 (3H, s, CH3). 13C-NMR (MeOD, 100 MHz) δ: 190.60, 155.88, 145.16, 142.30 114.59, 109.98, 108.57, 56.89, 56.18, 55.71, 45.34, 38.31, 33.34, 28.28, 20.08, 19.52, 11.65. IR (KBr) v, cm−1: 1682 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C15H20N2 + H]: 288.1964; Found: 288.1962.
(E)-N-(1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ylidene)pyridin-2-amine (
7l) [
33]. Yield: 39%, oil.
1H-NMR (MeOD, 400 MHz) δ: 6.75–8.35 (4H, m, phenyl), 2.20–2.55 (1H, m, N=C-CH
a), 1.70–1.90 (4H, m, N=C-CH
b, CH, CH
2), 1.20–1.50 (2H, m, CH
2), 0.99 (3H, s, CH
3), 0.94 (3H, s, CH
3), 0.82 (3H, s, CH
3).
13C-NMR (MeOD, 100 MHz) δ: 185.33, 163.31, 148.45, 137.87 118.99, 115.07, 53.84, 46.83, 43.16, 35.88, 31.49, 26.78, 19.28, 18.78, 11.21. IR (KBr) v, cm
−1: 1681 (C=N). GC: 100, MS (
m/
z, %): 228 (45), 213 (72), 200 (100), 185 (65), 78 (48).
(E)-N-(1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ylidene)pyridin-4-amine (7m). Yield: 10%, oil. 1H-NMR (MeOD, 400 MHz) δ: 6.70–8.45 (4H, m, phenyl), 2.10–2.20 (1H, m, N=C-CHa), 1.65–1.92 (4H, m, N=C-CHb, CH, CH2), 1.20–1.50 (2H, m, CH2), 0.98 (3H, s, CH3), 0.94 (3H, s, CH3), 0.82 (3H, s, CH3). 13C-NMR (MeOD, 100 MHz) δ: 187.29, 160.06, 149.49, 115.31, 54.22, 43.76, 35.72, 31.64, 26.76, 18.50, 17.89, 15.00. IR (KBr) v, cm−1: 1682 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C15H20N2 + H]: 229.1705; Found: 229.1695.
(E)-N-(1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ylidene)methyl)thiazole (7n). Yield: 15%, oil. 1H-NMR (MeOD, 400 MHz) δ: 7.55 (2H, d, phenyl, J=3.6 Hz), 7.35 (2H, d, phenyl, J=3.6 Hz), 2.55–2.65 (1H, m, N=C-CHa), 2.19 (1H, d, N=C-CHb, J = 18.4 Hz), 2.06 (1H, t, CH, J = 4.4 Hz), 1.85-1.96 (2H, m, CH2), 1.30–1.50 (2H, m, CH2), 1.08 (3H, s, CH3), 1.02 (3H, s, CH3), 0.85 (3H, s, CH3). 13C-NMR (MeOD, 100 MHz) δ: 194.58, 173.30, 140.90, 118.48, 57.07, 45.58, 39.64, 33.11, 28.00, 20.09, 19.45, 11.65. IR (KBr) v, cm−1: 1643 (C=N). HRMS (ESI, +H): Theoretical mass calculated for [C13H18N2S + H]: 235.1269; Found: 235.1268.