The Role of Lipopolysaccharide-Induced Extracellular Vesicles in Cardiac Cell Death
Abstract
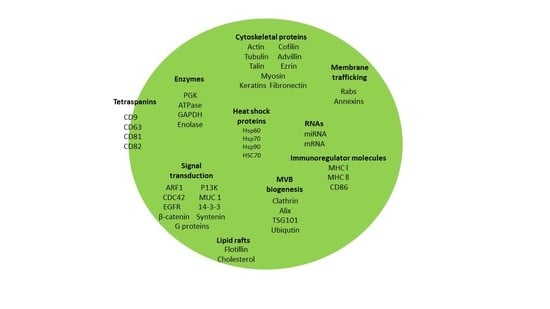
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Treatment with LPS
2.3. Cell Viability Using Trypan Blue Dye
2.4. Exosome Purification and Isolation
2.5. Exosome Characterization
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Statistical Analysis
3. Results
3.1. Viability of Cardiomyocytes after LPS Treatment
3.2. Exosome Characterization after LPS Treatment
3.3. Analysis of Exosome-Associated Proteins in LPS-Treated AC16 Cells via ELISA
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Accessibility
References
- Peleg, A.Y.; Hooper, D.C. Hospital-acquired infections due to gram-negative bacteria. N. Engl. J. Med. 2010, 362, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.F.; Sa-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in gram-negative bacteria during chronic infection. Fems Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Meredith, T.C.; Kahne, D. On the essentiality of lipopolysaccharide to gram-negative bacteria. Curr. Opin. Microbiol. 2013, 16, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C.; Trent, M.S. Biosynthesis and export of bacterial lipopolysaccharides. Annu. Rev. Biochem. 2014, 83, 99–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Quinn, P.J. Endotoxins: Lipopolysaccharides of gram-negative bacteria. Sub-Cell. Biochem. 2010, 53, 3–25. [Google Scholar]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef]
- Tran, A.X.; Trent, M.S.; Whitfield, C. The lpta protein of escherichia coli is a periplasmic lipid a-binding protein involved in the lipopolysaccharide export pathway. J. Biol. Chem. 2008, 283, 20342–20349. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef]
- Davidson, S.M.; Takov, K.; Yellon, D.M. Exosomes and cardiovascular protection. Cardiovasc. Drugs Ther. 2017, 31, 77–86. [Google Scholar] [CrossRef]
- Crenshaw, B.J.; Sims, B.; Matthews, Q.L. Biological function of exosomes as diagnostic markers and therapeutic delivery vehicles in carcinogenesis and infectious diseases. In Nanomedicines; Intech Open Limited: London, UK, 2018. [Google Scholar]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Gartz, M.; Strande, J.L. Examining the paracrine effects of exosomes in cardiovascular disease and repair. J. Am. Heart Assoc. 2018, 7, e007954. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.B.; Bell, C.R.; Bibb, K.E.; Gu, L.; Coats, M.T.; Matthews, Q.L. Pathogens and their effect on exosome biogenesis and composition. Biomedicines 2018, 6, 79. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, G.; Neuer, A.L.; Herrmann, I.K. Extracellular vesicles—A promising avenue for the detection and treatment of infectious diseases? Eur. J. Pharm. Biopharm. 2017, 118, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.; Vicencio, J.M.; Yellon, D.M.; Davidson, S.M. Microvesicles and exosomes: New players in metabolic and cardiovascular disease. J. Endocrinol. 2016, 228, R57–R71. [Google Scholar] [CrossRef]
- Bellin, G.; Gardin, C.; Ferroni, L.; Chachques, J.C.; Rogante, M.; Mitrecic, D.; Ferrari, R.; Zavan, B. Exosome in cardiovascular diseases: A complex world full of hope. Cells 2019, 8, 166. [Google Scholar] [CrossRef]
- Chen, X.; Qian, B.; Kong, X.; Hao, J.; Ye, Y.; Yang, K.; Xu, T.; Zhang, F. A20 protects neuronal apoptosis stimulated by lipopolysaccharide-induced microglial exosomes. Neurosci. Lett. 2019, 712, 134480. [Google Scholar] [CrossRef]
- Wang, G.; Jin, S.; Ling, X.; Li, Y.; Hu, Y.; Zhang, Y.; Huang, Y.; Chen, T.; Lin, J.; Ning, Z.; et al. Proteomic profiling of lps-induced macrophage-derived exosomes indicates their involvement in acute liver injury. Proteomics 2019, 19, e1800274. [Google Scholar] [CrossRef]
- Yang, Y.; Boza-Serrano, A.; Dunning, C.J.R.; Clausen, B.H.; Lambertsen, K.L.; Deierborg, T. Inflammation leads to distinct populations of extracellular vesicles from microglia. J. Neuroinflamm. 2018, 15, 168. [Google Scholar] [CrossRef]
- Bell, C.R. Microbial Assessment of Human Heart and Brain Samples. Unpublished Work. 2019. [Google Scholar]
- Wilson, J.W.; Schurr, M.J.; LeBlanc, C.L.; Ramamurthy, R.; Buchanan, K.L.; Nickerson, C.A. Mechanisms of bacterial pathogenicity. Postgrad. Med J. 2002, 78, 216–224. [Google Scholar] [CrossRef]
- Bari, R.; Guo, Q.; Xia, B.; Zhang, Y.H.; Giesert, E.E.; Levy, S.; Zheng, J.J.; Zhang, X.A. Tetraspanins regulate the protrusive activities of cell membrane. Biochem. Biophys. Res. Commun. 2011, 415, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Rohlena, J.; Volger, O.L.; van Buul, J.D.; Hekking, L.H.; van Gils, J.M.; Bonta, P.I.; Fontijn, R.D.; Post, J.A.; Hordijk, P.L.; Horrevoets, A.J. Endothelial cd81 is a marker of early human atherosclerotic plaques and facilitates monocyte adhesion. Cardiovasc. Res. 2009, 81, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Hein, S.; Kostin, S.; Heling, A.; Maeno, Y.; Schaper, J. The role of the cytoskeleton in heart failure. Cardiovasc. Res. 2000, 45, 273–278. [Google Scholar] [CrossRef]
- Davidson, S.M.; Yellon, D.M. Exosomes and cardioprotectioncritical analysis. Mol. Asp. Med. 2018, 60, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Ehler, E. Actin-associated proteins and cardiomyopathy—the “unknown” beyond troponin and tropomyosin. Biophys. Rev. 2018, 10, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Menasche, G.; Feldmann, J.; Houdusse, A.; Desaymard, C.; Fischer, A.; Goud, B.; de Saint Basile, G. Biochemical and functional characterization of rab27a mutations occurring in griscelli syndrome patients. Blood 2003, 101, 2736–2742. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, E.; Severin, F.; Backer, J.M.; Hyman, A.A.; Zerial, M. Rab5 regulates motility of early endosomes on microtubules. Nat. Cell Biol. 1999, 1, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Hume, A.N.; Collinson, L.M.; Rapak, A.; Gomes, A.Q.; Hopkins, C.R.; Seabra, M.C. Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J. Cell Biol. 2001, 152, 795–808. [Google Scholar] [CrossRef]
- Davidson, S.M.; Riquelme, J.A.; Zheng, Y.; Vicencio, J.M.; Lavandero, S.; Yellon, D.M. Endothelial cells release cardioprotective exosomes that may contribute to ischaemic preconditioning. Sci. Rep. 2018, 8, 15885. [Google Scholar] [CrossRef]
- Wu, R.; Gao, W.; Yao, K.; Ge, J. Roles of exosomes derived from immune cells in cardiovascular diseases. Front. Immunol. 2019, 10, 648. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, J.M.; Kim, J.; Hur, J.; Park, S.; Kim, K.; Shin, H.J.; Chwae, Y.J. Molecular mechanisms of biogenesis of apoptotic exosome—like vesicles and their roles as damage-associated molecular patterns. Proc. Natl. Acad. Sci. USA 2018, 115, E11721–E11730. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bell, C.R.; Jones, L.B.; Crenshaw, B.J.; Kumar, S.; Rowe, G.C.; Sims, B.; Javan, G.T.; Matthews, Q.L. The Role of Lipopolysaccharide-Induced Extracellular Vesicles in Cardiac Cell Death. Biology 2019, 8, 69. https://doi.org/10.3390/biology8040069
Bell CR, Jones LB, Crenshaw BJ, Kumar S, Rowe GC, Sims B, Javan GT, Matthews QL. The Role of Lipopolysaccharide-Induced Extracellular Vesicles in Cardiac Cell Death. Biology. 2019; 8(4):69. https://doi.org/10.3390/biology8040069
Chicago/Turabian StyleBell, Courtnee’ R., Leandra B. Jones, Brennetta J. Crenshaw, Sanjay Kumar, Glenn C. Rowe, Brian Sims, Gulnaz T. Javan, and Qiana L. Matthews. 2019. "The Role of Lipopolysaccharide-Induced Extracellular Vesicles in Cardiac Cell Death" Biology 8, no. 4: 69. https://doi.org/10.3390/biology8040069
APA StyleBell, C. R., Jones, L. B., Crenshaw, B. J., Kumar, S., Rowe, G. C., Sims, B., Javan, G. T., & Matthews, Q. L. (2019). The Role of Lipopolysaccharide-Induced Extracellular Vesicles in Cardiac Cell Death. Biology, 8(4), 69. https://doi.org/10.3390/biology8040069