Abiotic Deposition of Fe Complexes onto Leptothrix Sheaths
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Medium, and Culturing
2.2. Colony-Forming Unit (cfu) Test to Examine Growth of Cell Population
2.3. Lysozyme-EDTA-SDS Bacteriolysis
2.4. Determination of Fe(II) and Total Fe Concentration in Culture Medium with O-Phenanthroline
2.5. Scanning Electron Microscopy (SEM) and Energy Dispersive X-Ray Spectroscopy (EDX)
2.6. Transmission Electron Microscopy (TEM) Imaging and EDX Elemental Mapping
3. Results
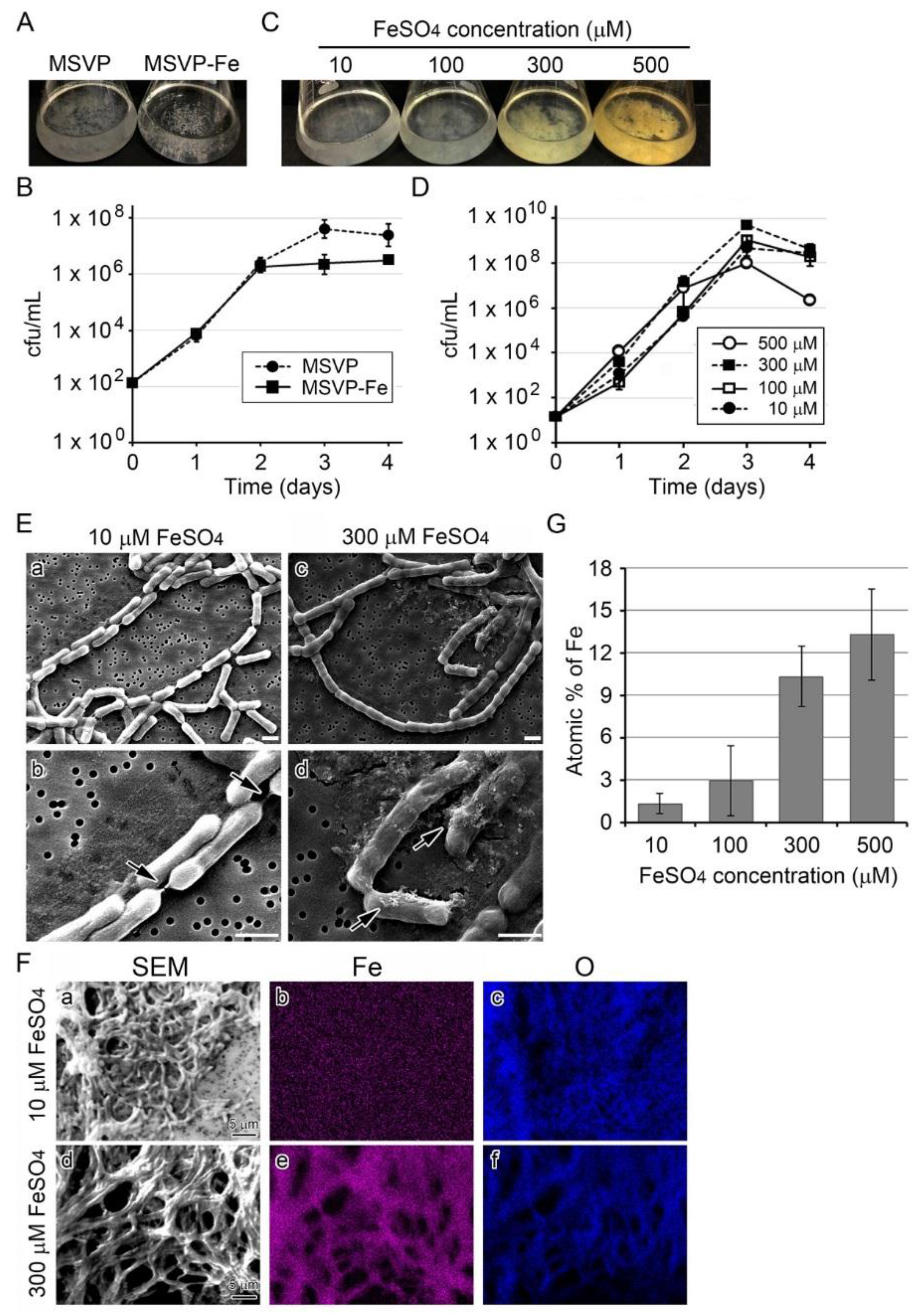
3.1. Influence of Fe(II) Concentration in Medium on Growth of SP-6 Cells
3.2. Influence of Fe(II) Concentration on Fe Deposition onto Sheaths
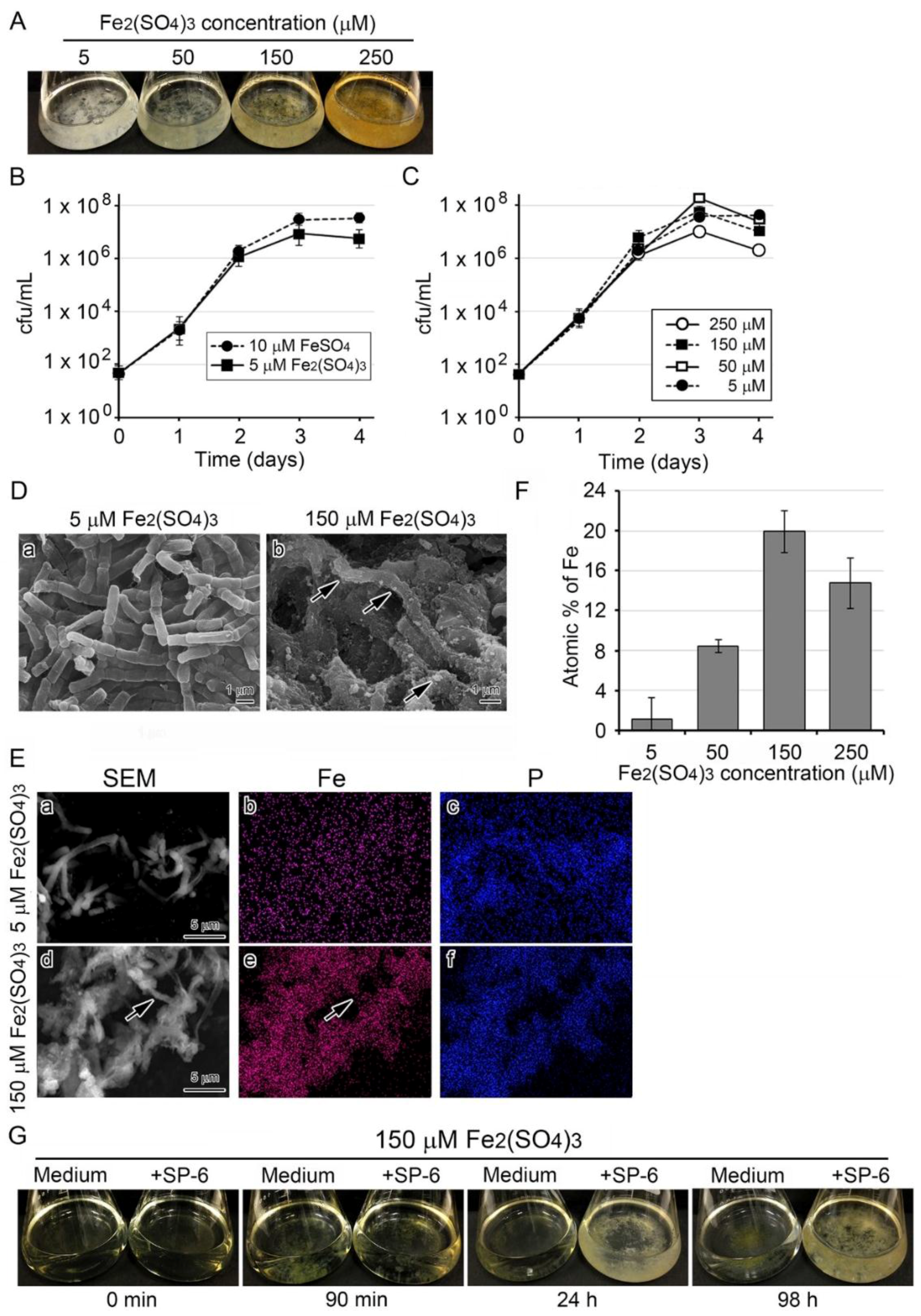
3.3. Deposition of Fe-Containing Precipitates onto Sheaths in Media Containing Higher Concentrations of the Fe Source
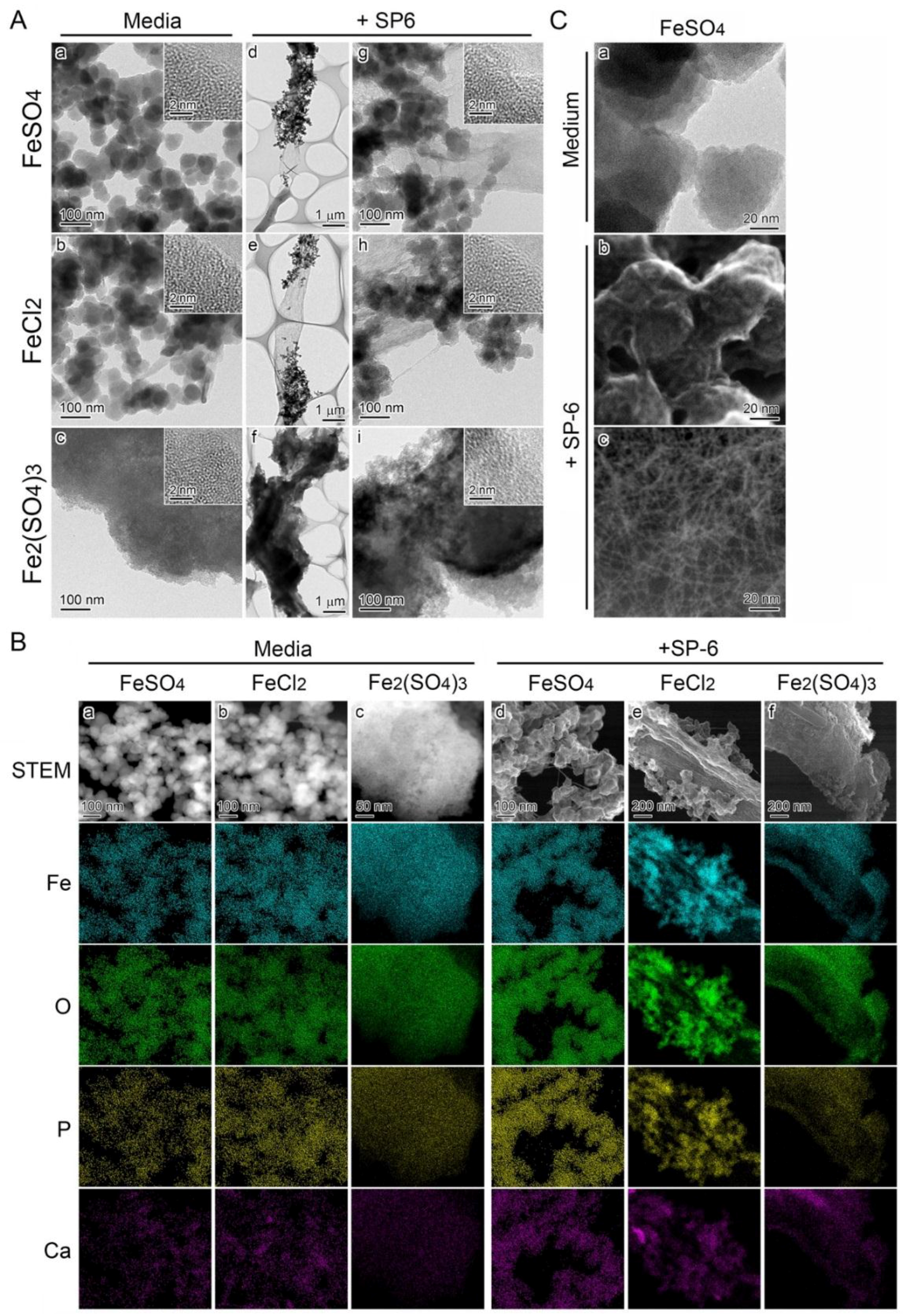
3.4. Recruitment of Precipitates in Media Containing Fe(III) for Fe Deposition onto Sheaths
3.5. Deposition of Media-Formed Fe Precipitates onto Initial Sheaths
3.6. Fe Deposition onto Sheaths Unrelated to Bacterial Cells
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| cfu | Colony-forming unit |
| DIC | Differential interference contrast microscope |
| PH | Phase-contrast microscope |
| SEM | Scanning electron microscopy |
| EDX | Energy dispersive X-ray spectroscopy |
| STEM | Scanning transmission electron microscopy |
| HAADF | High angle annular dark-field |
| HRETM | High-resolution transmission electron microscopy |
References
- Ghiorse, W.C.; Hirsch, P. An ultrastructural study of iron and manganese deposition associated with extracellular polymers of pedomicrobium-like budding bacteria. Arch. Microbiol. 1979, 123, 213–226. [Google Scholar] [CrossRef]
- Spring, S. The general Leptothrix and Sphaerotilus. Prokaryotes 2006, 5, 758–777. [Google Scholar]
- Furutani, M.; Suzuki, T.; Ishihara, H.; Hashimoto, H.; Kunoh, H.; Takada, J. Initial assemblage of bacterial saccharic fibrils and element deposition to form an immature sheath in cultured Leptothrix sp. strain OUMS1. Minerals 2011, 1, 157–166. [Google Scholar] [CrossRef]
- Ishihara, H.; Suzuki, T.; Hashimoto, H.; Kunoh, H.; Takada, J. Initial parallel arrangement of extracellular fibrils holds a key for sheath frame construction by Leptothrix sp. strain OUMS1. Minerals 2013, 3, 73–81. [Google Scholar] [CrossRef]
- Emerson, D.; Ghiorse, W.C. Ultrastructure and chemical composition of the sheath of Leptothrix discophora SP-6. J. Bacteriol. 1993, 175, 7808–7818. [Google Scholar] [PubMed]
- Suzuki, T.; Hashimoto, H.; Ishihara, H.; Kasai, T.; Kunoh, H.; Takada, J. Structural and spatial associations between Fe, O, and C in the network structure of the Leptothrix ochracea sheath surface. Appl. Environ. Microbiol. 2011, 77, 7873–7875. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Ishihara, H.; Furutani, M.; Shiraishi, T.; Kunoh, H.; Takada, J. A novel method for culturing of Leptothrix sp. strain OUMS1 in natural conditions. Minerals 2012, 2, 118–128. [Google Scholar] [CrossRef]
- Angelova, R.; Groudeva, V.; Slavov, L.; Iliev, M.; Nedkov, I.; Sziklai-László, I.; Krezhov, K. Investigation of iron-containing products from natural and laboratory cultivated Sphaerotilus-Leptothrix bacteria. J. Biol. Phys. 2015, 4, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Corstjens, P.L.; de Vrind, J.P.; Westbroek, P.; de Vrind-de Jong, E.W. Enzymatic iron oxidation by Leptothrix discophora: Identification of an iron-oxidizing protein. Appl. Environ. Microbiol. 1992, 58, 450–454. [Google Scholar] [PubMed]
- Adams, L.F.; Ghiorse, W.C. Characterization of extracellular Mn2+-oxidizing activity and isolation of an Mn2+-oxidizing protein from Leptothrix discophora SS-1. J. Bacteriol. 1987, 169, 1279–1285. [Google Scholar] [PubMed]
- Boogerd, F.C.; de Vrind, J.P. Manganese oxidation by Leptothrix discophora. J. Bacteriol. 1987, 169, 489–494. [Google Scholar] [PubMed]
- Emerson, D.; Ghiorse, W.C. Isolation, cultural maintenance, and taxonomy of a sheath-forming strain of Leptothrix discophora and characterization of manganese-oxidizing activity associated with the sheath. Appl. Environ. Microbiol. 1992, 58, 4001–4010. [Google Scholar] [PubMed]
- De Vrind-de Jong, E.W.; Corstjens, P.L.; Kempers, E.S.; Westbroek, P.; de Vrind, J.P. Oxidation of manganese and iron by Leptothrix discophora: Use of N,N,N′,N′-tetramethyl-p-phenylenediamine as an indicator of metal oxidation. Appl. Environ. Microbiol. 1990, 56, 3458–3462. [Google Scholar] [PubMed]
- Emerson, D.; Fleming, E.J.; McBeth, J.M. Iron-oxidizing bacteria: An environmental and genomic perspective. Annu. Rev. Microbiol. 2010, 64, 561–583. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.G.; Schultze, S.; Witten, T.C.; Fyfe, W.S.; Beveridge, T.J. Metal interactions with microbial biofilms in acidic and neutral pH environments. Appl. Environ. Microbiol. 1989, 55, 1249–1257. [Google Scholar] [PubMed]
- Toner, B.M.; Santelli, C.M.; Marcus, M.A.; Wirth, R.; Chan, C.S.; McCollon, T.; Bach, W.; Edwards, K.J. Biogenic iron oxyhydroxide formation at mid-ocean ridge hydrothermal vents: Juan de Fuca Ridge. Geochim. Cosmochim. Acta 2009, 73, 388–403. [Google Scholar] [CrossRef]
- Rogers, S.R.; Anderson, J.J. Measurement of growth and iron deposition in Sphaerotilus discophorus. J. Bacteriol. 1976, 126, 257–263. [Google Scholar] [PubMed]
- Vollrath, S.; Behrends, T.; Koch, C.B.; van Cappellen, P. Effects of temperature on rates and mineral products of microbial Fe(II) oxidation by Leptothrix cholodnii at microaerobic conditions. Geochim. Cosmochim. Acta 2013, 108, 107–124. [Google Scholar] [CrossRef]
- Nelson, Y.M.; Lion, L.W.; Ghiorse, W.C.; Shuler, M.L. Production of biogenic Mn oxides by Leptothrix discophora SS-1 in a chemically defined growth medium and evaluation of their Pb adsorption characteristics. Appl. Environ. Microbiol. 1999, 65, 175–180. [Google Scholar] [PubMed]
- Sawayama, M.; Suzuki, T.; Hashimoto, H.; Kasai, T.; Furutani, M.; Miyata, N.; Kunoh, H.; Takada, J. Isolation of a Leptothrix strain, OUMS1, from ocherous deposits in groundwater. Curr. Microbiol. 2011, 63, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kunoh, T.; Nakatsuka, D.; Hashimoto, H.; Tamura, K.; Kunoh, H.; Takada, J. Use of iron powder to obtain high yields of Leptothrix sheaths in culture. Minerals 2015, 5, 335–345. [Google Scholar] [CrossRef]
- Singer, P.C.; Stumm, W. Acidic mine drainage: The rate-determining step. Science 1970, 167, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; Pergamon Press: Oxford, UK, 1996. [Google Scholar]
- Emerson, D.; Garen, R.E.; Ghiorse, W.C. Formation of Metallogenium-like structures by a manganese-oxiding fungus. Arch. Microbiol. 1989, 151, 223–231. [Google Scholar] [CrossRef]
- Kunoh, T.; Suzuki, T.; Shiraishi, T.; Kunoh, H.; Takada, J. Treatment of Leptothrix cells with ultrapure water poses a threat to their viability. Biology 2015, 4, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Luman, C.R.; Castellano, F. Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 25–39. [Google Scholar]
- Chan, C.S.; Fakra, S.C.; Edwards, D.C.; Emerson, D.; Banfield, J.F. Iron oxyhydroxide mineralization on microbial extracellular polysaccharides. Geochim. Cosmochim. Acta. 2009, 73, 3807–3818. [Google Scholar] [CrossRef]
- Chan, C.S.; de Stasio, G.; Welch, S.A.; Girasole, M.; Frazer, B.H.; Nesterova, M.V.; Fakra, S.; Banfeld, J.F. Microbial polysaccharides template assembly of nanocyrstal fibers. Science 2004, 303, 1656–1658. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.S.; McAllister, S.M.; Leavitt, A.H.; Glazer, B.T.; Krepski, S.T.; Emerson, D. The architecture of iron microbial mats reflects the adaptation of chemolithotrophic iron oxidation in freshwater and marine environments. Front. Microbiol. 2016. [Google Scholar] [CrossRef]
- Vollrath, S.; Behrends, T.; van Cappellen, P. Oxygen Dependency of Neutrophilic Fe(II) Oxidation by Leptothrix Differs from Abiotic Reaction. Geomicrobiol. J. 2012, 29, 550–560. [Google Scholar] [CrossRef]
- Hashimoto, H.; Kobayashi, G.; Sakuma, R.; Fujii, T.; Hayashi, N.; Kanno, R.; Takano, M.; Takada, J. Bacterial nanometric amorphous Fe-based oxide: A potential lithium-ion battery anode material. ACS Appl. Mater. Interfaces 2014, 6, 5374–5378. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, R.; Hashimoto, H.; Kobayashi, G.; Fujii, T.; Nakanishi, M.; Kanno, R.; Takano, M.; Takada, J. High-rate performance of a bacterial iron-oxide electrode material for lithium-ion battery. Mater. Lett. 2015, 139, 414–417. [Google Scholar] [CrossRef]
- Ema, T.; Miyazaki, Y.; Kozuki, I.; Sakai, T.; Hashimoto, H.; Takada, J. Highly active lipase immobilized on biogenous iron oxide via an organic bridging group: The dramatic effect of the immobilization support on enzymatic function. Green Chem. 2011, 13, 3187–3195. [Google Scholar] [CrossRef]
- Ema, T.; Miyazaki, Y.; Taniguchi, T.; Takada, J. Robust porphyrin catalysts immobilized on biogenous iron oxide for the repetitive conversions of epoxides and CO2 into cyclic carbonates. Green Chem. 2013, 15, 2485–2492. [Google Scholar] [CrossRef]
- Mandai, K.; Korenaga, T.; Ema, T.; Sakai, T.; Furutani, M.; Hashimoto, H.; Takada, J. Biogenous iron oxide-immobilized palladium catalyst for the solvent-free Suzuki-Miyaura coupling reaction. Tetrahedron Lett. 2012, 53, 329–332. [Google Scholar] [CrossRef]
- Hashimoto, H.; Asaoka, H.; Nakano, T.; Kusano, Y.; Ishihara, H.; Ikeda, Y.; Nakanishi, M.; Fujii, T.; Yokoyama, T.; Horiishi, N.; et al. Preparation, microstructure, and color tone of microtubule material composed of hematite/amorphous-silicate nonocomposite from iron oxide of bacterial origin. Dyes. Pigm. 2012, 95, 639–643. [Google Scholar] [CrossRef]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunoh, T.; Hashimoto, H.; McFarlane, I.R.; Hayashi, N.; Suzuki, T.; Taketa, E.; Tamura, K.; Takano, M.; El-Naggar, M.Y.; Kunoh, H.; et al. Abiotic Deposition of Fe Complexes onto Leptothrix Sheaths. Biology 2016, 5, 26. https://doi.org/10.3390/biology5020026
Kunoh T, Hashimoto H, McFarlane IR, Hayashi N, Suzuki T, Taketa E, Tamura K, Takano M, El-Naggar MY, Kunoh H, et al. Abiotic Deposition of Fe Complexes onto Leptothrix Sheaths. Biology. 2016; 5(2):26. https://doi.org/10.3390/biology5020026
Chicago/Turabian StyleKunoh, Tatsuki, Hideki Hashimoto, Ian R. McFarlane, Naoaki Hayashi, Tomoko Suzuki, Eisuke Taketa, Katsunori Tamura, Mikio Takano, Mohamed Y. El-Naggar, Hitoshi Kunoh, and et al. 2016. "Abiotic Deposition of Fe Complexes onto Leptothrix Sheaths" Biology 5, no. 2: 26. https://doi.org/10.3390/biology5020026





