Root-Zone Glyphosate Exposure Adversely Affects Two Ditch Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Procedures
2.3. Plant Measurements
2.4. Data Analyses
3. Results
3.1. Chlorophyll Content Index
3.2. Root-to-Shoot Biomass Ratios
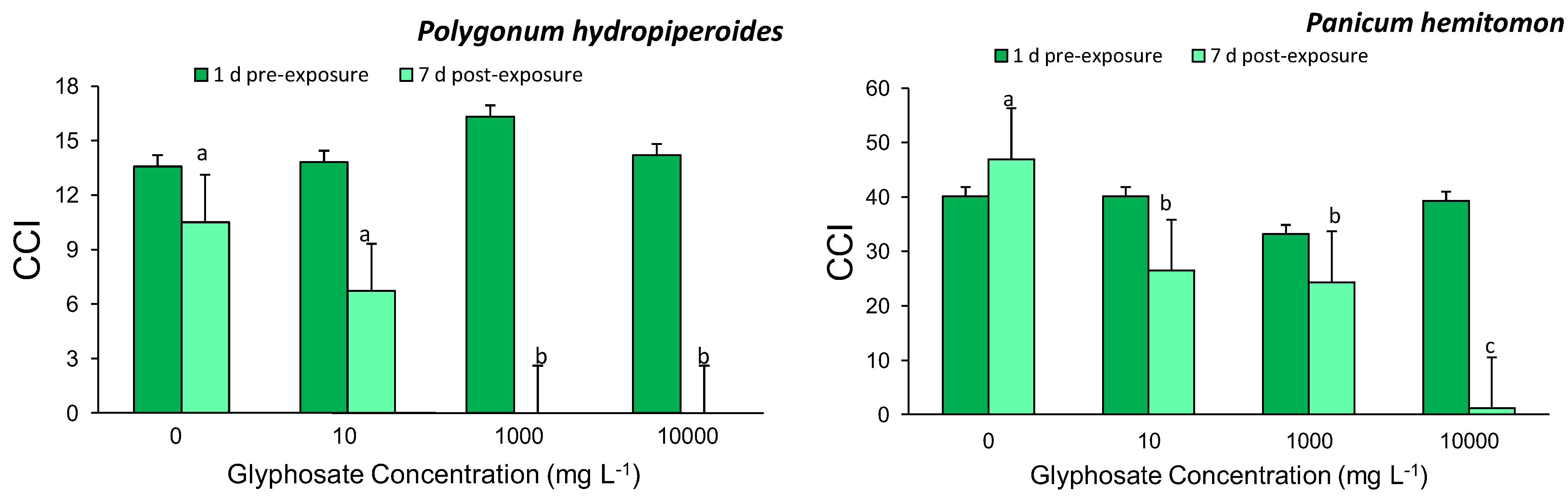

3.3. Survival
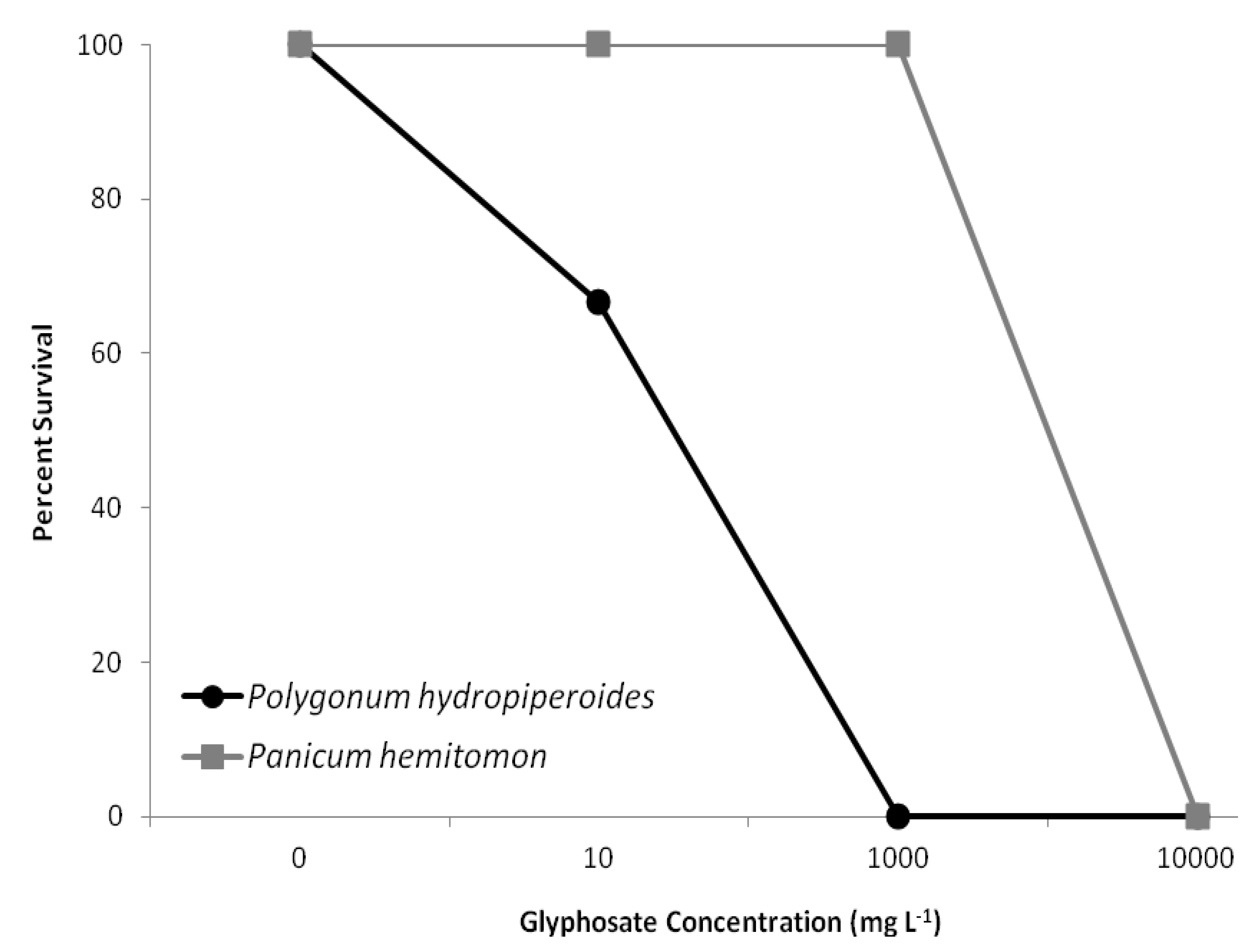
4. Discussion and Conclusions
Acknowledgments
Conflicts of Interest
References
- Battaglin, W.A.; Kolpin, D.W.; Scribner, E.A.; Kuivila, K.M.; Sandstrom, M.W. Glyphosate, other herbicides, and transformation products in Midwestern streams, 2002. J. Am. Water Resour. Assoc. 2005, 41, 323–332. [Google Scholar] [CrossRef]
- Pollegioni, L.; Schonbruun, E.; Siehl, D. Molecular basis of glyphosate resistance—Different approaches through protein engineering. FEBS J. 2011, 278, 2753–2766. [Google Scholar] [CrossRef]
- Grube, A.; Donaldson, D.; Kiely, T.; Wu, L. Pesticides Industry Sales and Usage 2006 and 2007 Market Estimates; EPA 733-R-11-001, US Environmental Protection Agency, Office of Chemical Safety and Pollution Prevention: Washington, DC, USA, 2011. [Google Scholar]
- De Snoo, G.R.; van der Poll, R.J. Effect of herbicide drift on adjacent boundary vegetation. Agric. Ecosyst. Environ. 1999, 73, 1–6. [Google Scholar] [CrossRef]
- White, A.L.; Boutin, C. Herbicidal effects on nontarget vegetation: Investigating the limitations of current pesticide registration guidelines. Environ. Toxicol. Chem. 2007, 26, 2634–2643. [Google Scholar] [CrossRef]
- Dalton, R.L.; Boutin, C. Comparison of the effects of glyphosate and atrazine herbicides on nontarget plants grown singly and in microcosms. Environ. Toxicol. Chem. 2010, 29, 2304–2315. [Google Scholar] [CrossRef]
- Vereecken, H. Mobility and leaching of glyphosate: A review. Pest Manag. Sci. 2005, 61, 1139–1151. [Google Scholar] [CrossRef]
- Weaver, L.M.; Herrmann, K.M. Dynamics of the shikimate pathway in plants. Trends Plant Sci. 1997, 2, 346–351. [Google Scholar] [CrossRef]
- Gruys, K.J.; Sikorski, J.A. Inhibitors of tryptophan, phenylalanine, and tyrosine biosynthesis as herbicides. In Plant Amino Acids: Biochemistry and Biotechnology; Singh, K., Ed.; Marcel Dekker: New York, NY, USA, 1999; pp. 357–384. [Google Scholar]
- Hoagland, R.E.; Duke, S.O. Biochemical effects of glyphosate [N-(Phosphonomethyl)glycine]. In Biochemical Responses Induced by Herbicides; Moreland, D.E., St. John, J.B., Hess, F.D., Eds.; American Chemical Society: Washington, DC, USA, 1982; pp. 175–205. [Google Scholar]
- Giesy, J.P.; Dobson, S.; Solomon, K.R. Ecotoxicological risk assessment for Roundup® herbicide. Rev. Environ. Contam. Toxicol. 2000, 167, 35–120. [Google Scholar]
- Pierce, S.C.; Pezeshki, S.R. Vegetation in agricultural ditches: Limitations to establishment, productivity and ecosystem functioning. In Agricultural Drainage Ditches: Mitigation Wetlands for the 21st Century; Moore, M.T., Kröger, R., Eds.; Research Signpost: Kerala, India, 2010; pp. 75–106. [Google Scholar]
- USEPA Pesticides: Environmental Effects. Available online: http://www.epa.gov/oppefed1/ecorisk_ders/toera_analysis_exp.htm/ (accessed on 4 January 2012).
- Bouldin, J.L.; Farris, J.L.; Moore, M.T.; Cooper, C.M. Vegetative and structural characteristics of agricultural drainages in the Mississippi Delta landscape. Environ. Pollut. 2004, 132, 403–411. [Google Scholar] [CrossRef]
- USDA NRCS PLANTS Database. Available online: http://plants.usda.gov/ (accessed on 4 January 2012).
- Moore, M.T.; Kröger, R. Evaluating plant species-specific contributions to nutrient mitigation in drainage ditch mesocosms. Water Air Soil Pollut. 2011, 217, 445–454. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Gimsing, A.L. Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: A review. Pest Manag. Sci. 2008, 64, 441–456. [Google Scholar] [CrossRef]
- SPSS Inc. SPSS 12.0 for Windows, Release 12.0.1; SPSS Inc.: Chicago, IL, USA, 2004. [Google Scholar]
- Alister, C.; Kogan, M.; Pino, I. Differential phytotoxicity of glyphosate in maize seedlings following applications to roots or shoot. Weed Res. 2005, 45, 27–32. [Google Scholar] [CrossRef]
- Beisel, K.G.; Jahnke, S.; Hofmann, D.; Köppchen, S.; Schurr, U.; Matsubara, S. Continuous turnover of carotenes and chlorophyll a in mature leaves of Arabidopsis revealed by 14CO2 pulse-chase labeling[OA]. Plant Physiol. 2010, 152, 2188–2199. [Google Scholar] [CrossRef]
- Rankins, A.; Shaw, D.; Douglas, J. Response of perennial grasses potentially used as filter strips to selected postemergence herbicides. Weed Technol. 2005, 19, 73–77. [Google Scholar] [CrossRef]
- Stehle, S.; Elsaesser, D.; Gregoire, C.; Imfeld, G.; Niehaus, E.; Passeport, E.; Payraudeau, S.; Shafer, R.B.; Tournebize, J.; Shulz, R. Pesticide risk mitigation by vegetated treatment systems: A meta-analysis. J. Environ. Qual. 2011, 40, 1068–1080. [Google Scholar] [CrossRef]
- Syversen, N.; Bechmann, M. Vegetative buffer zones as pesticide filters for simulated surface runoff. Ecol. Eng. 2004, 22, 175–184. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saunders, L.E.; Koontz, M.B.; Pezeshki, R. Root-Zone Glyphosate Exposure Adversely Affects Two Ditch Species. Biology 2013, 2, 1488-1496. https://doi.org/10.3390/biology2041488
Saunders LE, Koontz MB, Pezeshki R. Root-Zone Glyphosate Exposure Adversely Affects Two Ditch Species. Biology. 2013; 2(4):1488-1496. https://doi.org/10.3390/biology2041488
Chicago/Turabian StyleSaunders, Lyndsay E., Melissa B. Koontz, and Reza Pezeshki. 2013. "Root-Zone Glyphosate Exposure Adversely Affects Two Ditch Species" Biology 2, no. 4: 1488-1496. https://doi.org/10.3390/biology2041488
APA StyleSaunders, L. E., Koontz, M. B., & Pezeshki, R. (2013). Root-Zone Glyphosate Exposure Adversely Affects Two Ditch Species. Biology, 2(4), 1488-1496. https://doi.org/10.3390/biology2041488



