Systemic Effects of a Phage Cocktail on Healthy Weaned Piglets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteriophages
2.2. Animals and Experimental Design
2.3. Data Recording and Sample Collection
2.4. Endotoxin Assay
2.5. Growth Performance Evaluation
2.6. Serum Antioxidant Capacity
2.7. Intestinal Histomorphology
2.8. Intestinal Chyme Digestive Enzyme Activity Evaluation
2.9. The levels of Serum and Intestinal Mucosal Inflammatory Factors, Immunoglobulin and Toll-Like Receptors Determination
2.10. Intestinal Barrier Function Evaluation
2.11. Fecal Bacterial Community Analysis
2.12. Intestinal Chyme Short-Chain Fatty Acids (SCFAs) Determination
2.13. Statistical Analysis
3. Results
3.1. Endotoxin Levels in Phage Stock Solutions and Drinking Water
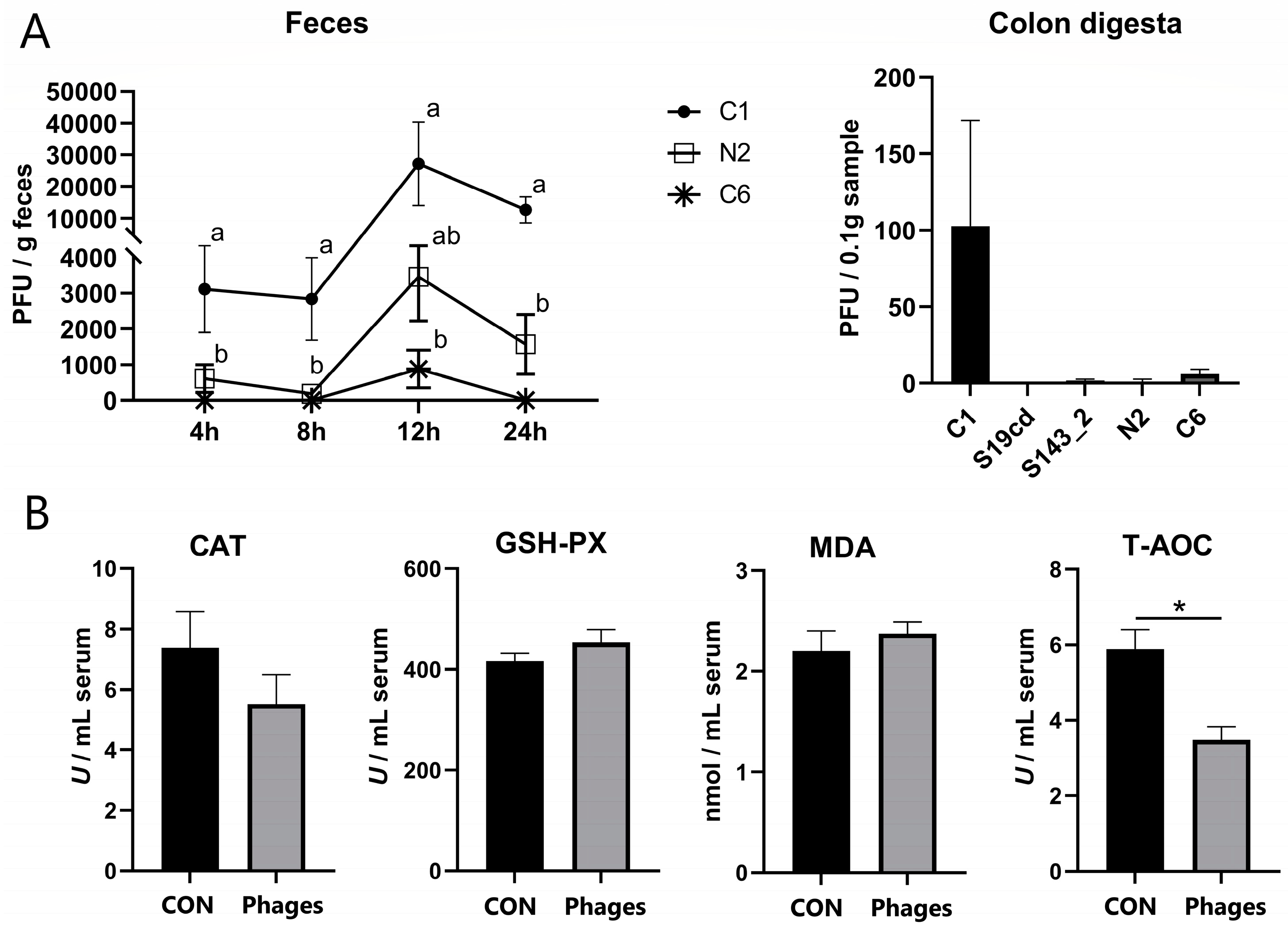
3.2. Fecal and Intestinal Phage Quantification
3.3. Effects of Phage Cocktail on Growth Performance, Routine Blood Parameters, and Antioxidant Capacity of Piglets
3.4. Effects of Phage Cocktail on Intestinal Digesta pH, Digestive Enzyme Activity, and Intestinal Morphology of Piglets
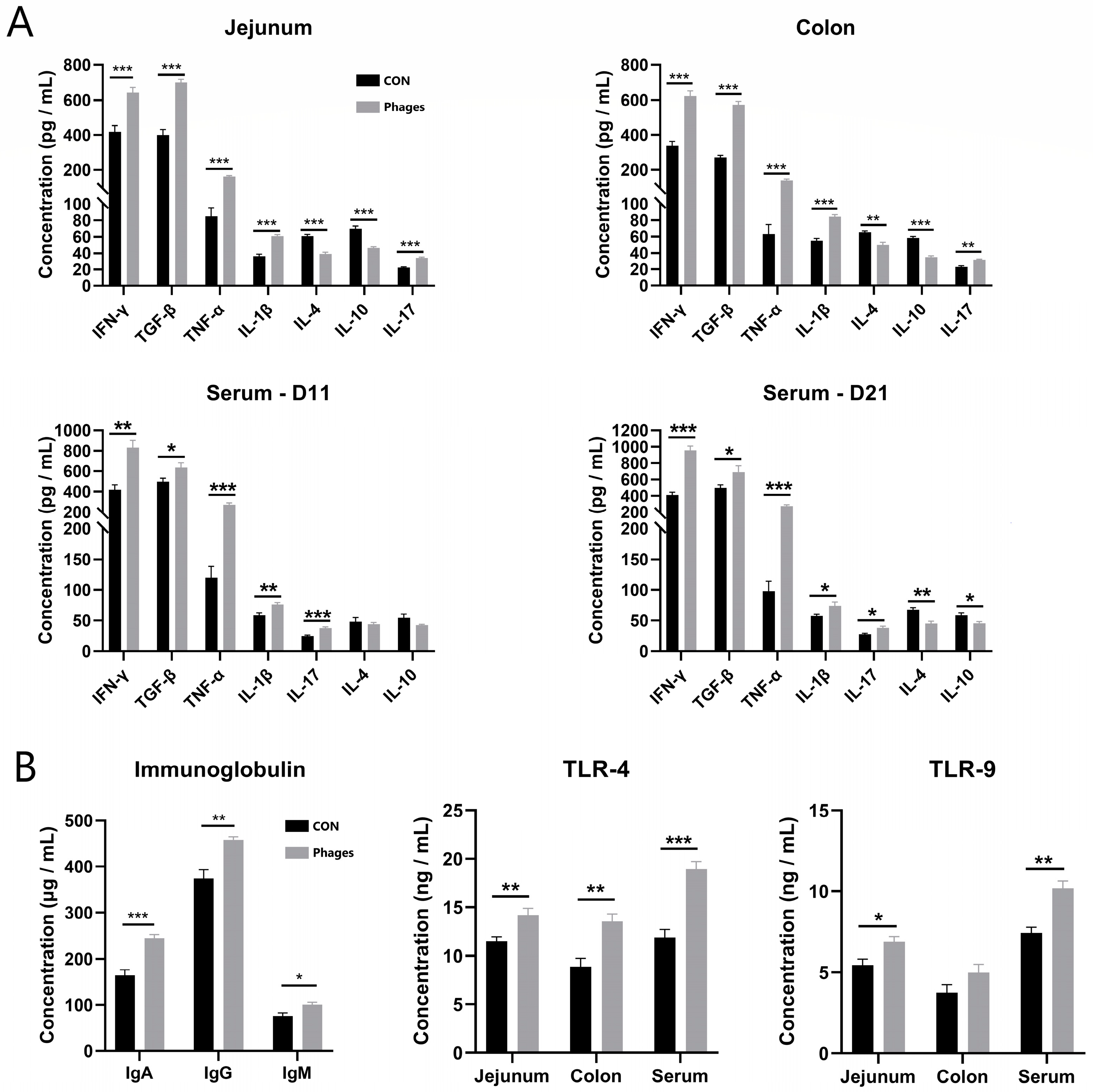
3.5. Effects of Phage Cocktail on Inflammatory Cytokines and Immunoglobulin Levels in Piglets
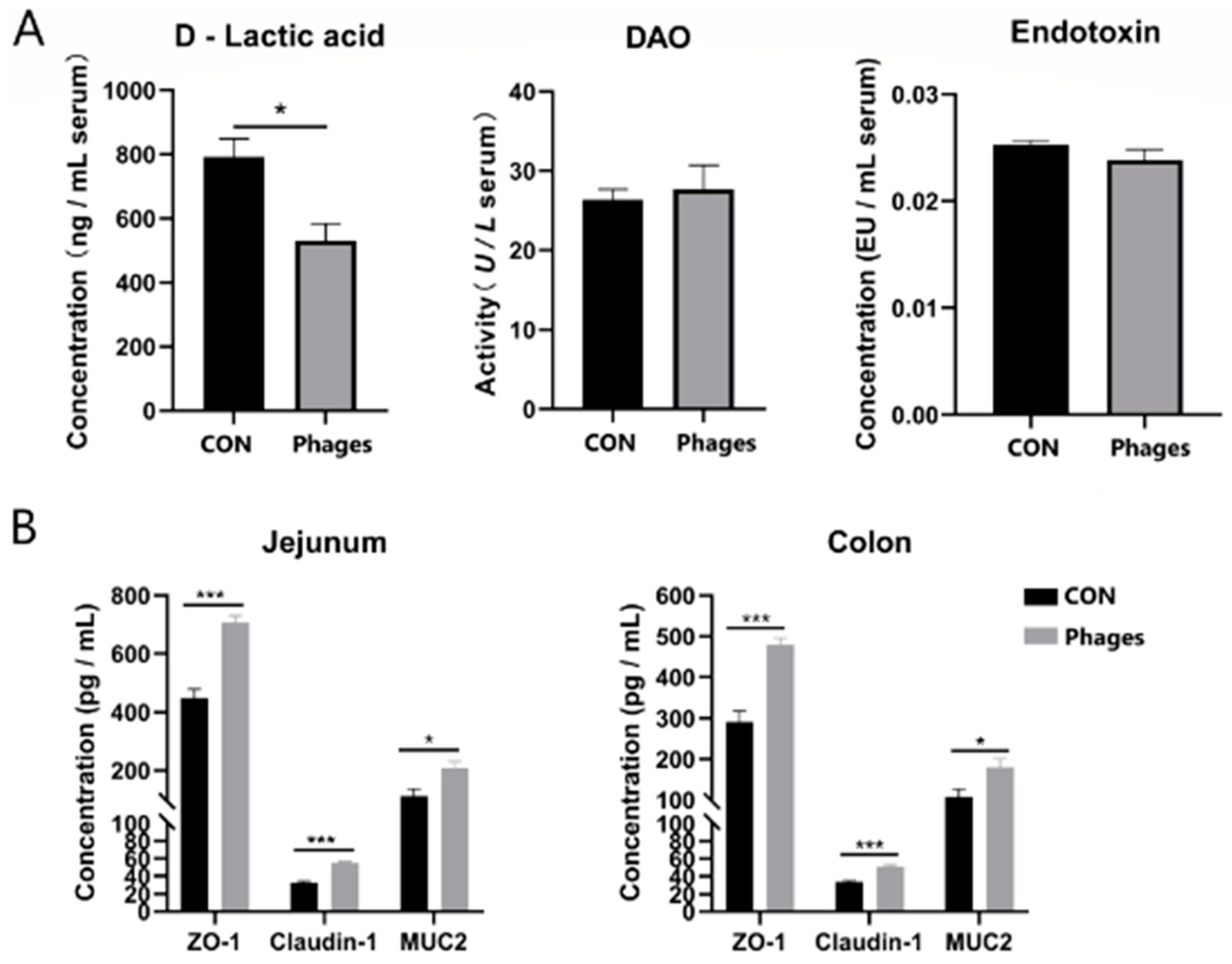
3.6. Effects of Phage Cocktails on Intestinal Barrier-Related Indices in Piglets
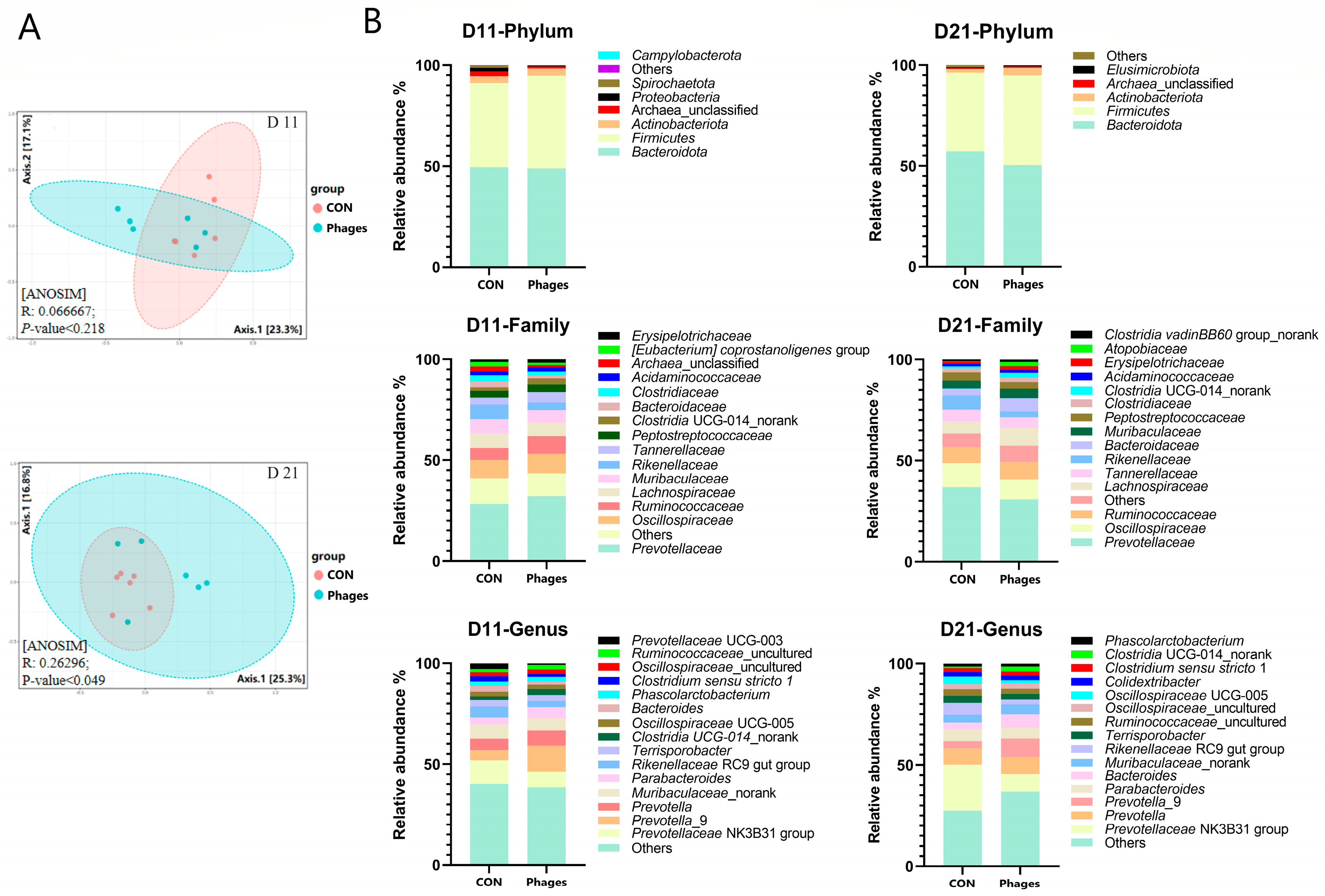
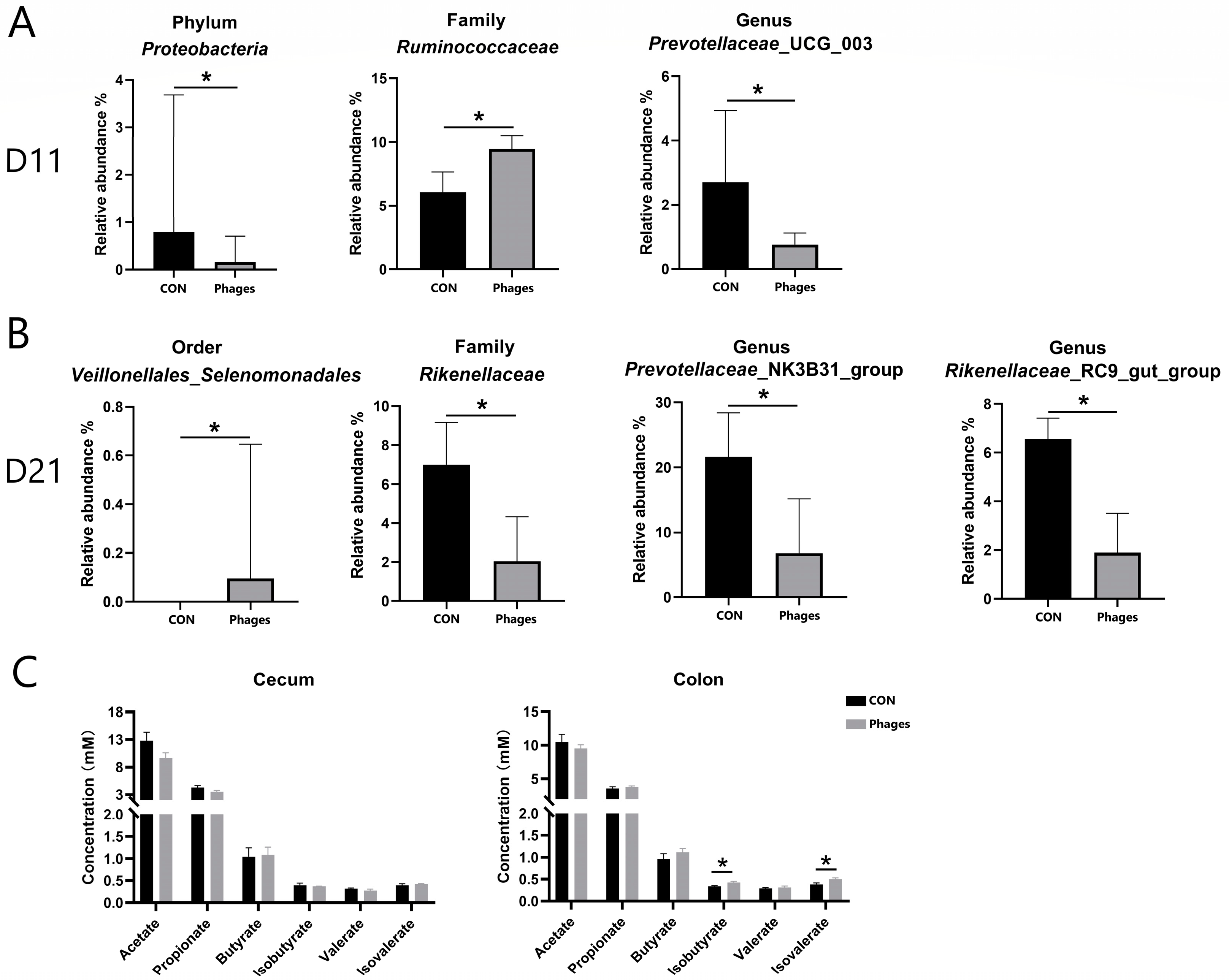
3.7. Effects of Phage Cocktail on Fecal Bacteria Community
4. Discussion
4.1. Most Phages Are Deactivated in the Gastrointestinal Passage
4.2. Phages Can Alter the Host Immune Status of Piglets
4.3. Phages May Promote the Intestinal Health of Piglets
4.4. Phages Alter the Intestinal Microbiota Structure Even without Host Bacteria
4.5. The Complex Health Effects of Phages Require More Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohr, K.I. History of Antibiotics Research. Curr. Top. Microbiol. Immunol. 2016, 398, 237–272. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. The Science of Antibiotic Discovery. Cell 2020, 181, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence-Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef] [PubMed]
- Kakasis, A.; Panitsa, G. Bacteriophage therapy as an alternative treatment for human infections. A comprehensive review. Int. J. Antimicrob. Agents 2019, 53, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Summers, W.C. Bacteriophage therapy. Annu. Rev. Microbiol. 2001, 55, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Gordillo Altamirano, F.L.; Barr, J.J. Phage Therapy in the Postantibiotic Era. Clin. Microbiol. Rev. 2019, 32, e00066-18. [Google Scholar] [CrossRef] [PubMed]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef]
- Roach, D.R.; Leung, C.Y.; Henry, M.; Morello, E.; Singh, D.; Di Santo, J.P.; Weitz, J.S.; Debarbieux, L. Synergy between the Host Immune System and Bacteriophage Is Essential for Successful Phage Therapy against an Acute Respiratory Pathogen. Cell Host Microbe 2017, 22, 38–47.e4. [Google Scholar] [CrossRef]
- Sarker, S.A.; Sultana, S.; Reuteler, G.; Moine, D.; Descombes, P.; Charton, F.; Bourdin, G.; McCallin, S.; Ngom-Bru, C.; Neville, T.; et al. Oral Phage Therapy of Acute Bacterial Diarrhea With Two Coliphage Preparations: A Randomized Trial in Children From Bangladesh. eBioMedicine 2016, 4, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.B.; Yoo, A.N.; Lee, W.J.; Shin, M.K.; Jung, M.H.; Shin, S.W.; Cho, Y.W.; Yoo, H.S. Effect of bacteriophage in enterotoxigenic Escherichia coli (ETEC) infected pigs. J. Vet. Med. Sci. 2012, 74, 1037–1039. [Google Scholar] [CrossRef]
- Yen, M.; Cairns, L.S.; Camilli, A. A cocktail of three virulent bacteriophages prevents Vibrio cholerae infection in animal models. Nat. Commun. 2017, 8, 14187. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.D.; Park, J.H. Characterization and application of phages isolated from sewage for reduction of Escherichia coli O157:H7 in biofilm. LWT-Food Sci. Technol. 2015, 60, 571–577. [Google Scholar] [CrossRef]
- Viazis, S.; Labuza, T.P.; Diez-Gonzalez, F. Bacteriophage Mixture Inactivation Kinetics against Escherichia coli O157:H7 on Hard Surfaces. J. Food Saf. 2015, 35, 66–74. [Google Scholar] [CrossRef]
- Abuladze, T.; Li, M.; Menetrez, M.Y.; Dean, T.; Senecal, A.; Sulakvelidze, A. Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef by Escherichia coli O157:H7. Appl. Environ. Microbiol. 2008, 74, 6230–6238. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.J. A bacteriophages journey through the human body. Immunol. Rev. 2017, 279, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Majewska, J.; Beta, W.; Lecion, D.; Hodyra-Stefaniak, K.; Klopot, A.; Kazmierczak, Z.; Miernikiewicz, P.; Piotrowicz, A.; Ciekot, J.; Owczarek, B.; et al. Oral Application of T4 Phage Induces Weak Antibody Production in the Gut and in the Blood. Viruses 2015, 7, 4783–4799. [Google Scholar] [CrossRef] [PubMed]
- Dor-On, E.; Solomon, B. Targeting glioblastoma via intranasal administration of Ff bacteriophages. Front. Microbiol. 2015, 6, 530. [Google Scholar] [CrossRef]
- Rouse, M.D.; Stanbro, J.; Roman, J.A.; Lipinski, M.A.; Jacobs, A.; Biswas, B.; Regeimbal, J.; Henry, M.; Stockelman, M.G.; Simons, M.P. Impact of Frequent Administration of Bacteriophage on Therapeutic Efficacy in an A. baumannii Mouse Wound Infection Model. Front. Microbiol. 2020, 11, 414. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhou, B.; Zhu, W. Pathogenic Escherichia coli-Specific Bacteriophages and Polyvalent Bacteriophages in Piglet Guts with Increasing Coliphage Numbers after Weaning. Appl. Environ. Microbiol. 2021, 87, e0096621. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Y.; Zhang, Y.; Yuan, W.; Wang, D.; Zhu, W. Biological and genomic characterization of a polyvalent bacteriophage (S19cd) strongly inhibiting Salmonella enterica serovar Choleraesuis. Vet. Microbiol. 2023, 284, 109822. [Google Scholar] [CrossRef]
- Gavric, D.; Knezevic, P. Optimized Method for Pseudomonas aeruginosa Integrative Filamentous Bacteriophage Propagation. Front. Microbiol. 2021, 12, 707815. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Ling, H. Drinking water management for pigs. Swine Prod. 2006, 5, 53–56. (In Chinese) [Google Scholar]
- Seo, B.J.; Song, E.T.; Lee, K.; Kim, J.W.; Jeong, C.G.; Moon, S.H.; Son, J.S.; Kang, S.H.; Cho, H.S.; Jung, B.Y.; et al. Evaluation of the broad-spectrum lytic capability of bacteriophage cocktails against various Salmonella serovars and their effects on weaned pigs infected with Salmonella typhimurium. J. Vet. Med. Sci. 2018, 80, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.P.; Gyles, C.L.; Huff, W.E.; Ojha, S.; Huff, G.R.; Rath, N.C.; Donoghue, A.M. Bacteriophages for prophylaxis and therapy in cattle, poultry and pigs. Anim. Health Res. Rev. 2008, 9, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Almeida, G.M.F.; Laanto, E.; Ashrafi, R.; Sundberg, L.R. Bacteriophage Adherence to Mucus Mediates Preventive Protection against Pathogenic Bacteria. mBio 2019, 10, e01984-19. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid. Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef] [PubMed]
- Ihara, S.; Hirata, Y.; Koike, K. TGF-beta in inflammatory bowel disease: A key regulator of immune cells, epithelium, and the intestinal microbiota. J. Gastroenterol. 2017, 52, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Moaaz, M.; Youssry, S.; Elfatatry, A.; El Rahman, M.A. Th17/Treg cells imbalance and their related cytokines (IL-17, IL-10 and TGF-beta) in children with autism spectrum disorder. J. Neuroimmunol. 2019, 337, 577071. [Google Scholar] [CrossRef] [PubMed]
- Gogokhia, L.; Buhrke, K.; Bell, R.; Hoffman, B.; Brown, D.G.; Hanke-Gogokhia, C.; Ajami, N.J.; Wong, M.C.; Ghazaryan, A.; Valentine, J.F.; et al. Expansion of Bacteriophages Is Linked to Aggravated Intestinal Inflammation and Colitis. Cell Host Microbe 2019, 25, 285–299 e288. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Z.; Zou, T.; Chen, J.; Li, G.; Zheng, L.; Li, S.; You, J. Bacteriophage as an Alternative to Antibiotics Promotes Growth Performance by Regulating Intestinal Inflammation, Intestinal Barrier Function and Gut Microbiota in Weaned Piglets. Front. Vet. Sci. 2021, 8, 623899. [Google Scholar] [CrossRef]
- Gorski, A.; Borysowski, J.; Miedzybrodzki, R. Bacteriophage Interactions With Epithelial Cells: Therapeutic Implications. Front. Microbiol. 2020, 11, 631161. [Google Scholar] [CrossRef] [PubMed]
- Huh, H.; Wong, S.; St Jean, J.; Slavcev, R. Bacteriophage interactions with mammalian tissue: Therapeutic applications. Adv. Drug Deliv. Rev. 2019, 145, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Jariah, R.O.A.; Hakim, M.S. Interaction of phages, bacteria, and the human immune system: Evolutionary changes in phage therapy. Rev. Med. Virol. 2019, 29, e2055. [Google Scholar] [CrossRef] [PubMed]
- Duerkop, B.A.; Hooper, L.V. Resident viruses and their interactions with the immune system. Nat. Immunol. 2013, 14, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Sweere, J.M.; Van Belleghem, J.D.; Ishak, H.; Bach, M.S.; Popescu, M.; Sunkari, V.; Kaber, G.; Manasherob, R.; Suh, G.A.; Cao, X.; et al. Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Science 2019, 363, eaat9691. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Gu, M.J.; Kye, Y.-C.; Ju, Y.-J.; Hong, R.; Ju, D.B.; Pyung, Y.J.; Han, S.H.; Park, B.-C.; Yun, C.-H. Bacteriophage EK99P-1 alleviates enterotoxigenic Escherichia coli K99-induced barrier dysfunction and inflammation. Sci. Rep. 2022, 12, 941. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Y.; Lv, P.; Huang, J.; Tai, R.; Jin, X.; Wang, J.; Wang, X. Salmonella Phages Affect the Intestinal Barrier in Chicks by Altering the Composition of Early Intestinal Flora: Association With Time of Phage Use. Front. Microbiol. 2022, 13, 947640. [Google Scholar] [CrossRef]
- Cadwell, K.; Bichet, M.C.; Adderley, J.; Avellaneda-Franco, L.; Magnin-Bougma, I.; Torriero-Smith, N.; Gearing, L.J.; Deffrasnes, C.; David, C.; Pepin, G.; et al. Mammalian cells internalize bacteriophages and use them as a resource to enhance cellular growth and survival. PLoS Biol. 2023, 21, e3002341. [Google Scholar] [CrossRef]
- Howe, K.L.; Reardon, C.; Wang, A.; Nazli, A.; McKay, D.M. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability. Am. J. Pathol. 2005, 167, 1587–1597. [Google Scholar] [CrossRef]
- Kinugasa, T.; Sakaguchi, T.; Gu, X.; Reinecker, H.C. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology 2000, 118, 1001–1011. [Google Scholar] [CrossRef]
- Brabec, T.; Vobořil, M.; Schierová, D.; Valter, E.; Šplíchalová, I.; Dobeš, J.; Březina, J.; Dobešová, M.; Aidarova, A.; Jakubec, M.; et al. IL-17-driven induction of Paneth cell antimicrobial functions protects the host from microbiota dysbiosis and inflammation in the ileum. Mucosal Immunol. 2023, 16, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T. Lysis from without. Bacteriophage 2011, 1, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kong, Q.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. A High-Fat Diet Increases Gut Microbiota Biodiversity and Energy Expenditure Due to Nutrient Difference. Nutrients 2020, 12, 3197. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, E.; Roos, S.; Liu, H.Y.; Lindberg, J.E. Fermentable non-starch polysaccharides increases the abundance of Bacteroides-Prevotella-Porphyromonas in ileal microbial community of growing pigs. Animal 2014, 8, 1777–1787. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J.; et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Borysowski, J.; Wierzbicki, P.; Kłosowska, D.; Korczak-Kowalska, G.; Weber-Dabrowska, B.; Górski, A. The effects of T4 and A3/R phage preparations on whole-blood monocyte and neutrophil respiratory burst. Viral Immunol. 2010, 23, 541–544. [Google Scholar] [CrossRef]
- Van Belleghem, J.D.; Dąbrowska, K.; Vaneechoutte, M.; Barr, J.J.; Bollyky, P.L. Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System. Viruses 2018, 11, 10. [Google Scholar] [CrossRef]
| Phage Strain | Endotoxin Concentration (EU/mL) | Average Daily Gavage Volume (μL) |
|---|---|---|
| C1 | 96,794.73 ± 4038.27 | 12 |
| S19cd | 39,451.71 ± 1149.41 | 148.53 |
| S143_2 | 72,135.35 ± 2369.81 | 85.88 |
| C6 | 11,043.06 ± 584.79 | 147.53 |
| N2 | 61,158.41 ± 1109.76 | 196.94 |
| water | 72.46 ± 3.82 | \ |
| Segment | CON Group | Phages Group | p-Value |
|---|---|---|---|
| Jejunum | 6.52 + 0.15 a | 6.93 + 0.08 b | 0.030 |
| Cecum | 6.57 + 0.19 | 6.64 + 0.09 | 0.751 |
| Colon | 6.50 + 0.16 | 6.61 + 0.08 | 0.538 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Lin, Y.; Zhu, W. Systemic Effects of a Phage Cocktail on Healthy Weaned Piglets. Biology 2024, 13, 271. https://doi.org/10.3390/biology13040271
Liu Y, Lin Y, Zhu W. Systemic Effects of a Phage Cocktail on Healthy Weaned Piglets. Biology. 2024; 13(4):271. https://doi.org/10.3390/biology13040271
Chicago/Turabian StyleLiu, Yankun, Yan Lin, and Weiyun Zhu. 2024. "Systemic Effects of a Phage Cocktail on Healthy Weaned Piglets" Biology 13, no. 4: 271. https://doi.org/10.3390/biology13040271






