Thermostable Human Basic Fibroblast Growth Factor (TS-bFGF) Engineered with a Disulfide Bond Demonstrates Superior Culture Outcomes in Human Pluripotent Stem Cell
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Computational Design of Thermostable-bFGF (TS-bFGF)
2.2. TS-bFGF Construction and Production
2.3. Determination of Thermal Stability
2.4. Cell Proliferation Assay Using NIH/3T3 Cell Line
2.5. Cell Culture
2.6. Luciferase Activity Assays Comparing WT-Bfgf and TS-Bfgf
2.7. Cell Growth Kinetics
2.8. RNA Isolation and Reverse Transcription-Quantitative Polymerase Chain Reaction
2.9. Cell Cycle Assay
2.10. Alkaline Phosphatase (AP) Activity Test
2.11. Immunofluorescence Staining
2.12. Spontaneous Differentiation
2.13. Hematopoietic Progenitor Cell (HPS) Differentiation
2.14. Fluorescence-Activated Cell Sorting (FACS)
2.15. Generation of iPSCs from Mesenchymal Stem Cells (MSC)
2.16. Statistical Analysis
3. Results
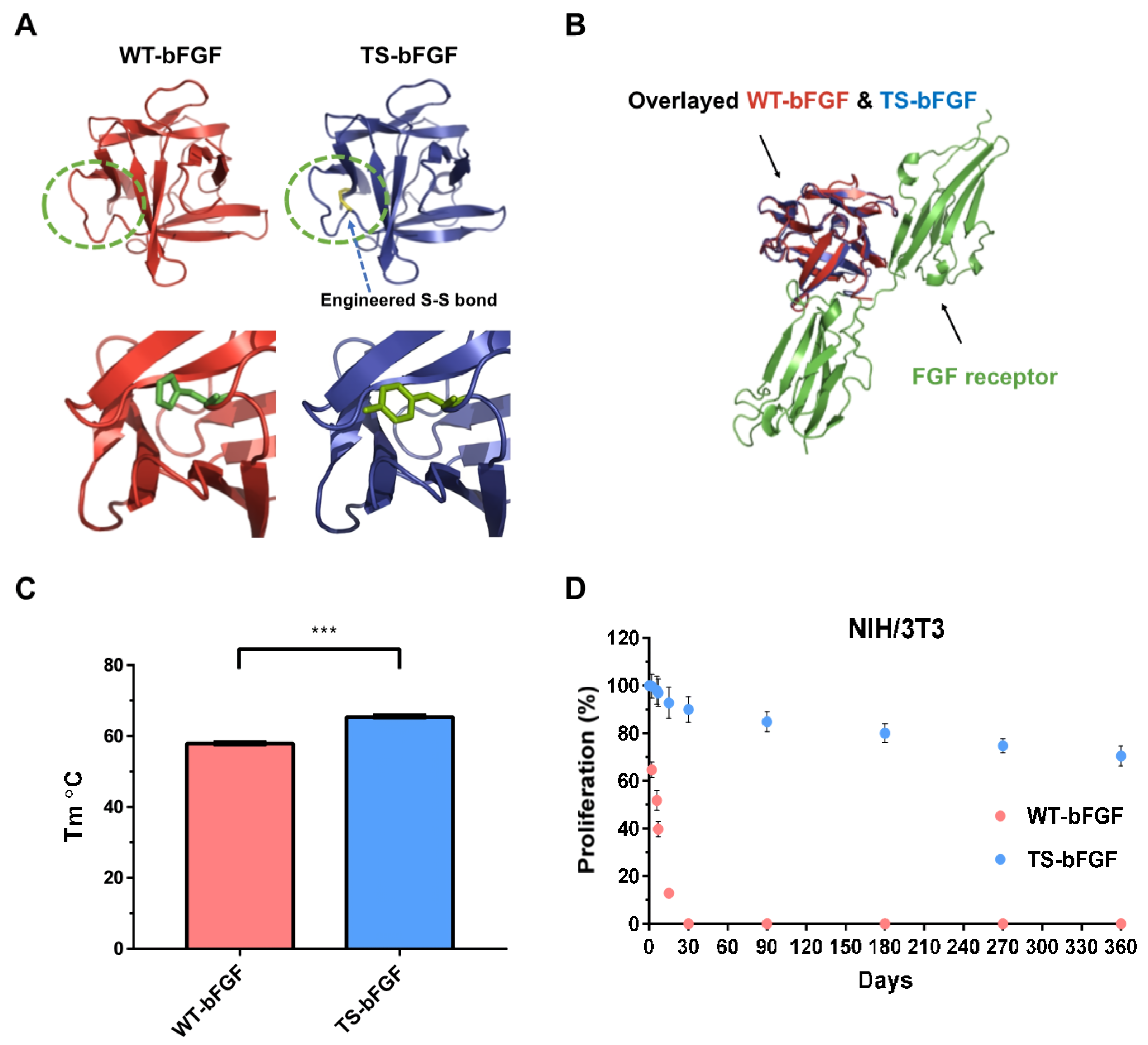
3.1. Schematic Diagram of Growth Factor Construction and Comparative Evaluation of Activity
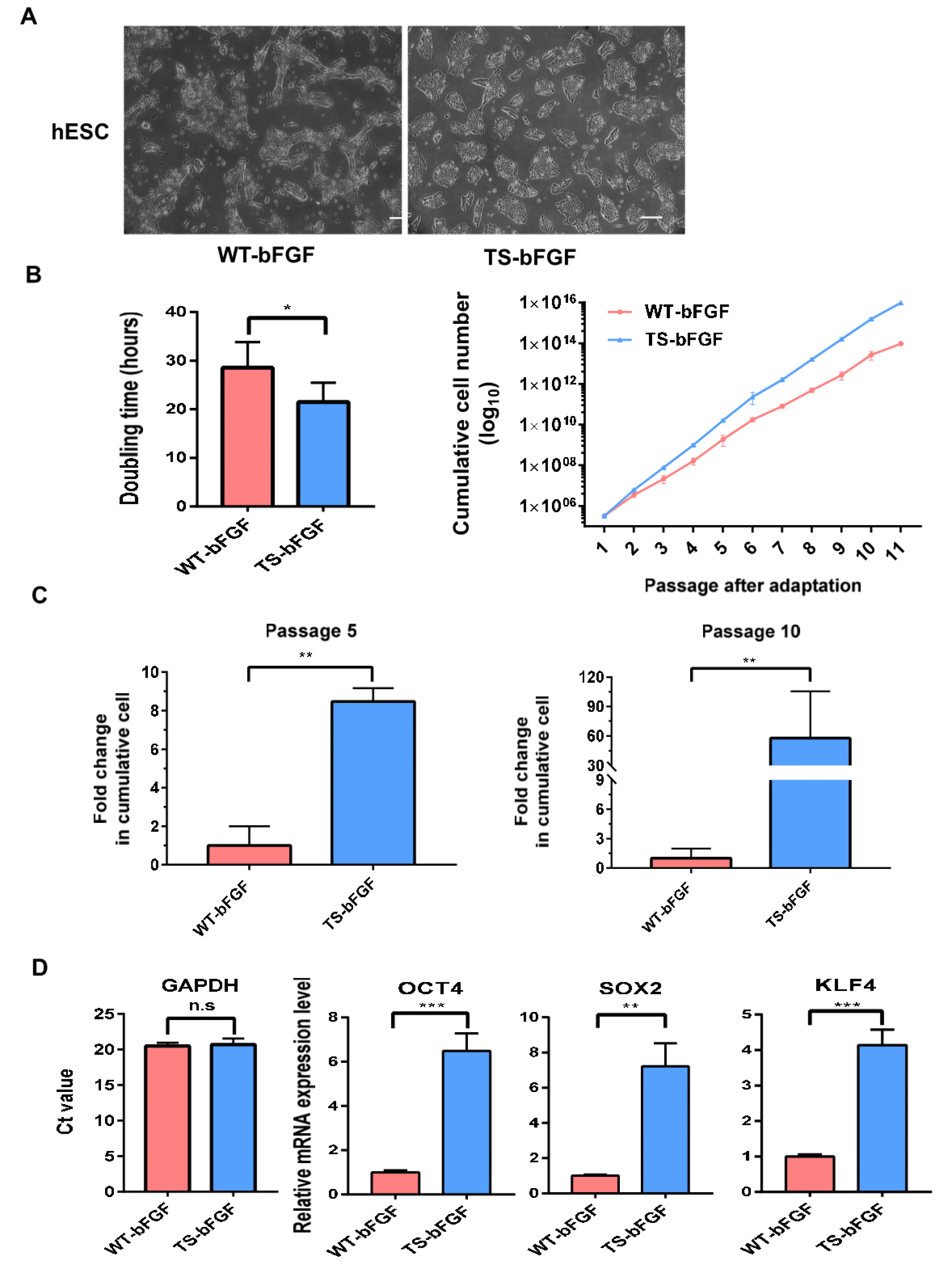
3.2. TS-bFGF Increases Embryonic Stem Cell Growth Kinetics
3.3. Effect of TS-bFGF on hESC Stemness Function
3.4. High Rate of Spontaneous Trilineage Differentiation Potential in TS-bFGF-Treated Embryoid Bodies


3.5. TS-bFGF Improved Differentiation of hESCs to Hematopoietic Progenitor Cells (HPCs)
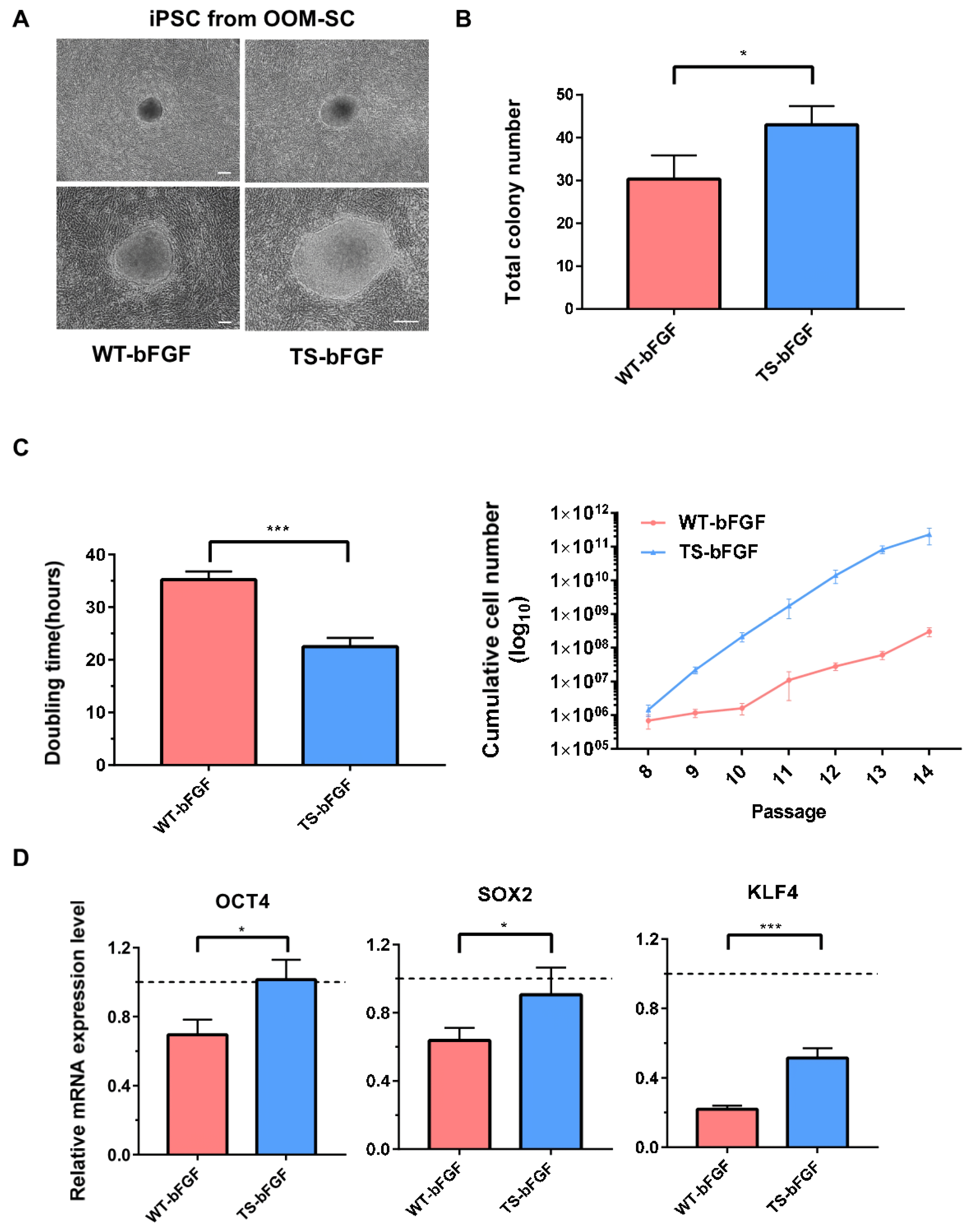
3.6. Effects of TS-bFGF on the Proliferation and Stemness of Induced Pluripotent Stem Cells (iPSCs)
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hwang, D.-Y. Feeder-free growth of undifferentiated human embryonic stem cells. In Human Embryonic and Induced Pluripotent Stem Cells; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–12. [Google Scholar]
- Goumans, M.J.; Ward-van Oostwaard, D.; Wianny, F.; Savatier, P.; Zwijsen, A.; Mummery, C. Mouse embryonic stem cells with aberrant transforming growth factor β signalling exhibit impaired differentiation in vitro and in vivo. Differentiation 1998, 63, 101–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Yin, S.; Zeng, H.; Li, H.; Wan, X. Regulation of Embryonic Stem Cell Self-Renewal. Life 2022, 12, 1151. [Google Scholar] [CrossRef]
- Vazin, T.; Freed, W.J. Human embryonic stem cells: Derivation, culture, and differentiation: A review. Restor. Neurol. Neurosci. 2010, 28, 589–603. [Google Scholar] [CrossRef]
- Beattie, G.M.; Lopez, A.D.; Bucay, N.; Hinton, A.; Firpo, M.T.; King, C.C.; Hayek, A. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells 2005, 23, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Bendall, S.C.; Stewart, M.H.; Menendez, P.; George, D.; Vijayaragavan, K.; Werbowetski-Ogilvie, T.; Ramos-Mejia, V.; Rouleau, A.; Yang, J.; Bossé, M. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature 2007, 448, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- James, D.; Levine, A.J.; Besser, D.; Hemmati-Brivanlou, A. TGFβ/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development 2005, 132, 1273–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pébay, A.; Wong, R.C.; Pitson, S.M.; Wolvetang, E.J.; Peh, G.S.L.; Filipczyk, A.; Koh, K.L.; Tellis, I.; Nguyen, L.T.; Pera, M.F. Essential roles of sphingosine-1-phosphate and platelet-derived growth factor in the maintenance of human embryonic stem cells. Stem Cells 2005, 23, 1541–1548. [Google Scholar] [CrossRef]
- Sato, N.; Meijer, L.; Skaltsounis, L.; Greengard, P.; Brivanlou, A.H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 2004, 10, 55–63. [Google Scholar] [CrossRef]
- Farooq, M.; Khan, A.W.; Kim, M.S.; Choi, S. The role of fibroblast growth factor (FGF) signaling in tissue repair and regeneration. Cells 2021, 10, 3242. [Google Scholar] [CrossRef]
- Weinberger, L.; Ayyash, M.; Novershtern, N.; Hanna, J.H. Dynamic stem cell states: Naive to primed pluripotency in rodents and humans. Nat. Rev. Mol. Cell Biol. 2016, 17, 155–169. [Google Scholar] [CrossRef]
- Van Hoof, D.; Braam, S.R.; Dormeyer, W.; Ward-van Oostwaard, D.; Heck, A.J.; Krijgsveld, J.; Mummery, C.L. Feeder-free monolayer cultures of human embryonic stem cells express an epithelial plasma membrane protein profile. Stem Cells 2008, 26, 2777–2781. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Inokuma, M.S.; Denham, J.; Golds, K.; Kundu, P.; Gold, J.D.; Carpenter, M.K. Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 2001, 19, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Rosler, E.; Jiang, J.; Lebkowski, J.S.; Gold, J.D.; O’Sullivan, C.; Delavan-Boorsma, K.; Mok, M.; Bronstein, A.; Carpenter, M.K. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells 2005, 23, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Annex, B.H.; Laham, R.J.; Kleiman, N.; Henry, T.; Dauerman, H.; Udelson, J.E.; Gervino, E.V.; Pike, M.; Whitehouse, M. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: Double-blind, randomized, controlled clinical trial. Circulation 2002, 105, 788–793. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurthy, R.; Manning, M.C. The stability factor: Importance in formulation development. Curr. Pharm. Biotechnol. 2002, 3, 361–371. [Google Scholar] [CrossRef]
- Plotnikov, A.N.; Hubbard, S.R.; Schlessinger, J.; Mohammadi, M. Crystal structures of two FGF-FGFR complexes reveal the determinants of ligand-receptor specificity. Cell 2000, 101, 413–424. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.M.; Lee, K.-M.; Kim, H.J.; Park, I.K.; Kang, H.J.; Shin, H.-C.; Baek, D.; Choi, Y.; Park, K.H.; Lee, J.W. Effects of structurally stabilized EGF and bFGF on wound healing in type I and type II diabetic mice. Acta Biomater. 2018, 66, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [Green Version]
- Krieger, E.; Joo, K.; Lee, J.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins Struct. Funct. Bioinform. 2009, 77, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Schymkowitz, J.; Borg, J.; Stricher, F.; Nys, R.; Rousseau, F.; Serrano, L. The FoldX web server: An online force field. Nucleic Acids Res. 2005, 33, W382–W388. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.-H.; Song, H.-D.; Kang, D.-K.; Chang, S.-I.; Kim, M.-K.; Cho, K.-H.; Scherga, H.A.; Shin, H.-C. Role of the surface loop on the structure and biological activity of angiogenin. BMB Rep. 2009, 42, 829–833. [Google Scholar] [CrossRef] [Green Version]
- Olsson, U.; Wolf-Watz, M. Overlap between folding and functional energy landscapes for adenylate kinase conformational change. Nat. Commun. 2010, 1, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, K.M.; Dayem, A.A.; Choi, Y.; Lee, Y.; An, J.; Gil, M.; Lee, S.; Kwak, H.J.; Vellingirl, B.; Shin, H.J. High Therapeutic and Esthetic Properties of Extracellular Vesicles Produced from the Stem Cells and Their Spheroids Cultured from Ocular Surgery-Derived Waste Orbicularis Oculi Muscle Tissues. Antioxidants 2021, 10, 1292. [Google Scholar] [CrossRef]
- Meng, G.; Rancourt, D.E. Derivation and maintenance of undifferentiated human embryonic stem cells. In Human Embryonic Stem Cells Handbook; Springer: Berlin/Heidelberg, Germany, 2012; pp. 69–80. [Google Scholar]
- Francis, K.; Wei, L. Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death Dis. 2010, 1, e22. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Pu, J. Differentiation and application of human pluripotent stem cells derived cardiovascular cells for treatment of heart diseases: Promises and challenges. Front. Cell Dev. Biol. 2021, 9, 658088. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Yan, L.; Wang, X.; Li, E.; Murphy, K.; Vaccaro, K.; Li, Y.; Xu, R.-H. Concise review: Mesenchymal stem cells derived from human pluripotent cells, an unlimited and quality-controllable source for therapeutic applications. Stem Cells 2019, 37, 572–581. [Google Scholar] [CrossRef] [Green Version]
- Boward, B.; Wu, T.; Dalton, S. Concise review: Control of cell fate through cell cycle and pluripotency networks. Stem Cells 2016, 34, 1427–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperger, J.M.; Chen, X.; Draper, J.S.; Antosiewicz, J.E.; Chon, C.H.; Jones, S.B.; Brooks, J.D.; Andrews, P.W.; Brown, P.O.; Thomson, J.A. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc. Natl. Acad. Sci. USA 2003, 100, 13350–13355. [Google Scholar] [CrossRef] [Green Version]
- Tonge, P.D.; Andrews, P.W. Retinoic acid directs neuronal differentiation of human pluripotent stem cell lines in a non-cell-autonomous manner. Differentiation 2010, 80, 20–30. [Google Scholar] [CrossRef]
- Poh, Y.-C.; Chen, J.; Hong, Y.; Yi, H.; Zhang, S.; Chen, J.; Wu, D.C.; Wang, L.; Jia, Q.; Singh, R. Generation of organized germ layers from a single mouse embryonic stem cell. Nat. Commun. 2014, 5, 4000. [Google Scholar] [CrossRef] [Green Version]
- Spelke, D.P.; Ortmann, D.; Khademhosseini, A.; Ferreira, L.; Karp, J.M. Methods for embryoid body formation: The microwell approach. In Embryonic Stem Cell Therapy for Osteo-Degenerative Diseases; Springer: Berlin/Heidelberg, Germany, 2011; pp. 151–162. [Google Scholar]
- Lim, W.F.; Inoue-Yokoo, T.; Tan, K.S.; Lai, M.I.; Sugiyama, D. Hematopoietic cell differentiation from embryonic and induced pluripotent stem cells. Stem Cell Res. Ther. 2013, 4, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidney, L.E.; Branch, M.J.; Dunphy, S.E.; Dua, H.S.; Hopkinson, A. Concise review: Evidence for CD34 as a common marker for diverse progenitors. Stem Cells 2014, 32, 1380–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Benington, L.; Rajan, G.; Locher, C.; Lim, L.Y. Fibroblast growth factor 2—A review of stabilisation approaches for clinical applications. Pharmaceutics 2020, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, P.; Bednar, D.; Vanacek, P.; Balek, L.; Eiselleova, L.; Stepankova, V.; Sebestova, E.; Kunova Bosakova, M.; Konecna, Z.; Mazurenko, S. Computer-assisted engineering of hyperstable fibroblast growth factor 2. Biotechnol. Bioeng. 2018, 115, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Dallas, M.; Balhouse, B.; Navarro, D.; Reid, K.; Mackowski, M. Improving Cell Culture Outcomes through Stabilized bFGF: Enhanced 2D and 3D Culture Using Gibco™ Heat Stable Recombinant Human Basic Fibroblast Growth Factor from Thermo Fisher Scientific. Genet. Eng. Biotechnol. News 2018, 38, 22–23. [Google Scholar] [CrossRef]
- Protze, S.I.; Lee, J.H.; Keller, G.M. Human pluripotent stem cell-derived cardiovascular cells: From developmental biology to therapeutic applications. Cell Stem Cell 2019, 25, 311–327. [Google Scholar] [CrossRef]
- Ntege, E.H.; Sunami, H.; Shimizu, Y. Advances in regenerative therapy: A review of the literature and future directions. Regen. Ther. 2020, 14, 136–153. [Google Scholar] [CrossRef]
- Doğan, A. Embryonic stem cells in development and regenerative medicine. In Cell Biology and Translational Medicine; Stem Cells in Regenerative Medicine: Advances and Challenges; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1, pp. 1–15. [Google Scholar]
- Kim, J.Y.; Nam, Y.; Rim, Y.A.; Ju, J.H. Review of the current trends in clinical trials involving induced pluripotent stem cells. Stem Cell Rev. Rep. 2022, 18, 142–154. [Google Scholar] [CrossRef]


| Gene | Primer Sequence 1 (5′ → 3′) | Product Size (bp) | Accession No. |
|---|---|---|---|
| GAPDH | GTCTCCTCTGACTTCAACAGCG (F) ACCACCCTGTTGCTGTAGCCAA (R) | 131 | NM_001357943.2 |
| OCT4 | CCTGAAGCAGAAGAGGATCACC (F) AAAGCGGCAGATGGTCGTTTGG(R) | 106 | NM_203289.6 |
| SOX2 | GCTACAGCATGATGCAGGACCA (F) TCTGCGAGCTGGTCATGGAGTT (R) | 135 | NM_003106.4 |
| KLF4 | CATCTCAAGGCACACCTGCGAA (F) TCGGTCGCATTTTTGGCACTGG (R) | 156 | NM_001314052.2 |
| HAND1 | CAAGGATGCACAGTCTGGCGAT (F) GCAGGAGGAAAACCTTCGTGCT (R) | 117 | NM_004821.3 |
| ACTA2 | CTATGCCTCTGGACGCACAACT (F) CAGATCCAGACGCATGATGGCA (R) | 115 | NM_001406462.1 |
| PAX6 | CTGAGGAATCAGAGAAGACAGGC (F) ATGGAGCCAGATGTGAAGGAGG (R) | 131 | NM_000280.6 |
| MIXL1 | CCCGACATCCACTTGCGCGAG (F) GGAAGGATTTCCCACTCTGACG (R) | 118 | NM_031944.3 |
| SOX1 | GAGTGGAAGGTCATGTCCGAGG (F) CCTTCTTGAGCAGCGTCTTGGT (R) | 136 | NM_005986.3 |
| TUBB3 | TCAGCGTCTACTACAACGAGGC (F) GCCTGAAGAGATGTCCAAAGGC (R) | 120 | NM_001197181.2 |
| TBXT | CCTTCAGCAAAGTCAAGCTCACC(F) TGAACTGGGTCTCAGGGAAGCA (R) | 153 | NM_047419269.1 |
| NES | TCAAGATGTCCCTCAGCCTGGA (F) AAGCTGAGGGAAGTCTTGGAGC (R) | 106 | NM_006617.2 |
| GATA4 | GCGGTGCTTCCAGCAACTCCA (F) GACATCGCACTGACTGAGAACG (R) | 139 | NM_001308093.3 |
| SOX17 | ACGCTTTCATGGTGTGGGCTAAG (F) GTCAGCGCCTTCCACGACTTG (R) | 112 | NM_022454.4 |
| AFP | GCAGAGGAGATGTGCTGGATTG (F) CGTGGTCAGTTTGCAGCATTCTG (R) | 113 | NM_001354717.2 |
| HNF4A | GGTGTCCATACGCATCCTTGAC (F) AGCCGCTTGATCTTCCCTGGAT (R) | 144 | NM_000457.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kang, G.-H.; Lim, K.M.; Shin, Y.; Song, K.; Park, S.; An, J.; Kim, D.Y.; Shin, H.-C.; Cho, S.-G. Thermostable Human Basic Fibroblast Growth Factor (TS-bFGF) Engineered with a Disulfide Bond Demonstrates Superior Culture Outcomes in Human Pluripotent Stem Cell. Biology 2023, 12, 888. https://doi.org/10.3390/biology12060888
Kim S, Kang G-H, Lim KM, Shin Y, Song K, Park S, An J, Kim DY, Shin H-C, Cho S-G. Thermostable Human Basic Fibroblast Growth Factor (TS-bFGF) Engineered with a Disulfide Bond Demonstrates Superior Culture Outcomes in Human Pluripotent Stem Cell. Biology. 2023; 12(6):888. https://doi.org/10.3390/biology12060888
Chicago/Turabian StyleKim, Sejong, Geun-Ho Kang, Kyung Min Lim, Yeokyung Shin, Kwonwoo Song, Sangrok Park, Jongyub An, Dae Young Kim, Hang-Cheol Shin, and Ssang-Goo Cho. 2023. "Thermostable Human Basic Fibroblast Growth Factor (TS-bFGF) Engineered with a Disulfide Bond Demonstrates Superior Culture Outcomes in Human Pluripotent Stem Cell" Biology 12, no. 6: 888. https://doi.org/10.3390/biology12060888






