Transfer RNA Mutation Associated with Type 2 Diabetes Mellitus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Search Strategy
3. Structure and Function of Transfer RNA Genes
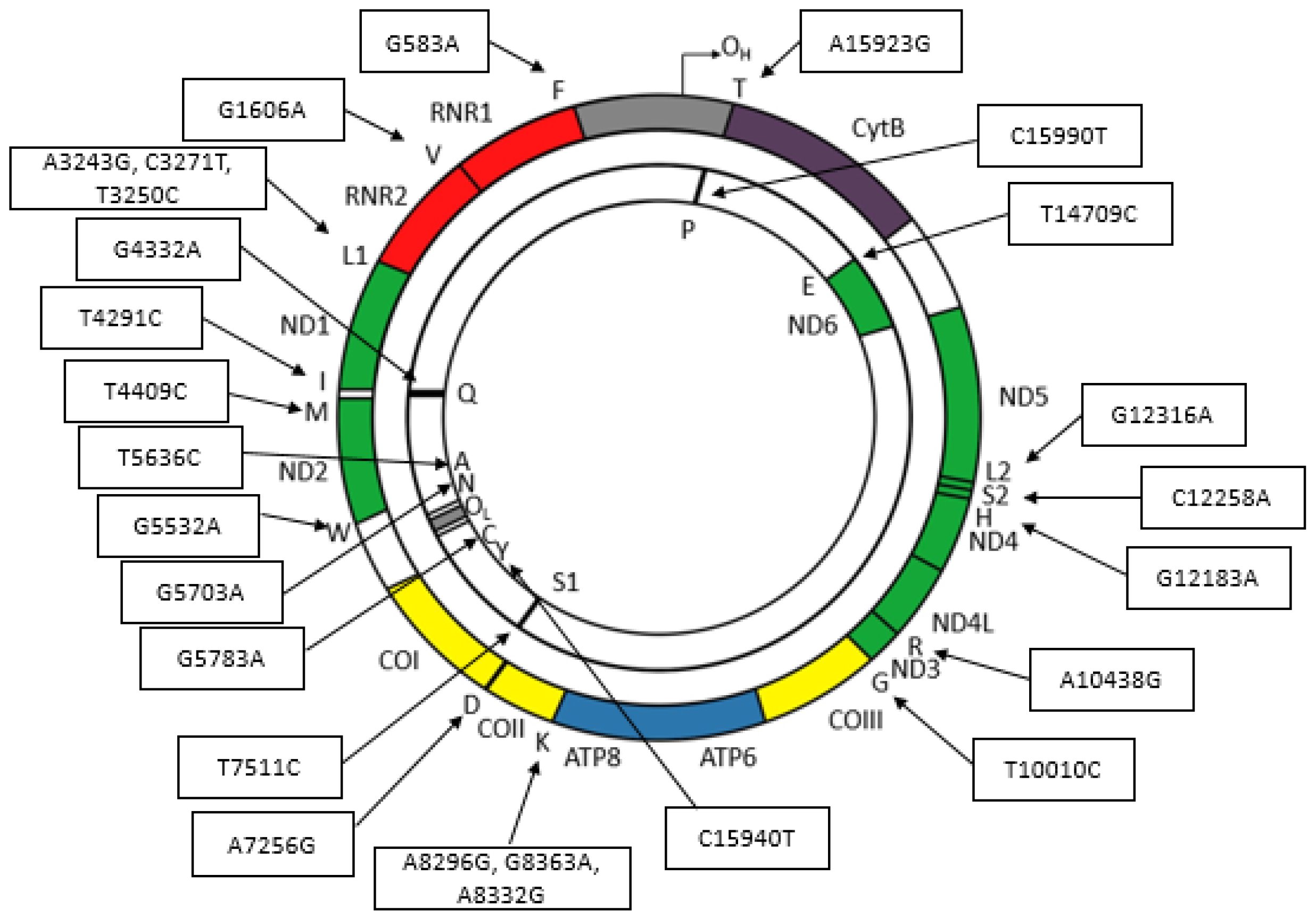
4. Some Diseases Associated with Mitochondrial Transfer RNA Mutations
5. Association between Transfer RNA Mutations and Type 2 Diabetes Mellitus
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suzuki, T.; Nagao, A.; Suzuki, T. Human Mitochondrial Trnas: Biogenesis, Function, Structural Aspects, and Diseases. Annu. Rev. Genet. 2011, 45, 299–329. [Google Scholar] [CrossRef]
- Stapulionis, R.; Deutscher, M.P. A Channeled TRNA Cycle during Mammalian Protein Synthesis. Proc. Natl. Acad. Sci. USA 1995, 92, 7158–7161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konovalova, S.; Tyynismaa, H. Mitochondrial Aminoacyl-TRNA Synthetases in Human Disease. Mol. Genet. Metab. 2013, 108, 206–211. [Google Scholar] [CrossRef]
- Smith, D.; Yarus, M. Transfer RNA Structure and Coding Specificity. I. Evidence That a D-Arm Mutation Reduces TRNA Dissociation from the Ribosome. J. Mol. Biol. 1989, 206, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Florentz, C.; Sohm, B.; Tryoen-Tóth, P.; Pütz, J.; Sissler, M. Human Mitochondrial TRNAs in Health and Disease. Cell. Mol. Life Sci. 2003, 60, 1356–1375. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Momoi, M.Y.; Tominaga, K.; Momoi, T.; Nihei, K.; Yanagisawa, M.; Kagawa, Y.; Ohta, S. A Point Mutation In The Mitochondrial Trnaleu(Uur) Gene In Me/As (Mitochondrial Myopathy, Encephalopathy, Lactic Acidosis And Stroke-Like Episodes). Biochem. Biophys. Res. Commun. 1990, 173, 816–822. [Google Scholar] [CrossRef]
- Shoffner, J.M.; Lott, M.T.; Lezza, A.M.S.; Seibel, P.; Ballinger, S.W.; Wallace, D.C. Myoclonic Epilepsy and Ragged-Red Fiber Disease (MERRF) Is Associated with a Mitochondrial DNA TRNALys Mutation. Cell 1990, 61, 931–937. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, B.; Huang, S.; Jia, W.; Yu, D. The TRNA-Associated Dysregulation in Diabetes Mellitus. Metabolism 2019, 94, 9–17. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2021; Volume 102, ISBN 9782930229980. [Google Scholar]
- MITOMAP. MITOMAP. Available online: https://www.mitomap.org/MITOMAP (accessed on 25 February 2023).
- Nelson, D.L.; Cox, M.M. Principles of Biochemistry, 6th ed.; Winslow, S., Ed.; WHFreeman and Company: New York, NY, USA, 2013; ISBN 9781429234146. [Google Scholar]
- Lin, L.; Zhang, D.; Jin, Q.; Teng, Y.; Yao, X.; Zhao, T.; Xu, X.; Jin, Y. Mutational Analysis of Mitochondrial Trna Genes in 200 Patients with Type 2 Diabetes Mellitus. Int. J. Gen. Med. 2021, 14, 5719–5735. [Google Scholar] [CrossRef] [PubMed]
- Helm, M.; Brulé, H.; Friede, D.; Giegé, R.; Pütz, D.; Florentz, C. Search for Characteristic Structural Features of Mammalian Mitochondrial TRNAs. Rna 2000, 6, 1356–1379. [Google Scholar] [CrossRef] [Green Version]
- Yarham, J.W.; Elson, J.L.; Blakely, E.L.; Mcfarland, R.; Taylor, R.W. Mitochondrial TRNA Mutations and Disease. Wiley Interdiscip. Rev. RNA 2010, 1, 304–324. [Google Scholar] [CrossRef] [PubMed]
- Kanai, A. Evolutionary Biology: Exobiology and Evolutionary Mechanisms; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9783642382123. [Google Scholar]
- He, Q.; He, X.; Xiao, Y.; Zhao, Q.; Ye, Z.; Cui, L.; Chen, Y.; Guan, M.X. Tissue-Specific Expression Atlas of Murine Mitochondrial TRNAs. J. Biol. Chem. 2021, 297, 100960. [Google Scholar] [CrossRef]
- Toh, Y.; Hori, H.; Tomita, K.; Ueda, T.; Watanabe, K. Transfer RNA Synthesis and Regulation. eLS 2009. [Google Scholar] [CrossRef]
- Phizicky, E.M.; Hopper, A.K. TRNA Biology Charges to the Front. Genes Dev. 2010, 24, 1832–1860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chujo, T.; Tomizawa, K. Human Transfer RNA Modopathies: Diseases Caused by Aberrations in Transfer RNA Modifications. FEBS J. 2021, 288, 7096–7122. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Gao, B.; Huang, J. Mitochondrial Cardiomyopathy: The Roles of Mt-TRNA Mutations. J. Clin. Med. 2022, 11, 6431. [Google Scholar] [CrossRef]
- El Yacoubi, B.; Bailly, M.; De Crécy-Lagard, V. Biosynthesis and Function of Posttranscriptional Modifications of Transfer RNAs. Annu. Rev. Genet. 2012, 46, 69–95. [Google Scholar] [CrossRef]
- Suzuki, T.; Suzuki, T.; Wada, T.; Saigo, K.; Watanabe, K. Taurine as a Constituent of Mitochondrial TRNAs: New Insights into the Functions of Taurine and Human Mitochondrial Diseases. EMBO J. 2002, 21, 6581–6589. [Google Scholar] [CrossRef] [Green Version]
- McFarland, R.; Elson, J.L.; Taylor, R.W.; Howell, N.; Douglass, T.M. Assigning Pathogenicity to Mitochondrial TRNA Mutations: When ‘Definitely Maybe’ Is Not Good Enough. Trends Genet. 2004, 20, 591–596. [Google Scholar] [CrossRef]
- Lant, J.T.; Berg, M.D.; Heinemann, I.U.; Brandl, C.J.; O’Donoghue, P. Pathways to Disease from Natural Variations in Human Cytoplasmic TRNAs. J. Biol. Chem. 2019, 294, 5294–5308. [Google Scholar] [CrossRef] [Green Version]
- Scaglia, F.; Wong, L.J.C. Human Mitochondrial Transfer RNAs: Role of Pathogenic Mutation in Disease. Muscle Nerve 2008, 37, 150–171. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, V.; Pitceathly, R.D.S.; Turnbull, D.M.; Taylor, R.W.; Sweeney, M.G.; Mudanohwo, E.E.; Rahman, S.; Hanna, M.G.; McFarland, R. The UK MRC Mitochondrial Disease Patient Cohort Study: Clinical Phenotypes Associated with the m.3243A>G Mutation—Implications for Diagnosis and Management. J. Neurol. Neurosurg. Psychiatry 2013, 84, 936–938. [Google Scholar] [CrossRef]
- De Laat, P.; Koene, S.; Van Den Heuvel, L.P.W.J.; Rodenburg, R.J.T.; Janssen, M.C.H.; Smeitink, J.A.M. Clinical Features and Heteroplasmy in Blood, Urine and Saliva in 34 Dutch Families Carrying the m.3243A > G Mutation. J. Inherit. Metab. Dis. 2012, 35, 1059–1069. [Google Scholar] [CrossRef] [Green Version]
- Maksum, I.P.; Sriwidodo, S.; Suprijana, O.; Natadisastra, G.; Nuswantara, S.; Noer, A.S. Identifikasi Mutasi Heteroplasmi A3243G DNA Mitokondria Dan Studi Pewarisan Maternal Pada Pasien Diabetes Melitus Tipe 2. Bionatura-J. Ilmu-Ilmu Hayati Fis. 2010, 12, 78–85. [Google Scholar]
- Li, D.; Liang, C.; Zhang, T.; Marley, J.L.; Zou, W.; Lian, M.; Ji, D. Pathogenic Mitochondrial DNA 3243A>G Mutation: From Genetics to Phenotype. Front. Genet. 2022, 13, 2915. [Google Scholar] [CrossRef]
- Iwanicka-Pronicka, K.; Pollak, A.; Skórka, A.; Lechowicz, U.; Pajdowska, M.; Furmanek, M.; Rzeski, M.; Korniszewski, L.; Skarzyński, H.; Płoski, R. Postlingual Hearing Loss as a Mitochondrial 3243A>G Mutation Phenotype. PLoS ONE 2012, 7, e44054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, T.; Nishio, S.Y.; Usami, S.I.; Takeichi, N.; Fukuda, S.; Namba, A.; Shinkawa, H.; Kobayashi, Y.; Sato, H.; Kawase, T.; et al. Frequency of Mitochondrial Mutations in Non-Syndromic Hearing Loss as Well as Possibly Responsible Variants Found by Whole Mitochondrial Genome Screening. J. Hum. Genet. 2014, 59, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Kameoka, K.; Isotani, H.; Tanaka, K.; Azukari, K.; Fujimura, Y.; Shiota, Y.; Sasaki, E.; Majima, M.; Furukawa, K.; Haginomori, S.; et al. Novel Mitochondrial DNA Mutation in TRNA Lys (8296A>G) Associated with Diabetes. Biochem. Biophys. Res. Commun. 1998, 245, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Akita, Y.; Koga, Y.; Iwanaga, R.; Wada, N.; Tsubone, J.; Nakamura, Y.; Kato, H. Fatal Hypertrophic Cardiomyopathy Associated with an A8296G Mutation in the Mitochondrial TRNA Lys Gene. Hum. Mutat. 2000, 15, 382. [Google Scholar] [CrossRef]
- Wilson, F.H.; Hariri, A.; Farhi, A.; Nelson-williams, C.; Raja, K.M.; Scheinman, S.J.; Lifton, R.P. A Cluster of Metabolic Defects Caused by Mutation in a Mitochondrial TRNA. Science 2004, 306, 1190–1194. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Xue, L.; Jiang, P.; Xu, M.; He, Y.; Shi, S.; Huang, Y.; He, J.; Mo, J.Q.; Guan, M. Mitochondrial TRNA Variants in Chinese Subjects With Coronary Heart. J. Am. Heart Assoc. 2014, 3, e000437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukuda, K.; Suzuki, Y.; Kameoka, K.; Osawa, N.; Goto, Y.; Katagiri, H.; Asano, T.; Yazaki, Y.; Oka, Y. Screening of Patients with Maternally Transmitted Diabetes for Mitochondrial Gene Mutations in the TRNALeu(UUR) Region. Diabet. Med. 1997, 14, 1032–1037. [Google Scholar] [CrossRef]
- Lynn, S.; Wardell, T.; Johnson, A.M.; Chinnery, P.F.; Daly, M.E.; Walker, M.; Turnbull, D.M. Mitochondrial Diabetes: Investigation and Identification of a Novel Mutation. Diabetes 1998, 47, 1800–1802. [Google Scholar] [CrossRef]
- Mansergh, F.C.; Millington-Ward, S.; Kennan, A.; Kiang, A.S.; Humphries, M.; Farrar, G.J.; Humphries, P.; Kenna, P.F. Retinitis Pigmentosa and Progressive Sensorineural Hearing Loss Caused by a C12258A Mutation in the Mitochondrial MTTS2 Gene. Am. J. Hum. Genet. 1999, 64, 971–985. [Google Scholar] [CrossRef] [Green Version]
- Tiranti, V.; Agruma, L.D. A Novel Mutation in the Mitochondrial TRNA Val Gene Associated with a Complex Neurological Presentation. Ann. Neurol. 1998, 43, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Sacconi, S.; Salviati, L.; Gooch, C.; Bonilla, E. Complex Neurologic Syndrome Associated With the G1606A Mutation of Mitochondrial DNA. Arch. Neurol. 2015, 59, 1013–1015. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, M.; Nishino, I.; Horai, S.; Nonaka, I.; Goto, Y.-I. Myoclonus Epilepsy Associated With Ragged-Red Fibers: A G-To-A Mutation At Nucleotide Pair 8363 In Mitochondrial TRNA Lys In Two Families. Muscle Nerve 1997, 20, 271–278. [Google Scholar] [CrossRef]
- Difabio, R.; Santorelli, F.M.; Nola, G.; Cricchi, F.; Masi, R.; Ingrosso, A.; Fattori, F.; Carrozzo, R.; Vanacore, N.; Pierelli, F.; et al. Neuromuscular Disorders Clinical and Audiological Follow up of a Family with the 8363G > A Mutation in the Mitochondrial DNA. Neuromuscul. Disord. 2009, 19, 291–296. [Google Scholar] [CrossRef]
- Mihailova, S.; Lukanov, C.; Naumova, E.; Simeonov, E.; Tincheva, R.; Toncheva, D. Mitochondrial DNA Mutations In Two Bulgarian Children with Autistic Spectrum Disorders. Balk. J. Med. Genet. 2013, 2, 47–53. [Google Scholar]
- Gal, A.; Pentelenyi, K.; Remenyi, V.; Pal, Z.; Csanyi, B.; Tomory, G.; Rasko, I. Novel Heteroplasmic Mutation in the Anticodon Stem of Mitochondrial TRNA Lys Associated with Dystonia and Stroke-like Episodes. Acta Neurol. Scand. 2010, 9, 252–256. [Google Scholar] [CrossRef]
- Pinto, M.; Moraes, C.T. Mitochondrial Genome Changes and Neurodegenerative Diseases ☆. Biochim. Biophys. Acta 2014, 1842, 1198–1207. [Google Scholar] [CrossRef] [Green Version]
- Inczedy-farkas, G.; Remenyi, V.; Gal, A.; Varga, Z.; Balla, P.; Udvardy-meszaros, A.; Bereznai, B.; Molnar, M.J. Psychiatric Symptoms of Patients with Primary Mitochondrial DNA Disorders. Behav. Brain Funct. 2012, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darin, N.; Kollberg, G.; Moslemi, A.; Tulinius, M.; Holme, E.; Gro, M.A. Mitochondrial Myopathy with Exercise Intolerance and Retinal Dystrophy in a Sporadic Patient with a G583A Mutation in the Mt TRNA Phe Gene. Neuromuscul. Disord. 2006, 16, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.G.; Nelson, I.P.; Wood, N.W. MELAS: A New Disease Associated Mitochondrial DNA Mutation and Evidence for Further Genetic Heterogeneity. J. Neurol. Neurosurg. Psychiatry 1998, 65, 512–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zsurka, G.; Hampel, K.G.; Nelson, I.; Jardel, C.; Mirandola, S.R.; Sassen, R.; Kornblum, C.; Marcorelles, P.; Lavoue, S.; Lombe, A.; et al. Severe Epilepsy as the Major Symptom of New Mutations in the Mitochondrial TRNA Phe Gene. Neurology 2010, 74, 507–512. [Google Scholar] [CrossRef]
- Nishigaki, Y.; Bonilla, E.; Shanske, S.; Gaskin, D.A.; DiMauro, S.; Hirano, M. Exercise-Induced Muscle “Burning,” Fatigue, and Hyper-CKemia: MtDNA T10010C Mutation in TRNAGly. Neurology 2002, 58, 1282–1285. [Google Scholar] [CrossRef]
- Bidooki, S.K.; Johnson, M.A.; Chrzanowska-Lightowlers, Z.; Bindoff, L.A.; Lightowlers, R.N. Intracellular Mitochondrial Triplasmy in a Patient with Two Heteroplasmic Base Changes. Am. J. Hum. Genet. 1997, 60, 1430–1438. [Google Scholar] [CrossRef] [Green Version]
- Crimi, M.; Galbiati, S.; Sciacco, M.; Bordoni, A.; Natali, M.G.; Raimondi, M.; Bresolin, N.; Comi, G. Pietro Mitochondrial-DNA Nucleotides G4298A and T10010C as Pathogenic Mutations: The Confirmation in Two New Cases. Mitochondrion 2004, 3, 279–283. [Google Scholar] [CrossRef]
- Uusimaa, J.; Finnilä, S.; Remes, A.M.; Rantala, H.; Vainionpää, L.; Hassinen, I.E.; Majamaa, K. Molecular Epidemiology of Childhood Mitochondrial Encephalomyopathies in a Finnish Population: Sequence Analysis of Entire MtDNA of 17 Children Reveals Heteroplasmic Mutations in TRNAArg, TRNAGlu, and TRNA Leu(UUR) Genes. Pediatrics 2004, 114, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Pancrudo, J.; Shanske, S.; Coku, J.; Lu, J.; Mardach, R.; Akman, O.; Krishna, S.; Bonilla, E.; DiMauro, S. Mitochondrial Myopathy Associated with a Novel Mutation in MtDNA. Neuromuscul. Disord. 2007, 17, 651–654. [Google Scholar] [CrossRef] [Green Version]
- Seneca, S.; Goemans, N.; Van Coster, R.; Givron, P.; Reybrouck, T.; Sciot, R.; Meulemans, A.; Smet, J.; Van Hove, J.L.K. A Mitochondrial TRNA Aspartate Mutation Causing Isolated Mitochondrial Myopathy. Am. J. Med. Genet. 2005, 137 A, 170–175. [Google Scholar] [CrossRef]
- Messmer, M.; Gaudry, A.; Sissler, M.; Florentz, C. Pathology-Related Mutation A7526G (A9G) Helps in the Understanding of the 3D Structural Core of Human Mitochondrial TRNAAsp. RNA 2009, 15, 1462–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bataillard, M.; Chatzoglou, E.; Rumbach, L.; Sternberg, D.; Tournade, A.; Laforêt, P.; Jardel, C.; Maisonobe, T.; Lombès, A. Atypical MELAS Syndrome Associated with a New Mitochondrial TRNA Glutamine Point Mutation. Neurology 2001, 56, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Hao, H. A Disease-Associated G5703A Mutation in Human Mitochondrial DNA Causes a Conformational Change and a Marked Decrease in Steady-State Levels of Mitochondrial TRNA Asn. Mol. Cell. Biol. 1997, 17, 6831–6837. [Google Scholar] [CrossRef] [Green Version]
- Vives-Bauza, C.; Del Toro, M.; Solano, A.; Montoya, J.; Andreu, A.L.; Roig, M. Genotype-Phenotype Correlation in the 5703G>A Mutation in the TRNAAsn Gene Ofmitochondrial DNA. J. Inherit.Metab. Dis. 2003, 26, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.-I.; Tojo, M.; Tohyama, J.; Horai, S.; Nonaka, I. A Novel Point Mutation in the Mitochondrial TRNALeu(UUR) Gene in a Family with Mitochondrial Myopathy. Ann. Neurol. 1992, 31, 672–675. [Google Scholar] [CrossRef]
- Akanuma, J.; Muraki, K.; Komaki, H.; Nonaka, I.; Goto, Y. Two Pathogenic Point Mutations Exist in the Authentic Mitochondrial Genome, Not in the Nuclear Pseudogene. J. Hum. Genet. 2000, 45, 337–341. [Google Scholar] [CrossRef] [Green Version]
- Moraes, C.T.; Ciacci, F.; Bonilla, E.; Ionasescu, V.; Schon, E.A.; Mauro, S. Di A Mitochondrial TRNA Anticodon Swap Associated with a Muscle Disease. Nat. Genet. 1993, 3, 73–96. [Google Scholar]
- Cardaioli, E.; Da Pozzo, P.; Malfatti, E.; Gallus, G.N.; Rubegni, A.; Malandrini, A.; Gaudiano, C.; Guidi, L.; Serni, G.; Berti, G.; et al. Chronic Progressive External Ophthalmoplegia: A New Heteroplasmic TRNALeu(CUN) Mutation of Mitochondrial DNA. J. Neurol. Sci. 2008, 272, 106–109. [Google Scholar] [CrossRef]
- Jones, C.N.; Jones, C.I.; Graham, W.D.; Agris, P.F.; Spremulli, L.L. A Disease-Causing Point Mutation in Human Mitochondrial TRNAMet Results in TRNA Misfolding Leading to Defects in Translational Initiation and Elongation. J. Biol. Chem. 2008, 283, 34445–34456. [Google Scholar] [CrossRef] [Green Version]
- Vissing, J.; Salamon, M.B.; Arlien-Søborg, P.; Nørby, S.; Manta, P.; DiMauro, S.; Schmalbruch, H. A New Mitochondrial TRNA(Met) Gene Mutation in a Patient with Dystrophic Muscle and Exercise Intolerance. Neurology 1998, 50, 1875–1878. [Google Scholar] [CrossRef] [PubMed]
- Maniura-Weber, K.; Taylor, R.W.; Johnson, M.A.; Chrzanowska-Lightowlers, Z.; Morris, A.A.M.; Charlton, C.P.J.; Turnbull, D.M.; Bindoff, L.A. A Novel Point Mutation in the Mitochondrial TRNATrp Gene Produces a Neurogastrointestinal Syndrome. Eur. J. Hum. Genet. 2004, 12, 509–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, K.L.; Aprille, J.R.; Ernst, S.G. Mitochondrial TRNAthr Mutation in Fatal Infantile Respiratory Enzyme Deficiency. Biochem. Biophys. Res. Commun. 1991, 176, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.L.; Ernst, S.G.; Rasmussen, C.; Dooling, E.C.; Aprille, J.R. Mitochondrial Disorder Associated with Newborn Cardiopulmonary Arrest. Pediatr. Res. 1993, 33, 433–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Miyauchi, K.; Harada, T.; Okita, R.; Takeshita, E.; Komaki, H.; Fujioka, K.; Yagasaki, H.; Goto, Y.I.; Yanaka, K.; et al. CO2-Sensitive TRNA Modification Associated with Human Mitochondrial Disease. Nat. Commun. 2018, 9, 1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulkes, T.; Siddiqui, A.; Hanna, M.G. A Novel Mutation in the Mitochondrial TRNA Tyr Gene Associated With Exercise Intolerance. Neurology 2000, 55, 1210–1212. [Google Scholar] [CrossRef]
- Pinós, T.; Marotta, M.; Gallardo, E.; Illa, I.; Díaz-Manera, J.; Gonzalez-Vioque, E.; García-Arumí, E.; Andreu, A.L.; Martí, R. A Novel Mutation in the Mitochondrial TRNAAla Gene (m.5636T>C) in a Patient with Progressive External Ophthalmoplegia. Mitochondrion 2011, 11, 228–233. [Google Scholar] [CrossRef]
- Hutchin, T.P.; Parker, M.J.; Young, I.D.; Davis, A.C.; Pulleyn, L.J.; Deeble, J.; Lench, N.J.; Markham, A.F.; Mueller, R.F. Short Reports A Novel Mutation in the Mitochondrial TRNA Ser (UCN) Gene in a Family with Non-Syndromic Sensorineural Hearing Impairment. J. Med. Genet. 2000, 37, 692–694. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Ishikawa, K.; Deng, J.; Heman-ackah, S.; Tamagawa, Y.; Yang, L.; Bai, Y.; Ichimura, K.; Guan, M. Maternally Inherited Nonsyndromic Hearing Loss Is Associated with the T7511C Mutation in the Mitochondrial TRNA. J. Med. Genet. 2005, 328, 32–37. [Google Scholar] [CrossRef]
- El-Hattab, A.W.; Scaglia, F. Mitochondrial Cytopathies. Cell Calcium 2016, 60, 199–206. [Google Scholar] [CrossRef]
- Doco-fenzy, M.; Mauran, P.; Lebrun, J.M.; Bock, S.; Bednarek, N.; Albuisson, J.; Ardalan, A.; Collot, N. A Child With Marcus Gunn Phenomenon and Multiple Congenital Anomalies. Am. J. Hum. Genet. 2006, 221, 212–221. [Google Scholar] [CrossRef]
- Wani, A.A.; Ahanger, S.H.; Bapat, S.A.; Rangrez, A.Y.; Hingankar, N.; Suresh, C.G.; Barnabas, S.; Patole, M.S.; Shouche, Y.S. Analysis of Mitochondrial DNA Sequences in Childhood Encephalomyopathies Reveals New Disease-Associated Variants. PLoS ONE 2007, 2, e942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crimi, M.; Galbiati, S.; Perini, M.P.; Bordoni, A.; Malferrari, G.; Sciacco, M.; Biunno, I.; Strazzer, S.; Moggio, M.; Bresolin, N.; et al. A Mitochondrial TRNAHis Gene Mutation Causing Pigmentary Retinopathy and Neurosensorial Deafness. Neurology 2003, 60, 1200–1203. [Google Scholar] [CrossRef] [PubMed]
- Perucca-lostanlen, D.; Taylor, R.W.; Narbonne, H.; De Camaret, B.M.; Hayes, C.M. Molecular and Functional Effects of the T14709C Point Mutation in the Mitochondrial DNA of a Patient with Maternally Inherited Diabetes and Deafness. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2002, 1588, 210–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ban, R.; Guo, J.H.; Pu, C.Q.; Shi, Q.; Liu, H.X.; Zhang, Y.T. A Novel Mutation of Mitochondrial T14709C Causes Myoclonic Epilepsy with Ragged Red Fibers Syndrome in a Chinese Patient. Chin. Med. J. 2018, 131, 1569–1574. [Google Scholar] [CrossRef]
- McFarland, R.; Schaefer, A.M.; Gardner, J.L.; Lynn, S.; Hayes, C.M.; Barron, M.J.; Walker, M.; Chinnery, P.F.; Taylor, R.W.; Turnbull, D.M. Familial Myopathy: New Insights into the T14709C Mitochondrial TRNA Mutation. Ann. Neurol. 2004, 55, 478–484. [Google Scholar] [CrossRef]
- Damore, M.E.; Speiser, P.W.; Slonim, A.E.; New, M.I.; Shanske, S.; Xia, W.; Santorelli, F.M.; Dimauro, S. Early Onset of Diabetes Mellitus Associated with the Mitochondrial DNA T14709C Point Mutation: Patient Report and Literature Review. J. Pediatr. Endocrinol. Metab. 1999, 213, 207–213. [Google Scholar] [CrossRef]
- Fitriyah, N.; Musthofa, M.W.; Rahayu, P.P. Mathematics Model of Diabetes Mellitus Illness without Genetic Factors with Treatment. Kaunia Integr. Interconnect. Islam Sci. 2021, 17, 21–25. [Google Scholar] [CrossRef]
- Association, A.D. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2015, 38, S10–S11. [Google Scholar] [CrossRef] [Green Version]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Stewart, J.B.; Chinnery, P.F. Extreme Heterogeneity of Human Mitochondrial DNA from Organelles to Populations. Nat. Rev. Genet. 2021, 22, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Paschou, S.A.; Papadopoulou-Marketou, N.; Chrousos, G.P.; Kanaka-Gantenbein, C. On Type 1 Diabetes Mellitus Pathogenesis. Endocr. Connect. 2018, 7, 38–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatimah, R.N. Diabetes Melitus Tipe 2. J. Major. 2015, 4, 93–101. [Google Scholar]
- Okaniawan, P.E.P.; Agustini, N.N.M. Penurunan Fungsi Kognitif Akibat Diabetes Melitus. Ganesha Med. J. 2021, 1, 28. [Google Scholar] [CrossRef]
- Picke, A.K.; Campbell, G.; Napoli, N.; Hofbauer, L.C.; Rauner, M. Update on the Impact of Type 2 Diabetes Mellitus on Bone Metabolism and Material Properties. Endocr. Connect. 2019, 8, R55–R70. [Google Scholar] [CrossRef]
- Tamarai, K.; Bhatti, J.S.; Reddy, P.H. Molecular and Cellular Bases of Diabetes: Focus on Type 2 Diabetes Mouse Model-TallyHo. Biochim. Biophys. Acta-Mol. Basis Dis. 2019, 1865, 2276–2284. [Google Scholar] [CrossRef]
- Prasad, R.B.; Groop, L. Genetics of Type 2 Diabetes—Pitfalls and Possibilities. Genes 2015, 6, 87–123. [Google Scholar] [CrossRef] [Green Version]
- De Andrade, P.B.M.; Rubi, B.; Frigerio, F.; Van Den Ouweland, J.M.W.; Maassen, J.A.; Maechler, P. Diabetes-Associated Mitochondrial DNA Mutation A3243G Impairs Cellular Metabolic Pathways Necessary for Beta Cell Function. Diabetologia 2006, 49, 1816–1826. [Google Scholar] [CrossRef]
- Ratna Pertiwi, K. Penerapan Teknologi DNA Dalam Identifikasi Forensik. J. Ilm. WUNY 2015, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, M.; Takei, M.; Ishii, H.; Sato, Y. Glucose-Stimulated Insulin Secretion: A Newer Perspective. J. Diabetes Investig. 2013, 4, 511–516. [Google Scholar] [CrossRef]
- Park, S.Y.; Gautier, J.F.; Chon, S. Assessment of Insulin Secretion and Insulin Resistance in Human. Diabetes Metab. J. 2021, 45, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K. Effects of Magnesium Deficiency on Mechanisms of Insulin Resistance in Type 2 Diabetes: Focusing on the Processes of Insulin Secretion and Signaling. Int. J. Mol. Sci. 2019, 20, 1351. [Google Scholar] [CrossRef] [Green Version]
- Tokarz, V.L.; MacDonald, P.E.; Klip, A. The Cell Biology of Systemic Insulin Function. J. Cell Biol. 2018, 217, 2273–2289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takano, C.; Ogawa, E.; Hayakawa, S. Insulin Resistance in Mitochondrial Diabetes. Biomolecules 2023, 13, 126. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative Stress, Insulin Signaling, and Diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Z.; Tseng, Y.; White, M.F. Insulin Signaling Meets Mitochondria in Metabolism. Trends Endocrinol. Metab. 2010, 21, 589–598. [Google Scholar] [CrossRef] [Green Version]
- Maksum, I.P.; Saputra, S.R.; Indrayati, N.; Yusuf, M.; Subroto, T. Bioinformatics Study of m.9053G>A Mutation at the ATP6 Gene in Relation to Type 2 Diabetes Mellitus and Cataract Diseases. Bioinform. Biol. Insights 2017, 11, 1177932217728515. [Google Scholar] [CrossRef] [Green Version]
- Destiarani, W.; Mulyani, R.; Yusuf, M.; Maksum, I.P. Molecular Dynamics Simulation of T10609C and C10676G Mutations of Mitochondrial ND4L Gene Associated With Proton Translocation in Type 2 Diabetes Mellitus and Cataract Patients. Bioinform. Biol. Insights 2020, 14, 1177932220978672. [Google Scholar] [CrossRef]
- Wei, F.Y.; Tomizawa, K. TRNA Modifications and Islet Function. Diabetes Obes. Metab. 2018, 20, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Kirino, Y.; Yasukawa, T.; Ohta, S.; Akira, S.; Ishihara, K.; Watanabe, K.; Suzuki, T. Codon-Specific Translational Defect Caused by a Wobble Modification Deficiency in Mutant TRNA from a Human Mitochondrial Disease. Proc. Natl. Acad. Sci. USA 2004, 101, 15070–15075. [Google Scholar] [CrossRef] [Green Version]
- Chinnery, P.F.; Johnson, M.A.; Wardell, T.M.; Hayes, C.; Brown, D.T.; Taylor, R.W.; Bindoff, L.A.; Turnbull, D.M.; Pf, C.; Ma, J.; et al. The Epidemiology of Pathogenic Mitochondrial DNA Mutations. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2000, 48, 188–193. [Google Scholar] [CrossRef]
- Majamaa, K.; Moilanen, J.S.; Uimonen, S.; Remes, A.M.; Salmela, P.I.; Majamaa-voltti, K.A.M.; Rusanen, H.; Sorri, M.; Peuhkurinen, K.J.; Hassinen, I.E. Epidemiology of A3243G, the Mutation for Mitochondrial Encephalomyopathy, Lactic Acidosis, and Strokelike Episodes: Prevalence of the Mutation in an Adult Population. Am. J. Hum. Genet. 1998, 63, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.M.; Ullah, H.; Raziq, A.; Khan, A.A.; Khan, M.W. Molecular Genetic Analysis of Leucine TRNA in Relevance to Type 2 Diabetes Mellitus. Clin. Diabetol. 2020, 9, 167–173. [Google Scholar] [CrossRef]
- Chomyn, A.; Martinuzzi, A.; Yoneda, M.; Daga, A.; Johnst, D.; Lai, S.T.; Nonaka, I.; Angelinit, C.; Atrardi, G. MELAS Mutation in MtDNA Binding Site for Transcription Termination Factor Causes Defects in Protein Synthesis and in Respiration but No Change in Levels of Upstream and Downstream Mature Transcripts. Proc. Natl. Acad. Sci. USA 1992, 89, 4221–4225. [Google Scholar] [CrossRef] [Green Version]
- Finsterer, J. Genetic, Pathogenetic, and Phenotypic Implications of the Mitochondrial A3243G TRNALeu(UUR) Mutation. Acta Neurol. Scand. 2007, 116, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wittenhagen, L.M.; Kelley, S.O. Dimerization of a Pathogenic Human Mitochondrial TRNA. Nat. Struct. Biol. 2002, 9, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Yao, Y.N.; Zheng, Y.G.; Xu, M.G.; Wang, E.D. Reduction of Mitochondrial TRNA Leu(UUR) Aminoacylation by Some MELAS-Associated Mutations. FEBS Lett. 2004, 578, 135–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maksum, I.P.; Maulana, A.F.; Yusuf, M.; Mulyani, R.; Destiarani, W.; Rustaman, R. Molecular Dynamics Simulation of a TRNA-Leucine Dimer with an A3243G Heteroplasmy Mutation in Human Mitochondria Using a Secondary Structure Prediction Approach. Indones. J. Chem. 2022, 22, 1043–1051. [Google Scholar] [CrossRef]
- Puspita, S.R.; Fariz, M.A.; Muhammad, Y.; Maksum Iman, P. Simulation Modeling of A3243G Mutations on TRNALeu (UUR) against Type 2 Diabetes Mellitus Using In Silico Method. Res. J. Chem. Environ. 2023, 27, 65–71. [Google Scholar] [CrossRef]
- Kirino, Y.; Goto, Y.I.; Campos, Y.; Arenas, J.; Suzuki, T. Specific Correlation between the Wobble Modification Deficiency in Mutant TRNAs and the Clinical Features of a Human Mitochondrial Disease. Proc. Natl. Acad. Sci. USA 2005, 102, 7127–7132. [Google Scholar] [CrossRef] [Green Version]
- Wilichowski, E.; Christoph Korenke, G.; Ruitenbeek, W.; De Meirleir, L.; Hagendorff, A.; Janssen, A.J.M.; Lissens, W.; Hanefeld, F. Pyruvate Dehydrogenase Complex Deficiency and Altered Respiratory Chain Function in a Patient with Kearns-Sayre/MELAS Overlap Syndrome and A3243G MtDNA Mutation. J. Neurol. Sci. 1998, 157, 206–213. [Google Scholar] [CrossRef]
- Helm, M.; Florentz, C.; Chomyn, A.; Attardi, G. Search for Differences in Post-Transcriptional Modification Patterns of Mitochondrial DNA-Encoded Wild-Type and Mutant Human TRNA(Lys) and TRNA(Leu(UUR)). Nucleic Acids Res. 1999, 27, 756–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizah, M.I.; Mulyani, R.; Maksum, I.P. Design and Optimization of PCR-RFLP Assay for Detection of G9053A and T15663C Mutation in Mitochondrial DNA. Res. J. Chem. Environ. 2023, 27, 1–5. [Google Scholar] [CrossRef]
- Biggin, A.; Henke, R.; Bennetts, B.; Thorburn, D.R.; Christodoulou, J. Mutation Screening of the Mitochondrial Genome Using Denaturing High-Performance Liquid Chromatography. Mol. Genet. Metab. 2005, 84, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Urata, M.; Wada, Y.; Kim, S.H.; Chumpia, W.; Kayamori, Y.; Hamasaki, N.; Kang, D. High-Sensitivity Detection of the A3243G Mutation of Mitochondrial DNA by a Combination of Allele-Specific PCR and Peptide Nucleic Acid-Directed PCR Clamping. Clin. Chem. 2004, 50, 2045–2051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.L.; Hua, X.Y.; Marsden, D.L.; Liu, D.; Kennaway, N.G.; Ngo, K.Y.; Haas, R.H. Diabetes and Mitochondrial Encephalomyopathy with Lactic Acidosis and Stroke-like Episodes (MELAS): Radiolabeled Polymerase Chain Reaction Is Necessary for Accurate Detection of Low Percentages of Mutation. J. Clin. Endocrinol. Metab. 1997, 82, 2826–2831. [Google Scholar] [CrossRef]
- White, H.E.; Durston, V.J.; Seller, A.; Fratter, C.; Harvey, J.F.; Cross, N.C.P. Accurate Detection and Quantitation of Heteroplasmic Mitochondrial Point Mutations by Pyrosequencing. Genet. Test. 2005, 9, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Urata, M.; Wakiyama, M.; Iwase, M.; Yoneda, M.; Kinoshita, S.; Hamasaki, N.; Kang, D. New Sensitive Method for the Detection of the A3243G Mutation of Human Mitochondrial Deoxyribonucleic Acid in Diabetes Mellitus Patients by Ligation-Mediated Polymerase Chain Reaction. Clin. Chem. 1998, 44, 2088–2093. [Google Scholar] [CrossRef]
- Hartati, Y.W.; Nur Topkaya, S.; Maksum, I.P.; Ozsoz, M. Sensitive Detection of Mitochondrial DNA A3243G TRNALeu Mutation via an Electrochemical Biosensor Using Meldola’s Blue as a Hybridization Indicator. Adv. Anal. Chem. 2013, 2013, 20–27. [Google Scholar] [CrossRef]
- Chandra, R.A.I.; Sriwidodo, A.D.; Diantini, A.; Maksum, I.P. Restriction Enzymes ApaI Analysis to Find A3243G Mutation in Indonesia Diabetes Mellitus Type II Patients. J. Med. Bioeng. 2015, 4, 492–496. [Google Scholar] [CrossRef] [Green Version]
- Liu, V.W.S.; Zhang, C.; Linnane, A.W.; Nagley, P. Quantitative Allele-Specific PCR: Demonstration of Age-Associated Accumulation in Human Tissues of the A→G Mutation at Nucleotide 3243 in Mitochondrial DNA. Hum. Mutat. 1997, 9, 265–271. [Google Scholar] [CrossRef]
- Sriwidodo, S.; Suprijana, O.; Subroto, T.; Maksum, I.P. Studi Mutasi Titik A3243G DNA Mitokondria Penyebab Maternally Mitokondria Penyebab Maternally. Maj. Ilmu Kefarmasian 2008, 3, 2. [Google Scholar] [CrossRef]
- Maksum, I.P.; Farhani, A.; Rachman, S.D.; Ngili, Y. Making of the A3243G Mutant Template through Site Directed Mutagenesis as Positive Control in PASA-Mismatch Three Bases. Int. J. PharmTech Res. 2013, 5, 441–450. [Google Scholar]
- Rong, E.; Wang, H.; Hao, S.; Fu, Y.; Ma, Y.; Wang, T. Heteroplasmy Detection of Mitochondrial DNA A3243G Mutation Using Quantitative Real-Time PCR Assay Based on TaqMan-MGB Probes. Biomed Res. Int. 2018, 2018, 1286480. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhang, S.; Guo, Q.; Zheng, H. Mitochondrial Diabetes Is Associated with TRNALeu(UUR) A3243G and ND6 T14502C Mutations. Diabetes Metab. Syndr. Obes. 2022, 15, 1687–1701. [Google Scholar] [CrossRef] [PubMed]
- Mulyani, R.; Yumna, N.; Maksum, I.P.; Subroto, T.; Hartati, Y.W. Optimization of Aptamer-Based Electrochemical Biosensor for ATP Detection Using Screen-Printed Carbon Electrode/Gold Nanoparticles (SPCE/AuNP). Indones. J. Chem. 2022, 22, 1256–1268. [Google Scholar] [CrossRef]

| Type of tRNA | Three-Letter Abbreviation | One-Letter Abbreviation | Gene | Codons | Size (bp) | Location in Genome |
|---|---|---|---|---|---|---|
| Phenylalanine | Phe | F | MT-TF | UUU UUC | 71 | 577–647 |
| Valine | Val | V | MT-TV | GUU GUC GUA GUG | 69 | 1602–1670 |
| Leucine (UUR) | Leu | L | MT-TL1 | UUA UUG | 75 | 3230–3304 |
| Isoleucine | Ile | I | MT-TI | AUU AUC AUA | 69 | 4263–4331 |
| Glutamic Acid | Glu | E | MT-TE | GAA GAG | 72 | 4329–4400 |
| Methionine | Met | M | MT-TM | AUG | 68 | 4402–4469 |
| Tryptophan | Trp | W | MT-TW | UGG | 68 | 5512–5579 |
| Alanine | Ala | A | MT-TA | GCU GCC GCA GCG | 69 | 5587–5655 |
| Asparagine | Asn | N | MT-TN | AAU AAC | 73 | 5657–5729 |
| Cysteine | Cys | C | MT-TC | UGU UGC | 66 | 5761–5826 |
| Tyrosine | Tyr | Y | MT-TY | UAU UAC | 66 | 5826–5891 |
| Serine (UCN) | Ser | S | MT-TS1 | UCU UCC UCA UCG | 72 | 7445–7516 |
| Aspartic Acid | Asp | D | MT-TD | GAU GAC | 68 | 7518–7585 |
| Lysine | Lys | K | MT-TK | AAA AAG | 70 | 8295–8364 |
| Glycine | Gly | G | MT-TG | GGU GGC GGA GGG | 68 | 9991–10058 |
| Arginine | Arg | R | MT-TR | AGA AGG | 65 | 10405–10469 |
| Histidine | His | H | MT-TH | CAU CAC | 69 | 12138–12206 |
| Serine (AGY) | Ser | S | MT-TS2 | AGA AGG | 59 | 12207–12265 |
| Leucine (CUN) | Leu | L | MT-TL2 | CUU CUC CUA CUG | 71 | 12266–12366 |
| Glutamine | Gln | Q | MT-TQ | CAA CAG | 69 | 14674–14742 |
| Threonine | Thr | T | MT-TT | ACU ACC ACA ACG | 66 | 15888–15953 |
| Proline | Pro | P | MT-TP | CCU CCC CCA CCG | 69 | 15955–16023 |
| Syndrome | Point Mutation | tRNA Gene | Diseases | References |
|---|---|---|---|---|
| Diabetes mellitus (DM) | 3243A>G | tRNALeu(UUR) | MIDD 1, MELAS 2, PEO 3, Leigh syndrome, hearing loss | [26,27,28,29,30,31] |
| 8296A>G | tRNALys | Cardiomyopathy | [32,33] | |
| 4291T>C | tRNAIle | Myopathy, hypomagnesemia, and hypokalemia | [34,35] | |
| 3271C>T | tRNALeu(UUR) | DM | [36] | |
| 12258C>A | tRNASer(AGY) | Hearing loss | [37,38] | |
| Encephalomyopathy | 1606G>A | tRNAVal | Hearing loss | [39,40] |
| 8363G>A | tRNALys | MERRF 4, autism, deafness | [41,42,43] | |
| 8332A>G | tRNALys | Dystonia, MELAS, hearing loss | [44,45,46] | |
| 583G>A | tRNAPhe | MELAS | [47,48,49] | |
| 10010T>C | tRNAGly | PEM 5 | [50,51,52] | |
| 10438A>G | tRNAArg | Progressive encephalopathy | [53,54] | |
| 7526A>G | tRNAAsp | MM | [55,56] | |
| 4332G>A | tRNAGln | MELAS | [57] | |
| Mitochondrial myopathy (MM) | 5703G>A | tRNAAsn | CPEO 6 | [58,59] |
| 3250T>C | tRNALeu(UUR) | MM, CPEO | [60,61] | |
| 15990C>T | tRNAPro | MM | [62] | |
| 12316G>A | tRNALeu(CUN) | CPEO | [63] | |
| 4409T>C | tRNAMet | MM | [64,65] | |
| 5532G>A | tRNATrp | Gastrointestinal syndrome | [66] | |
| 15923A>G | tRNAThr | LIMM 7 | [67,68,69] | |
| 15940T>G | tRNATyr | Exercise intolerance | [70] | |
| 5636T>C | tRNAAla | PEO | [71] | |
| Deafness | 7511T>C | tRNASer(UCN) | SNHL 8 | [72,73] |
| 5783G>A | tRNACys | Myopathy, SNHL | [74,75,76] | |
| 12183G>A | tRNAHis | SNHL, retinitis pigmentosa | [77] | |
| 14709T>C | tRNAGlu | Mental retardation, cerebellar dysfunction, MIDD, MERRF | [48,78,79,80,81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmadanthi, F.R.; Maksum, I.P. Transfer RNA Mutation Associated with Type 2 Diabetes Mellitus. Biology 2023, 12, 871. https://doi.org/10.3390/biology12060871
Rahmadanthi FR, Maksum IP. Transfer RNA Mutation Associated with Type 2 Diabetes Mellitus. Biology. 2023; 12(6):871. https://doi.org/10.3390/biology12060871
Chicago/Turabian StyleRahmadanthi, Fanny Rizki, and Iman Permana Maksum. 2023. "Transfer RNA Mutation Associated with Type 2 Diabetes Mellitus" Biology 12, no. 6: 871. https://doi.org/10.3390/biology12060871




