A Review of Contact Lens-Induced Limbal Stem Cell Deficiency
Abstract
:Simple Summary
Abstract
1. Introduction
2. Epidemiology
3. Pathophysiology
- Lens diameter, blinking;
- Volume and rate at which tears are produced/secreted;
- CL properties: lens-to-cornea fitting relationship, material composition, design (base curve/diameter combination, zone size, etc.).
4. Risk Factors
4.1. Contact Lens Properties
4.2. Factors Causing Dry Eye
4.3. Concomitant Ocular Disease
5. Diagnosis
5.1. Histologic Markers
5.2. Impression Cytology
5.3. In Vivo Confocal Microscopy
5.4. AS-OCT
5.5. OCT-Angiography (OCT-A)
6. Management
6.1. Conservative Management
6.2. Surgical Management
6.2.1. Superficial Keratectomy (SK)
6.2.2. Phototherapeutic Keratectomy (PTK)
6.2.3. Penetrating Keratoplasty (PK)
6.2.4. Amniotic Membrane Transplantation (AMT)
6.2.5. Autologous Limbal Stem Cell Transplantation
6.2.6. Narrow Strip Conjunctival Autograft
6.2.7. Simple Limbal Epithelial Transplantation (SLET)
6.2.8. Living-Related Conjunctival Limbal Allograft (Lr-CLAL)
6.2.9. Cadaver Donor Keratolimbal Allograft (Cd-KLAL)
6.2.10. Cultivated Limbal Epithelial Transplantation (CLET)
7. Current Gaps
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, V.; Tiwari, A.; Kethiri, A.R.; Sangwan, V.S. Current Perspectives of Limbal-Derived Stem Cells and its Application in Ocular Surface Regeneration and Limbal Stem Cell Transplantation. Stem Cells Transl. Med. 2021, 10, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Jiang, S.; Musayeva, A.; Pfeiffer, N.; Gericke, A. Corneal Epithelial Stem Cells–Physiology, Pathophysiology and Therapeutic Options. Cells 2021, 10, 2302. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.; González, S.; Roberts, J.S.; Robertson, S.Y.T.; Ruiz, M.; Zheng, J.; Deng, S.X. Human limbal epithelial stem cell regulation, bioengineering and function. Prog. Retin. Eye Res. 2021, 85, 100956. [Google Scholar] [CrossRef]
- Yazdanpanah, G.; Jabbehdari, S.; Djalilian, A.R. Limbal and corneal epithelial homeostasis. Curr. Opin. Ophthalmol. 2017, 28, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.; Chauhan, T.; Yung, M.; Tseng, C.-H.; Deng, S.X. Outcomes of Limbal Stem Cell Transplant: A Meta-analysis. JAMA Ophthalmol. 2020, 138, 660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, H.; Liu, Y.; Chen, S.; Cai, S.; Zhu, Y.; Guo, P. The Limbal Epithelial Progenitors in the Limbal Niche Environment. Int. J. Med. Sci. 2016, 13, 835–840. [Google Scholar] [CrossRef]
- Yeh, S.-I.; Chu, T.-W.; Cheng, H.-C.; Wu, C.-H.; Tsao, Y.-P. The use of autologous serum to reverse severe contact lens-induced limbal stem cell deficiency. Cornea 2020, 39, 736–741. [Google Scholar] [CrossRef]
- Rossen, J.; Amram, A.; Milani, B.; Park, D.; Harthan, J.; Joslin, C.; McMahon, T.; Djalilian, A. Contact lens-induced limbal stem cell deficiency. Ocul. Surf. 2016, 14, 419–434. [Google Scholar] [CrossRef]
- Deng, S.X.; Borderie, V.; Chan, C.C.; Dana, R.; Figueiredo, F.C.; Gomes, J.A.P.; Pellegrini, G.; Shimmura, S.; Kruse, F.E. Global consensus on definition, classification, diagnosis, and staging of limbal stem cell deficiency. Cornea 2019, 38, 364–375. [Google Scholar] [CrossRef]
- Kim, B.Y.; Riaz, K.M.; Riaz, K.M.; Bakhtiari, P.; Chan, C.C.; Welder, J.D.; Holland, E.J.; Basti, S.; Djalilian, A.R. Medically reversible limbal stem cell disease: Clinical features and management strategies. Ophthalmology 2014, 121, 2053–2058. [Google Scholar] [CrossRef]
- Jeng, B.H.; Halfpenny, C.P.; Meisler, D.M.; Stock, E.L. Management of Focal Limbal Stem Cell Deficiency Associated with Soft Contact Lens Wear. Cornea 2011, 30, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.; Tuft, S.; Liu, C.; Buckley, R. Limbal transplantation in the management of chronic contact-lens-associated epitheliopathy. Eye 1993, 7 Pt 5, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Kate, A.; Basu, S. A review of the diagnosis and treatment of limbal stem cell deficiency. Front. Med. 2022, 9, 836009. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Ting, D.S.J.; AlSaadi, A.; Said, D.G. Management of limbal stem cell deficiency by amnion-assisted conjunctival epithelial redirection using vacuum-dried amniotic membrane and fibrin glue. Br. J. Ophthalmol. 2023, 107, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Achong, R.A.; Caroline, P. Limbal stem cell deficiency in a contact lens-related case. Clin. Eye Vis. Care 1999, 11, 191–197. [Google Scholar] [CrossRef]
- Zainodin, E.L.; Ahmad Najmee, N.A.; Hamzah, F.N.; Saliman, N.H. Ocular Complications in Contact Lens Wear and the Risk Factors: A retrospective analysis. E-BPJ 2021, 6, 111–116. [Google Scholar] [CrossRef]
- Forister, J.F.Y.; Forister, E.F.; Yeung, K.K.; Ye, P.; Chung, M.Y.; Tsui, A.; Weissman, B.A. Prevalence of contact lens-related complications: UCLA contact lens study. Eye Contact Lens Sci. Clin. Pract. 2009, 35, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Cope, J.R.; Collier, S.A.; Rao, M.M.; Chalmers, R.; Mitchell, G.L.; Richdale, K.; Wagner, H.; Kinoshita, B.T.; Lam, D.Y.; Sorbara, L.; et al. Contact lens wearer demographics and risk behaviors for contact lens-related eye infections—United States, 2014. MMWR Morb. Mortal Wkly Rep. 2015, 64, 865–870. [Google Scholar] [CrossRef]
- Nichols, J.J.; Willcox, M.D.P.; Bron, A.J.; Belmonte, C.; Ciolino, J.B.; Craig, J.P.; Dogru, M.; Foulks, G.N.; Jones, L.; Nelson, J.D.; et al. The TFOS international workshop on contact lens discomfort: Executive summary. Investig. Ophthalmol. Vis. Sci. 2013, 54, TFOS7–TFOS13. [Google Scholar] [CrossRef]
- Gurnani, B.; Kaur, K. Contact Lens–Related Problems and Complications; National Library of Medicine: Bethesda, MD, USA, 2022. [Google Scholar]
- Kim, J.H.; Song, J.S.; Hyon, J.Y.; Chung, S.K.; Kim, T.J. A survey of contact lens-related complications in korea: The korean contact lens study society. J. Korean Ophthalmol. Soc. 2014, 55, 20. [Google Scholar] [CrossRef]
- Termote, K.; Schendel, S.; Moloney, G.; Holland, S.P.; Lange, A.P. Focal limbal stem cell deficiency associated with soft contact lens wear. Can. J. Ophthalmol. 2017, 52, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Martin, R. Corneal conjunctivalisation in long-standing contact lens wearers. Clin. Exp. Optom. 2007, 90, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.C.; Holland, E.J. Severe Limbal Stem Cell Deficiency from Contact Lens Wear: Patient Clinical Features. Am. J. Ophthalmol. 2013, 155, 544–549.e2. [Google Scholar] [CrossRef] [PubMed]
- Donisi, P.M.; Rama, P.; Fasolo, A.; Ponzin, D. Analysis of limbal stem cell deficiency by corneal impression cytology. Cornea 2003, 22, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Fuerst, D.J.; Sugar, J.; Worobec, S. Superior limbic keratoconjunctivitis associated with cosmetic soft contact lens wear. Arch. Ophthalmol. 1983, 101, 1214–1216. [Google Scholar] [CrossRef]
- Shen, C.; Chan, C.C.; Holland, E.J. Limbal stem cell transplantation for soft contact lens wear–related limbal stem cell deficiency. Am. J. Ophthalmol. 2015, 160, 1142–1149.e1. [Google Scholar] [CrossRef]
- Schornack, M. Limbal stem cell disease: Management with scleral lenses. Clin. Exp. Optom. 2011, 94, 592–594. [Google Scholar] [CrossRef]
- Sendele, D.D.; Kenyon, K.R.; Mobilia, E.F.; Rosenthal, P.; Steinert, R.; Hanninen, L.A. Superior limbic keratoconjunctivitis in contact lens wearers. Ophthalmology 1983, 90, 616–622. [Google Scholar] [CrossRef]
- Clinch, T.E.; Goins, K.M.; Cobo, L.M. Treatment of contact lens-related ocular surface disorders with autologous conjunctival transplantation. Ophthalmology 1992, 99, 634–638. [Google Scholar] [CrossRef]
- Zhang, X.; Jeyalatha, M.V.; Qu, Y.; He, X.; Ou, S.; Bu, J.; Jia, C.; Wang, J.; Wu, H.; Liu, Z.; et al. Dry eye management: Targeting the ocular surface microenvironment. Int. J. Med. Sci. 2017, 18, 1398. [Google Scholar] [CrossRef]
- Andrasko, G.; Ryen, K. Corneal staining and comfort observed with traditional and silicone hydrogel lenses and multipurpose solution combinations. Optom. J. Am. Optom. Assoc. 2008, 79, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.H.; Lally, J.M.; Hoong, L.D.; Huth, S.W. Lipophilic versus hydrodynamic modes of uptake and release by contact lenses of active entities used in multipurpose solutions. Contact Lens Anterior Eye 2010, 33, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Paugh, J.R.; Nguyen, A.L.; Hall, J.Q.; Krall, D.; Webb, J.R.; Ramsey, A.C.; Meadows, D.L. A preliminary study of silicone hydrogel lens material and care solution bioincompatibilities. Cornea 2011, 30, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Arentsen, J.J. Corneal neovascularization in contact lens wearers. Int. Ophthalmol. Clin. 1986, 26, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, S.E.; Jakobiec, F.A.; Theodore, F.H. Contact lens induced keratopathy. Ophthalmology 1984, 91, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, R.J.; Dassanayake, N.; Carey, C.; Stein, J.; Stone, R.; David, R. Corneal staining and subjective symptoms with multipurpose solutions as a function of time. Eye Contact Lens Sci. Clin. Pract. 2005, 31, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Lebow, K.A.; Schachet, J.L. Evaluation of corneal staining and patient preference with use of three multi-purpose solutions and two brands of soft contact lenses. Eye Contact Lens Sci. Clin. Pract. 2003, 29, 213–220. [Google Scholar] [CrossRef]
- Jones, L.; Powell, C.H. Uptake and release phenomena in contact lens care by silicone hydrogel lenses. Eye Contact Lens Sci. Clin. Pract. 2013, 39, 29–36. [Google Scholar] [CrossRef]
- Bradley, C.S.; Sicks, L.A.; Pucker, A.D. Common ophthalmic preservatives in soft contact lens care products: Benefits, complications, and a comparison to non-preserved solutions. OPTO Vol. 2021, 13, 271–285. [Google Scholar] [CrossRef]
- Tripathi, B.J.; Tripathi, R.C.; Kolli, S.P. Cytotoxicity of ophthalmic preservatives on human corneal epithelium. Lens Eye Toxic. Research. 1992, 9, 361–375. [Google Scholar]
- Nguyen, D.Q.; Srinivasan, S.; Hiscott, P.; Kaye, S.B. Thimerosal-Induced limbal stem cell failure: Report of a case and review of the literature. Eye Contact Lens Sci. Clin. Pract. 2007, 33, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Mowrey-McKee, M. Comparative Cytotoxicity Potential of Soft Contact Lens Care Products. Cutan. Ocul. Toxicol. 2005, 24, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; MacDougall, N.; Sorbara, L.G. Asymptomatic corneal staining associated with the use of Balafilcon Silicone-Hydrogel contact lenses disinfected with a polyaminopropyl biguanide-preserved care regimen. Optom. Vis. Sci. 2002, 79, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Young, G.; Keir, N.; Hunt, C.; Woods, C.A. Clinical evaluation of long-term users of two contact lens care preservative systems. Eye Contact Lens Sci. Clin. Pract. 2009, 35, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Lipener, C. A randomized clinical comparison of OPTI-FREE EXPRESS and ReNu MultiPLUS multipurpose lens care solutions. Adv. Ther. 2009, 26, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Choy, C.K.; Cho, P.; Boost, M.V. Cytotoxicity and effects on metabolism of contact lens care solutions on human corneal epithelium cells. Clin. Exp. Optom. 2012, 95, 198–206. [Google Scholar] [CrossRef]
- Lievens, C.W.; Kannarr, S.; Zoota, L.; Lemp, J. Lid papillae improvement with hydrogen peroxide lens care solution use. Optom. Vis. Sci. 2016, 93, 933–942. [Google Scholar] [CrossRef]
- Guillon, M.; Maissa, C.; Wong, S.; Patel, T.; Garofalo, R. The influence of lens care systems on eyelid tissue changes during silicone hydrogel contact lens wear. Contact Lens Anterior Eye 2018, 41, 362–368. [Google Scholar] [CrossRef]
- Tanti, N.C.; Jones, L.; Gorbet, M.B. Impact of multipurpose solutions released from contact lenses on corneal cells. Optom. Vis. Sci. 2011, 88, 483–492. [Google Scholar] [CrossRef]
- Truong, T.N.; Graham, A.D.; Lin, M.C. Factors in contact lens symptoms. Optom. Vis. Sci. 2014, 91, 133–141. [Google Scholar] [CrossRef]
- Ramasubramanian, V.S.; Meenatchi Sundaram, S.; Thomas, R.; Ramesh, S.V.; Raghuvir Pai, B.; Hazarika, M.; Khader, S.M.A.; Poojary, R.G.; Girish; Crasto, V.S. Finite element analysis of cornea and lid wiper during blink, with and without contact lens. J. Ophthalmol. 2022, 2022, 7930334. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, P.; Cotter, J.M.; Baum, J. Treatment of persistent corneal epithelial defect with extended wear of a fluid-ventilated gas-permeable scleral contact lens. Am. J. Ophthalmol. 2000, 130, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Papas, E.B. The significance of oxygen during contact lens wear. Contact Lens Anterior Eye 2014, 37, 394–404. [Google Scholar] [CrossRef]
- Lin, M.C.; Soliman, G.N.; Song, M.J.; Smith, J.P.; Lin, C.T.; Chen, Y.Q.; Polse, K.A. Soft contact lens extended wear affects corneal epithelial permeability: Hypoxic or mechanical etiology? Contact Lens Anterior Eye 2003, 26, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.S.; Weissman, B.A. Clinical Contact Lens Practice; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; 1102p. [Google Scholar]
- Holden, B.A.; Stephenson, A.; Stretton, S.; Sankaridurg, P.R.; O’Hare, N.; Jalbert, I.; Sweeney, D.F. Superior epithelial arcuate lesions with soft contact lens wear. Optom. Vis. Sci. 2001, 78, 9–12. [Google Scholar] [CrossRef]
- Carney, L.G.; Mainstone, J.C.; Quinn, T.G.; Hill, R.M. Rigid lens centration: Effects of lens design and material density. Int. Contact Lens Clin. 1996, 23, 6–12. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology: IUPAC Recommendations; Wiley-Blackwell: Hoboken, NJ, USA, 1997; 450p. [Google Scholar]
- Paugh, J.R.; Stapleton, F.; Keay, L.; Ho, A. Tear exchange under hydrogel contact lenses: Methodological considerations. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2813–2820. [Google Scholar]
- Garg, A.; Trinh, T.; Wong, B.M.; Mimouni, M.; Ramdass, S.; Liao, J.; Chandrakumar, M.; Slomovic, A.R.; Chan, C.C. Prosthetic replacement of the ocular surface ecosystem for limbal stem cell deficiency: A case series. Eye Contact Lens Sci. Clin. Pract. 2022, 48, 493–496. [Google Scholar] [CrossRef]
- Kim, K.H.; Deloss, K.S.; Hood, C.T. Prosthetic replacement of the ocular surface ecosystem (PROSE) for visual rehabilitation in limbal stem cell deficiency. Eye Contact Lens Sci. Clin. Pract. 2020, 46, 359–363. [Google Scholar] [CrossRef]
- Tran, N.; Graham, A.D.; Lin, M.C. Ethnic differences in dry eye symptoms: Effects of corneal staining and length of contact lens wear. Contact Lens Anterior Eye 2013, 36, 281–288. [Google Scholar] [CrossRef]
- Utheim, T.P. Limbal Epithelial Cell Therapy: Past, Present, and Future. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; pp. 3–43. [Google Scholar]
- Tan, D.T.; Ficker, L.A.; Buckley, R.J. Limbal transplantation. Ophthalmology 1996, 103, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Stenson, S. Superior limbic keratoconjunctivitis associated with soft contact lens wear. Arch. Ophthalmol. 1983, 101, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Puangsricharern, V.; Tseng, S.C.G. Cytologlogic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology 1995, 102, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.F.; Bron, A.J. Limbal palisades of Vogt. Trans. Am. Ophthalmol. Soc. 1982, 80, 155–171. [Google Scholar] [PubMed]
- Tseng, S.C.G. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch. Ophthalmol. 1998, 116, 431. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Gomes, J.A.; Singh, A. Corneal epithelial wound healing. Br. J. Ophthalmol. 1994, 78, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Sejpal, K.D. Characterization of limbal stem cell deficiency by in vivo laser scanning confocal microscopy. Arch. Ophthalmol. 2012, 130, 440. [Google Scholar] [CrossRef] [PubMed]
- D’Aversa, G.; Luchs, J.L.; Fox, M.J.; Rosenbaum, P.S.; Udell, I.J. Advancing wave-like epitheliopathy. Ophthalmology 1997, 104, 962–969. [Google Scholar] [CrossRef]
- Barbaro, V.; Ferrari, S.; Fasolo, A.; Pedrotti, E.; Marchini, G.; Sbabo, A.; Nettis, N.; Ponzin, D.; Di Iorio, E. Evaluation of ocular surface disorders: A new diagnostic tool based on impression cytology and confocal laser scanning microscopy. Br. J. Ophthalmol. 2009, 94, 926–932. [Google Scholar] [CrossRef]
- Rivas, L.; Oroza, M.A.; Perez-Esteban, A.; Murube-del-Castillo, J. Morphological changes in ocular surface in dry eyes and other disorders by impression cytology. Graefe’s Arch. Clin. Exp. Ophthalmol. 1992, 230, 329–334. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Kruse, F.E. Identification and characterization of limbal stem cells. Exp. Eye Res. 2005, 81, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Poli, M.; Burillon, C.; Auxenfans, C.; Rovere, M.-R.; Damour, O. Immunocytochemical diagnosis of limbal stem cell deficiency. Cornea 2015, 34, 817–823. [Google Scholar] [CrossRef] [PubMed]
- García, I.; Etxebarria, J.; Merayo-Lloves, J.; Torras, J.; Boto-de-los-Bueis, A.; Díaz-Valle, D.; Méndez-Fernández, R.; Acera, A.; Suárez-Cortés, T. Novel Molecular Diagnostic System of Limbal Stem Cell Deficiency Based onMUC5ACTranscript Detection in Corneal Epithelium by PCR-Reverse Dot Blot. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5643. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.P.; Srivastava, R.; Ghosh, A. Limbal stem cell study in contact lens wearers. Ann. Ophthalmol. Skokie Ill 2009, 41, 87–92. [Google Scholar]
- Miri, A.; Alomar, T.; Nubile, M.; Al-aqaba, M.; Lanzini, M.; Fares, U.; Said, D.G.; Lowe, J.; Dua, H.S. In vivo confocal microscopic findings in patients with limbal stem cell deficiency. Br. J. Ophthalmol. 2012, 96, 523–529. [Google Scholar] [CrossRef]
- Romano, A.; Espana, E.; Djalilian, A.; Yoo, S. En face optical coherence tomography imaging of corneal limbal stem cell niche. In Clinical En Face OCT Atlas; Jaypee Brothers Medical Publishers (P) Ltd.: New Delhi, India, 2013; p. 77. [Google Scholar]
- Zakaria, N.; Ní Dhubhghaill, S.; Taal, M.; Berneman, Z.; Koppen, C.; Tassignon, M.-J. Optical coherence tomography in cultivated limbal epithelial stem cell transplantation surgery. Asia-Pac. J. Ophthalmol. 2015, 4, 339–345. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Edwards, K.; Harkin, D.; Schmid, K.L. Central corneal basal cell density and nerve parameters in ocular surface disease and limbal stem cell deficiency: A review and meta-analysis. Br. J. Ophthalmol. 2020, 104, 1633–1639. [Google Scholar] [CrossRef]
- Chidambaranathan, G.P.; Mathews, S.; Panigrahi, A.K.; Mascarenhas, J.; Prajna, N.V.; Muthukkaruppan, V. In vivo Confocal Microscopic Analysis of Limbal Stroma in Patients with Limbal Stem Cell Deficiency. Cornea 2015, 34, 1478–1486. [Google Scholar] [CrossRef]
- Caro-Magdaleno, M.; Alfaro-Juárez, A.; Montero-Iruzubieta, J.; Fernández-Palacín, A.; Muñoz-Morales, A.; Castilla-Martino, M.A.; Spínola-Muñoz, C.; Rodríguez-de-la-Rúa, E. In vivo confocal microscopy indicates an inverse relationship between the sub-basal corneal plexus and the conjunctivalisation in patients with limbal stem cell deficiency. Br. J. Ophthalmol. 2019, 103, 327–331. [Google Scholar] [CrossRef]
- Liang, Q.; Le, Q.; Cordova, D.W.; Tseng, C.-H.; Deng, S.X. Corneal epithelial thickness measured using anterior segment optical coherence tomography as a diagnostic parameter for limbal stem cell deficiency. Am. J. Ophthalmol. 2020, 216, 132–139. [Google Scholar] [CrossRef]
- Grieve, K.; Ghoubay, D.; Georgeon, C.; Thouvenin, O.; Bouheraoua, N.; Paques, M.; Borderie, V.M. Three-dimensional structure of the mammalian limbal stem cell niche. Exp. Eye Res. 2015, 140, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.; Yang, Y.; Deng, S.X.; Xu, J. Correlation between the existence of the palisades of Vogt and limbal epithelial thickness in limbal stem cell deficiency. Clin. Exp. Ophthalmol. 2016, 45, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.; Shanbhag, S.S.; Donthineni, P.R.; Mishra, D.K.; Singh, V.; Basu, S. High-Resolution Optical Coherence Tomography Angiography Characteristics of Limbal Stem Cell Deficiency. Diagnostics 2021, 11, 1130. [Google Scholar] [CrossRef] [PubMed]
- Oie, Y.; Nishida, K. Evaluation of Corneal Neovascularization Using Optical Coherence Tomography Angiography in Patients with Limbal Stem Cell Deficiency. Cornea 2017, 36 (Suppl. S1), S72–S75. [Google Scholar] [CrossRef] [PubMed]
- Bizheva, K.; Hutchings, N.; Sorbara, L.; Moayed, A.A.; Simpson, T. In vivo volumetric imaging of the human corneo-scleral limbus with spectral domain OCT. Biomed. Opt. Express 2011, 2, 1794–1802. [Google Scholar] [CrossRef]
- Lim, L.; Wei, R.H. Laser in situ keratomileusis treatment for myopia in a patient with partial limbal stem cell deficiency. Eye Contact Lens Sci. Clin. Pract. 2005, 31, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Kumar, P. Oral omega-3 fatty acid treatment for dry eye in contact lens wearers. Cornea 2015, 34, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Seo, K.Y.; Rhim, T.; Kim, E.K. Effect of retinoic acid on epithelial differentiation and mucin expression in primary human corneal limbal epithelial cells. Curr. Eye Res. 2011, 37, 33–42. [Google Scholar] [CrossRef]
- Anderson, D.F. Amniotic membrane transplantation for partial limbal stem cell deficiency. Br. J. Ophthalmol. 2001, 85, 567–575. [Google Scholar] [CrossRef]
- Kinoshita, S.; Friend, J.; Thoft, R.A. Sex chromatin of donor corneal epithelium in rabbits. Investig. Ophthalmol. Vis. Sci. 1981, 21, 434–441. [Google Scholar]
- Tseng, S.C.; Tsai, R.J. Limbal transplantation for ocular surface reconstruction—A review. Fortschritte Ophthalmol. Z. Dtsch. Ophthalmol. Ges. 1991, 88, 236–242. [Google Scholar]
- Dua, H.S. Autologous limbal transplantation in patients with unilateral corneal stem cell deficiency. Br. J. Ophthalmol. 2000, 84, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, K.; Zhou, Y.; Wu, T.; Zhang, H.; Yang, Q.; Huang, Y.; Wang, L. Outcome of keratolimbal allograft transplantation with deep anterior lamellar keratoplasty for bilateral limbal stem cell deficiency. Front. Med. 2022, 9, 986194. [Google Scholar] [CrossRef]
- Han, S.B.; Ibrahim, F.N.I.M.; Liu, Y.-C.; Mehta, J.S. Efficacy of modified amnion-assisted conjunctival epithelial redirection (ACER) for partial limbal stem cell deficiency. Medicina 2021, 57, 369. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.; Ellies, P.; Anderson, D.F.; Touhami, A.; Grueterich, M.; Espana, E.M.; Ti, S.-E.; Goto, E.; Feuer, W.J.; Tseng, S.C. Long-term outcome of keratolimbal allograft with or without penetrating keratoplasty for total limbal stem cell deficiency. Ophthalmology 2002, 109, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Miri, A.; Elalfy, M.S.; Lencova, A.; Said, D.G. Amnion-assisted conjunctival epithelial redirection in limbal stem cell grafting. Br. J. Ophthalmol. 2016, 101, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Keivyon, K.R.; Tseng, S.C.G. Limbal autograft transplantation for ocular surface disorders. Ophthalmology 1989, 96, 709–723. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Sarnicola, E.; Holland, E.J. Long-Term Ocular Surface Stability in Conjunctival Limbal Autograft Donor Eyes. Cornea 2017, 36, 1031–1035. [Google Scholar] [CrossRef]
- Shanbhag, S.S.; Nikpoor, N.; Rao Donthineni, P.; Singh, V.; Chodosh, J.; Basu, S. Autologous limbal stem cell transplantation: A systematic review of clinical outcomes with different surgical techniques. Br. J. Ophthalmol. 2020, 104, 247–253. [Google Scholar] [CrossRef]
- Baradaran-Rafii, A.; Eslani, M.; Jamali, H.; Karimian, F.; Tailor, U.A.; Djalilian, A.R. Postoperative complications of conjunctival limbal autograft surgery. Cornea 2012, 31, 893–899. [Google Scholar] [CrossRef]
- Marchini, G.; Pedrotti, E.; Pedrotti, M.; Barbaro, V.; Di Iorio, E.; Ferrari, S.; Bertolin, M.; Ferrari, B.; Passilongo, M.; Fasolo, A.; et al. Long-term effectiveness of autologous cultured limbal stem cell grafts in patients with limbal stem cell deficiency due to chemical burns. Clin. Exp. Ophthalmol. 2012, 40, 255–267. [Google Scholar] [CrossRef]
- Chen, K.; Soleimani, M.; Koganti, R.; Cheraqpour, K.; Habeel, S.; Djalilian, A.R. Cell-based therapies for limbal stem cell deficiency: A literature review. Ann. Eye Sci. 2023, 8, 6. [Google Scholar] [CrossRef]
- Human medicines European public assessment report (EPAR): Holoclar, ex vivo expanded autologous human corneal epithelial cells containing stem cells, Stem Cell Transplantation, Corneal Diseases, Date of authorisation: 17/02/2015, Revision: 5, Status: Authorised. Case Med. Res. 2019, 75. [CrossRef]
- Nishiwaki-Dantas, M.C. Ipsilateral limbal translocation for treatment of partial limbal deficiency secondary to ocular alkali burn. Br. J. Ophthalmol. 2001, 85, 1031–1033. [Google Scholar] [CrossRef]
- Dupps, W.J.; Jeng, B.H., Jr.; Meisler, D.M. Narrow-Strip conjunctival autograft for treatment of pterygium. Ophthalmology 2007, 114, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.S.; Basu, S.; MacNeil, S.; Balasubramanian, D. Simple limbal epithelial transplantation (SLET): A novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br. J. Ophthalmol. 2012, 96, 931–934. [Google Scholar] [CrossRef]
- Jackson, C.J.; Myklebust Ernø, I.T.; Ringstad, H.; Tønseth, K.A.; Dartt, D.A.; Utheim, T.P. Simple limbal epithelial transplantation: Current status and future perspectives. Stem Cells Transl. Med. 2020, 9, 316–327. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Holland, E.J. Keratolimbal allograft. Curr. Opin. Ophthalmol. 2017, 28, 377–381. [Google Scholar] [CrossRef]
- Biber, J.M.; Skeens, H.M.; Neff, K.D.; Holland, E.J. The Cincinnati procedure: Technique and outcomes of combined living-related conjunctival limbal allografts and keratolimbal allografts in severe ocular surface failure. Cornea 2011, 30, 765–771. [Google Scholar] [CrossRef]
- Krakauer, M.; Welder, J.D.; Pandya, H.K.; Nassiri, N.; Djalilian, A.R. Adverse Effects of Systemic Immunosuppression in Keratolimbal Allograft. J. Ophthalmol. 2012, 2012, 576712. [Google Scholar] [CrossRef]
- Holland, E.J.; Mogilishetty, G.; Skeens, H.M.; Hair, D.B.; Neff, K.D.; Biber, J.M.; Chan, C.C. Systemic Immunosuppression in Ocular Surface Stem Cell Transplantation: Results of a 10-Year Experience. Cornea 2012, 31, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Rao, S. Limbal allografting from related live donors for corneal surface reconstruction. Ophthalmology 1999, 106, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Ilari, L.; Daya, S.M. Long-term outcomes of keratolimbal allograft for the treatment of severe ocular surface disorders. Ophthalmology 2002, 109, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Shimmura, S.; Shinozaki, N.; Holland, E.J.; Shimazaki, J. Clinical application of living-related conjunctival-limbal allograft. Am. J. Ophthalmol. 2002, 133, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Han, E.S.; Wee, W.R.; Lee, J.H.; Kim, M.K. Long-term outcome and prognostic factor analysis for keratolimbal allografts. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.J.; Schwartz, G.S. The evolution of epithelial transplantation for severe ocular surface disease and a proposed classification system. Cornea 1996, 15, 549–556. [Google Scholar] [CrossRef]
- Basu, S.; Fernandez, M.M.; Das, S.; Gaddipati, S.; Vemuganti, G.K.; Sangwan, V.S. Clinical outcomes of xeno-free allogeneic cultivated limbal epithelial transplantation for bilateral limbal stem cell deficiency. Br. J. Ophthalmol. 2012, 96, 1504–1509. [Google Scholar] [CrossRef]
- Miri, A.; Al-Deiri, B.; Dua, H.S. Long-term outcomes of autolimbal and allolimbal transplants. Ophthalmology 2010, 117, 1207–1213. [Google Scholar] [CrossRef]
- Thokala, P.; Singh, A.; Singh, V.K.; Rathi, V.M.; Basu, S.; Singh, V.; MacNeil, S.; Sangwan, V.S. Economic, clinical and social impact of simple limbal epithelial transplantation for limbal stem cell deficiency. Br. J. Ophthalmol. 2021, 106, 923–928. [Google Scholar] [CrossRef]
- Baylis, O.; Figueiredo, F.; Henein, C.; Lako, M.; Ahmad, S. 13 years of cultured limbal epithelial cell therapy: A review of the outcomes. J. Cell Biochem. 2011, 112, 993–1002. [Google Scholar] [CrossRef]
- Booranapong, W.; Kosrirukvongs, P.; Duangsa-ard, S.; Kasetsinsombat, K.; Sa-ngiamsuntorn, K.; Wongkajornsilp, A. Transplantation of autologous cultivated oral mucosal epithelial sheets for limbal stem cell deficiency at Siriraj Hospital: A case series. J. Med. Case Rep. 2022, 16, 298. [Google Scholar] [CrossRef] [PubMed]
| Congenital |
|
| Traumatic/acquired |
|
| Autoimmune |
|
| Idiopathic | |
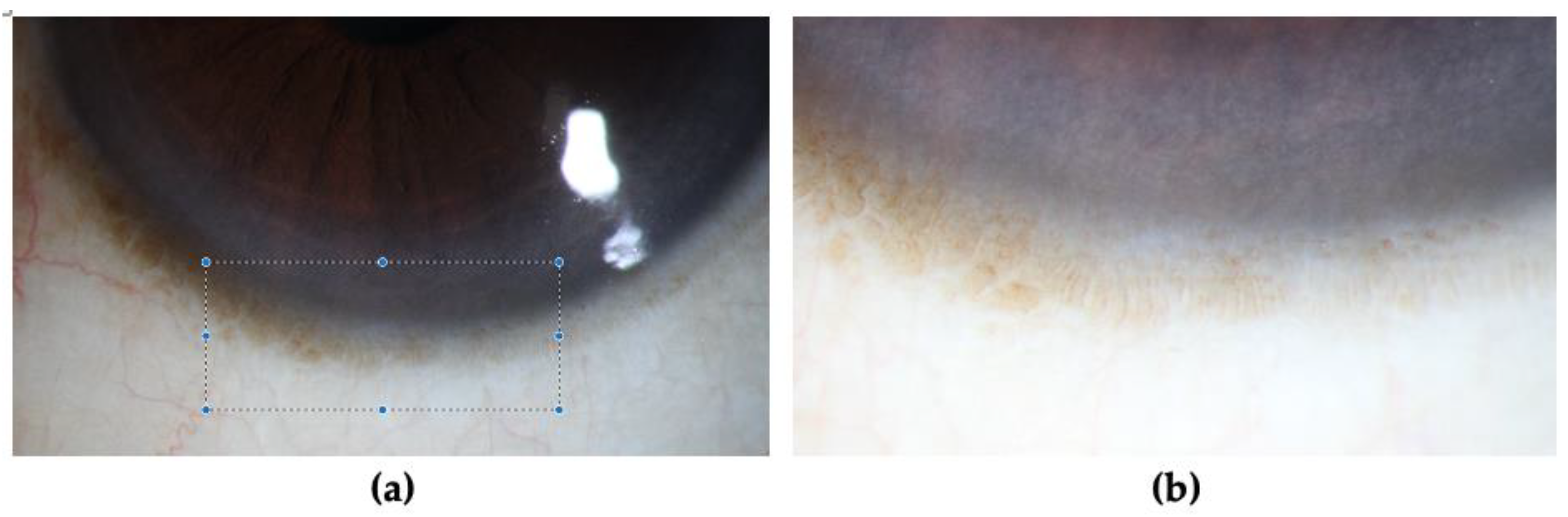
| Early | Fluorescein staining in a punctate pattern in a curve-like distribution: |
| Late | Punctate staining merges into a linear pattern.Eventually progresses into a centrally distributed confluent sheet [10,37]. |
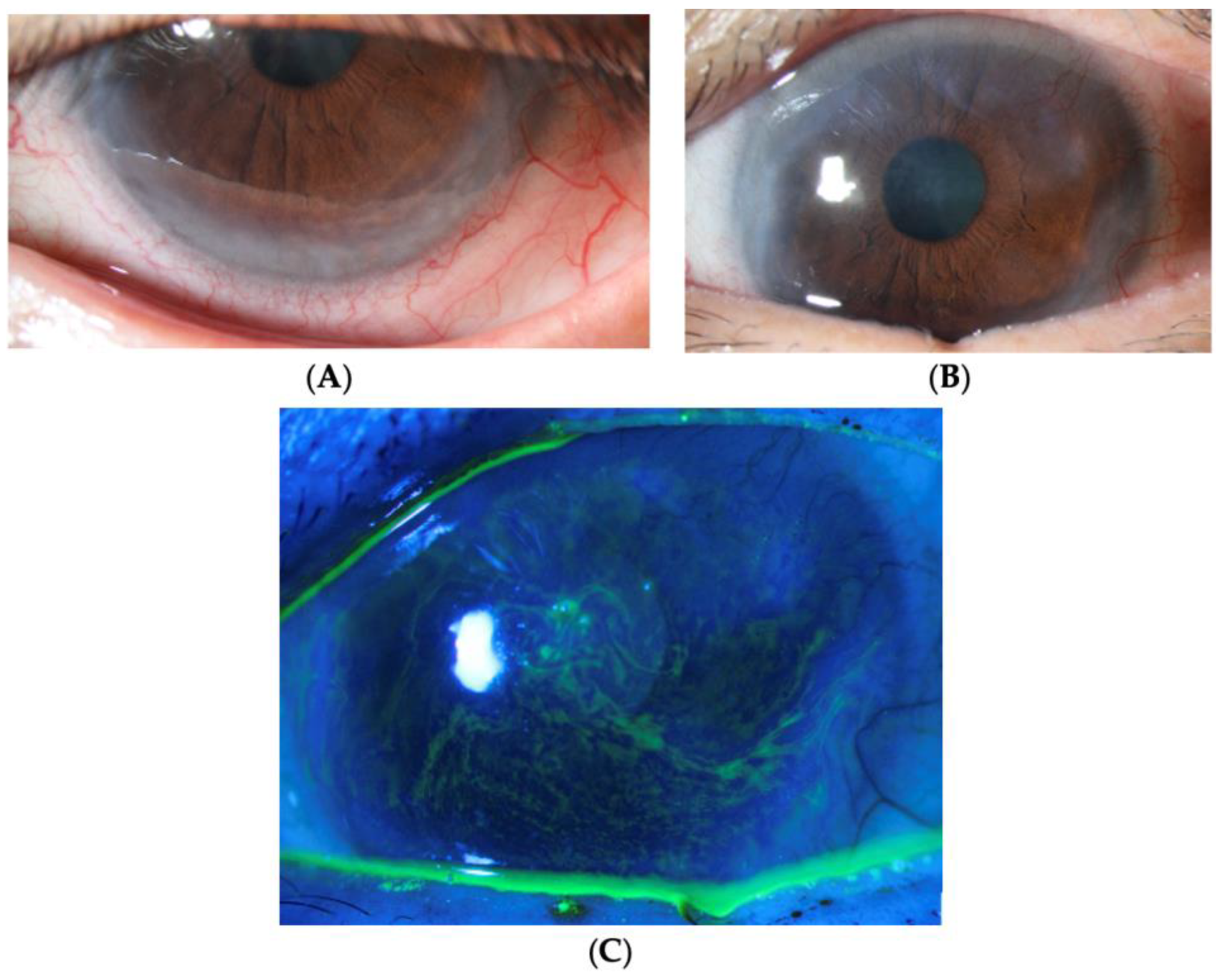
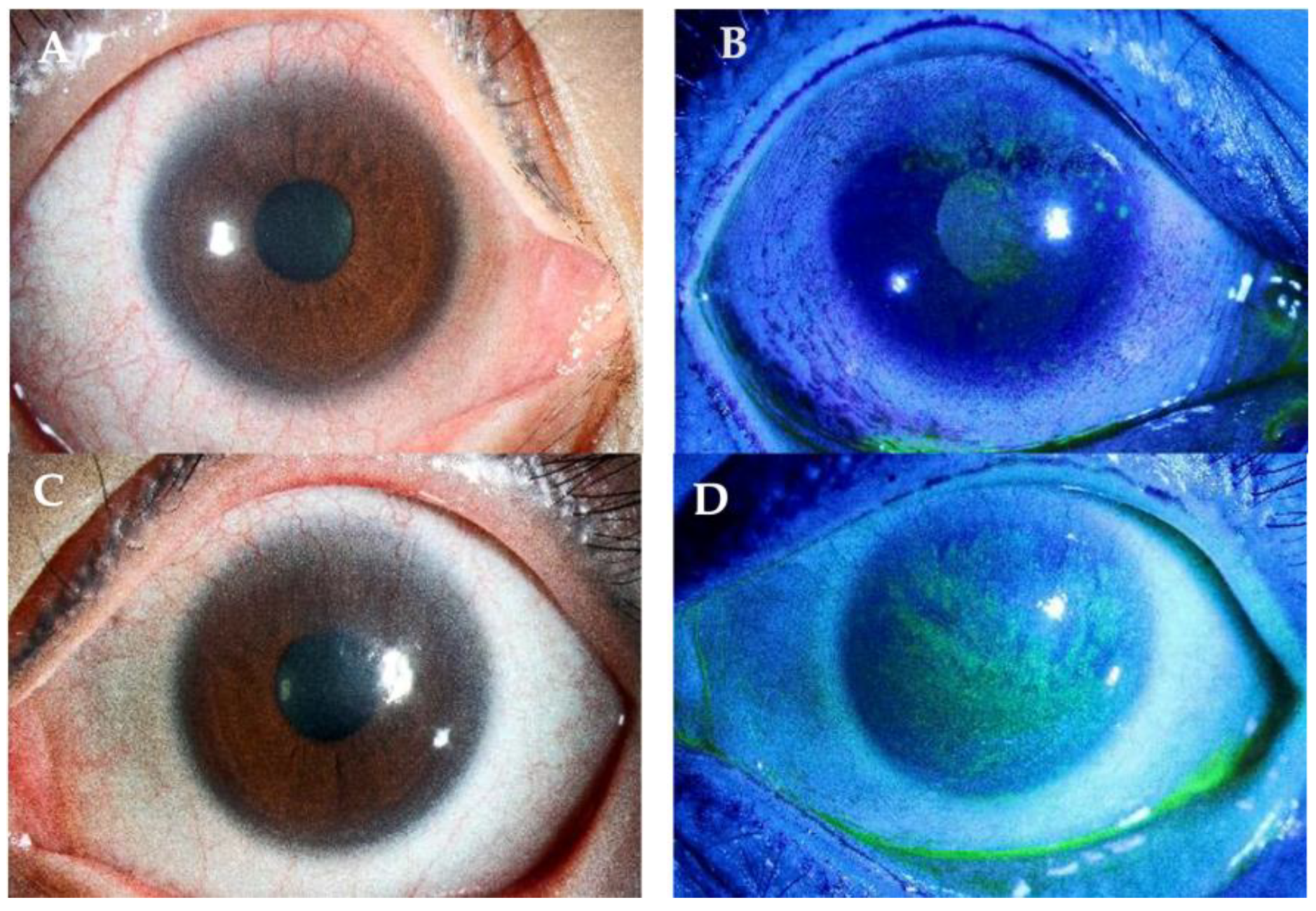
| Later-stage | Corneal pannus, which loosely refers to [10,28]:
|
| End-stage | Signs include [71,72]:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.F.; Yong, D.W.W.; Manotosh, R. A Review of Contact Lens-Induced Limbal Stem Cell Deficiency. Biology 2023, 12, 1490. https://doi.org/10.3390/biology12121490
Lee YF, Yong DWW, Manotosh R. A Review of Contact Lens-Induced Limbal Stem Cell Deficiency. Biology. 2023; 12(12):1490. https://doi.org/10.3390/biology12121490
Chicago/Turabian StyleLee, Yhu Fhei, Dayna Wei Wei Yong, and Ray Manotosh. 2023. "A Review of Contact Lens-Induced Limbal Stem Cell Deficiency" Biology 12, no. 12: 1490. https://doi.org/10.3390/biology12121490
APA StyleLee, Y. F., Yong, D. W. W., & Manotosh, R. (2023). A Review of Contact Lens-Induced Limbal Stem Cell Deficiency. Biology, 12(12), 1490. https://doi.org/10.3390/biology12121490






