Prostaglandin E2 Boosts the Hyaluronan-Mediated Increase in Inflammatory Response to Lipopolysaccharide by Enhancing Lyve1 Expression
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. RNA Analyses
2.4. Zymosan-Induced Peritonitis
2.5. FACS Analyses and Sorting
2.6. RNA Sequencing
2.7. Statistics
3. Results
3.1. Inhibition of mPGES-1 Reduces Lyve1 Expression in Macrophages during Resolution of Inflammation
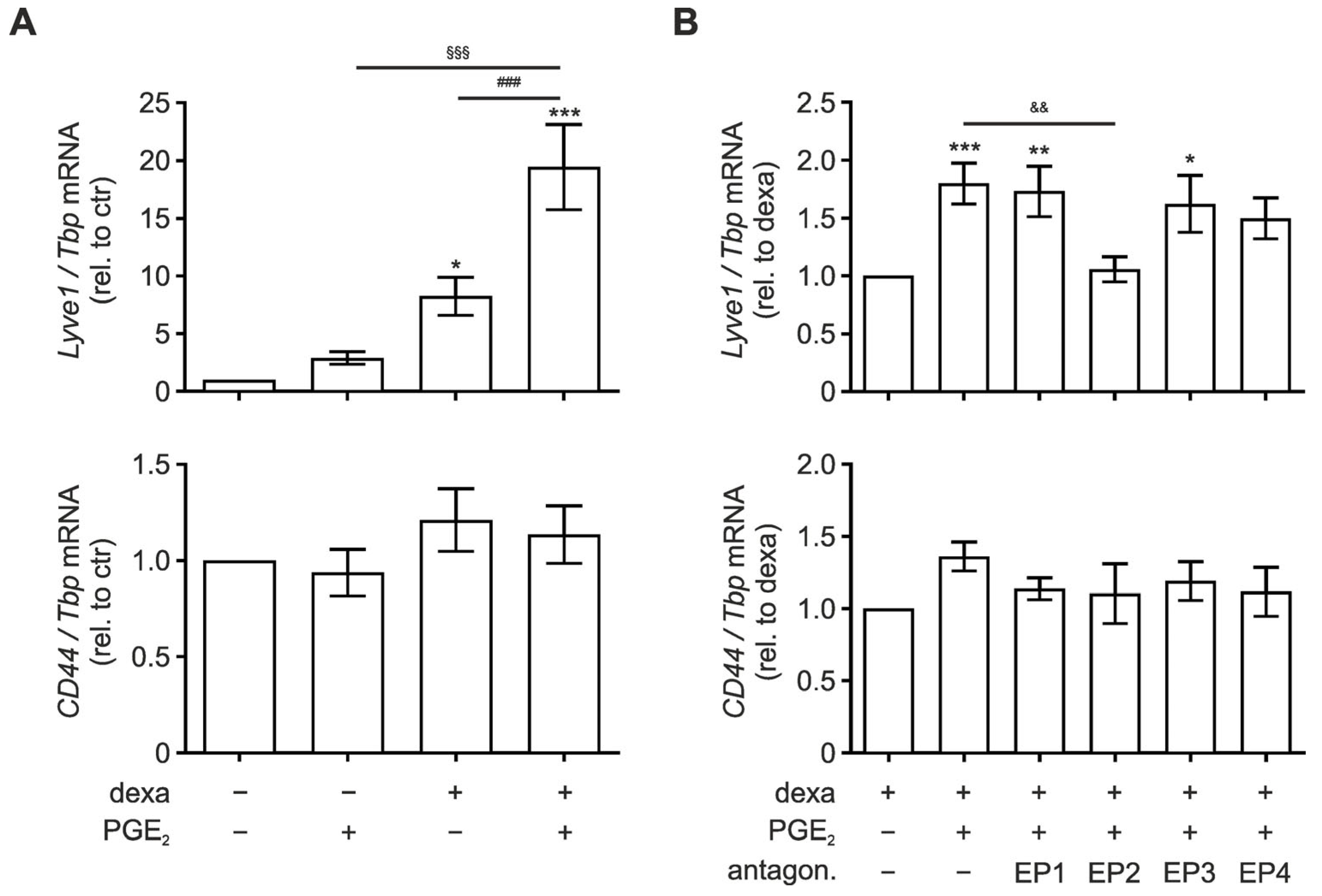
3.2. EP2 Signaling Enhances Lyve1 Expression
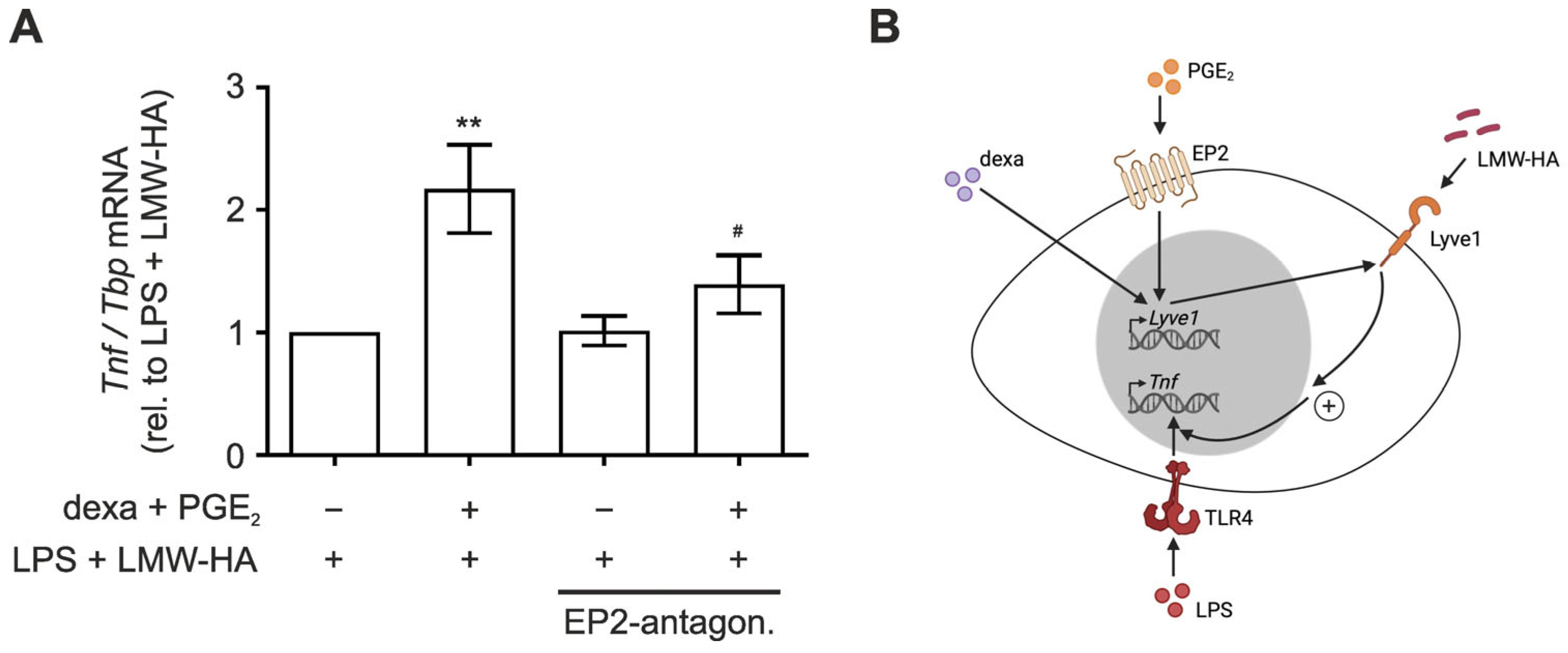
3.3. LMW-HA Enhances LPS-Induced TNF Expression in PGE2/Dexamethasone-Primed Macrophages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jentho, E.; Weis, S. DAMPs and Innate Immune Training. Front. Immunol. 2021, 12, 699563. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Schett, G.; Neurath, M.F. Resolution of Chronic Inflammatory Disease: Universal and Tissue-Specific Concepts. Nat. Commun. 2018, 9, 3261. [Google Scholar] [CrossRef]
- Sindrilaru, A.; Peters, T.; Wieschalka, S.; Baican, C.; Baican, A.; Peter, H.; Hainzl, A.; Schatz, S.; Qi, Y.; Schlecht, A.; et al. An Unrestrained Proinflammatory M1 Macrophage Population Induced by Iron Impairs Wound Healing in Humans and Mice. J. Clin. Investig. 2011, 121, 985–997. [Google Scholar] [CrossRef]
- Swirski, F.K.; Pittet, M.J.; Kircher, M.F.; Aikawa, E.; Jaffer, F.A.; Libby, P.; Weissleder, R. Monocyte Accumulation in Mouse Atherogenesis Is Progressive and Proportional to Extent of Disease. Proc. Natl. Acad. Sci. USA 2006, 103, 10340–10345. [Google Scholar] [CrossRef]
- Kasikara, C.; Doran, A.C.; Cai, B.; Tabas, I. The Role of Non-Resolving Inflammation in Atherosclerosis. J. Clin. Investig. 2018, 128, 2713–2723. [Google Scholar] [CrossRef]
- Newson, J.; Stables, M.; Karra, E.; Arce-Vargas, F.; Quezada, S.; Motwani, M.; Mack, M.; Yona, S.; Audzevich, T.; Gilroy, D.W. Resolution of Acute Inflammation Bridges the Gap between Innate and Adaptive Immunity. Blood 2014, 124, 1748–1764. [Google Scholar] [CrossRef]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 Macrophages and the Th1/Th2 Paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef]
- Chakarov, S.; Lim, H.Y.; Tan, L.; Lim, S.Y.; See, P.; Lum, J.; Zhang, X.-M.; Foo, S.; Nakamizo, S.; Duan, K.; et al. Two Distinct Interstitial Macrophage Populations Coexist across Tissues in Specific Subtissular Niches. Science 2019, 363, eaau0964. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The Chemokine System in Diverse Forms of Macrophage Activation and Polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Smigiel, K.S.; Parks, W.C. Macrophages, Wound Healing, and Fibrosis: Recent Insights. Curr. Rheumatol. Rep. 2018, 20, 17. [Google Scholar] [CrossRef]
- Zaynagetdinov, R.; Sherrill, T.P.; Kendall, P.L.; Segal, B.H.; Weller, K.P.; Tighe, R.M.; Blackwell, T.S. Identification of Myeloid Cell Subsets in Murine Lungs Using Flow Cytometry. Am. J. Respir. Cell Mol. Biol. 2013, 49, 180–189. [Google Scholar] [CrossRef]
- Lakschevitz, F.S.; Hassanpour, S.; Rubin, A.; Fine, N.; Sun, C.; Glogauer, M. Identification of Neutrophil Surface Marker Changes in Health and Inflammation Using High-Throughput Screening Flow Cytometry. Exp. Cell Res. 2016, 342, 200–209. [Google Scholar] [CrossRef]
- Blériot, C.; Chakarov, S.; Ginhoux, F. Determinants of Resident Tissue Macrophage Identity and Function. Immunity 2020, 52, 957–970. [Google Scholar] [CrossRef]
- Jablonski, K.A.; Amici, S.A.; Webb, L.M.; Ruiz-Rosado, J.D.D.; Popovich, P.G.; Partida-Sanchez, S.; Guerau-de-Arellano, M. Novel Markers to Delineate Murine M1 and M2 Macrophages. PLoS ONE 2015, 10, e0145342. [Google Scholar] [CrossRef]
- Banerji, S.; Ni, J.; Wang, S.-X.; Clasper, S.; Su, J.; Tammi, R.; Jones, M.; Jackson, D.G. LYVE-1, a New Homologue of the CD44 Glycoprotein, Is a Lymph-Specific Receptor for Hyaluronan. J. Cell Biol. 1999, 144, 789–801. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan in Tissue Injury and Repair. Annu. Rev. Cell Dev. Biol. 2007, 23, 435–461. [Google Scholar] [CrossRef]
- Ruppert, S.M.; Hawn, T.R.; Arrigoni, A.; Wight, T.N.; Bollyky, P.L. Tissue Integrity Signals Communicated by High-Molecular Weight Hyaluronan and the Resolution of Inflammation. Immunol. Res. 2014, 58, 186–192. [Google Scholar] [CrossRef]
- Baeva, L.F.; Lyle, D.B.; Rios, M.; Langone, J.J.; Lightfoote, M.M. Different Molecular Weight Hyaluronic Acid Effects on Human Macrophage Interleukin 1β Production: Effects of Lmw+Ha on Human Monocytes. J. Biomed. Mater. Res. 2014, 102, 305–314. [Google Scholar] [CrossRef]
- Cyphert, J.M.; Trempus, C.S.; Garantziotis, S. Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int. J. Cell Biol. 2015, 2015, 563818. [Google Scholar] [CrossRef]
- Zhang, G.; Gao, Y.; Zhao, Z.; Pyykko, I.; Zou, J. Low-Molecular-Weight Hyaluronic Acid Contributes to Noise-Induced Cochlear Inflammation. Audiol. Neurootol. 2023, 28, 380–393. [Google Scholar] [CrossRef]
- Dick, S.A.; Wong, A.; Hamidzada, H.; Nejat, S.; Nechanitzky, R.; Vohra, S.; Mueller, B.; Zaman, R.; Kantores, C.; Aronoff, L.; et al. Three Tissue Resident Macrophage Subsets Coexist across Organs with Conserved Origins and Life Cycles. Sci. Immunol. 2022, 7, eabf7777. [Google Scholar] [CrossRef]
- Wang, Y.; Chaffee, T.S.; LaRue, R.S.; Huggins, D.N.; Witschen, P.M.; Ibrahim, A.M.; Nelson, A.C.; Machado, H.L.; Schwertfeger, K.L. Tissue-Resident Macrophages Promote Extracellular Matrix Homeostasis in the Mammary Gland Stroma of Nulliparous Mice. eLife 2020, 9, e57438. [Google Scholar] [CrossRef]
- Anstee, J.E.; Feehan, K.T.; Opzoomer, J.W.; Dean, I.; Muller, H.P.; Bahri, M.; Cheung, T.S.; Liakath-Ali, K.; Liu, Z.; Choy, D.; et al. LYVE-1+ Macrophages Form a Collaborative CCR5-Dependent Perivascular Niche That Influences Chemotherapy Responses in Murine Breast Cancer. Dev. Cell 2023, 58, 1548–1561.e10. [Google Scholar] [CrossRef]
- Slysz, J.; Sinha, A.; DeBerge, M.; Singh, S.; Avgousti, H.; Lee, I.; Glinton, K.; Nagasaka, R.; Dalal, P.; Alexandria, S.; et al. Single-Cell Profiling Reveals Inflammatory Polarization of Human Carotid versus Femoral Plaque Leukocytes. JCI Insight 2023, 8, e171359. [Google Scholar] [CrossRef]
- Rappl, P.; Rösser, S.; Maul, P.; Bauer, R.; Huard, A.; Schreiber, Y.; Thomas, D.; Geisslinger, G.; Jakobsson, P.-J.; Weigert, A.; et al. Inhibition of MPGES-1 Attenuates Efficient Resolution of Acute Inflammation by Enhancing CX3CL1 Expression. Cell Death Dis. 2021, 12, 135. [Google Scholar] [CrossRef]
- Dollt, C.; Becker, K.; Michel, J.; Melchers, S.; Weis, C.-A.; Schledzewski, K.; Krewer, A.; Kloss, L.; Gebhardt, C.; Utikal, J.; et al. The Shedded Ectodomain of Lyve-1 Expressed on M2-like Tumor-Associated Macrophages Inhibits Melanoma Cell Proliferation. Oncotarget 2017, 8, 103682–103692. [Google Scholar] [CrossRef]
- Peach, R.; Hollenbaugh, D.; Stamenkovic, I.; Aruffo, A. Identification of Hyaluronic Acid Binding Sites in the Extracellular Domain of CD44. J. Cell Biol. 1993, 122, 257–264. [Google Scholar] [CrossRef]
- Noble, P.W.; McKee, C.M.; Cowman, M.; Shin, H.S. Hyaluronan Fragments Activate an NF-Kappa B/I-Kappa B Alpha Autoregulatory Loop in Murine Macrophages. J. Exp. Med. 1996, 183, 2373–2378. [Google Scholar] [CrossRef]
- Collins, S.L.; Black, K.E.; Chan-Li, Y.; Ahn, Y.-H.; Cole, P.A.; Powell, J.D.; Horton, M.R. Hyaluronan Fragments Promote Inflammation by Down-Regulating the Anti-Inflammatory A2a Receptor. Am. J. Respir. Cell Mol. Biol. 2011, 45, 675–683. [Google Scholar] [CrossRef]
- Shi, Q.; Zhao, L.; Xu, C.; Zhang, L.; Zhao, H. High Molecular Weight Hyaluronan Suppresses Macrophage M1 Polarization and Enhances IL-10 Production in PM2.5-Induced Lung Inflammation. Molecules 2019, 24, 1766. [Google Scholar] [CrossRef]
- Bonet, I.J.M.; Khomula, E.V.; Araldi, D.; Green, P.G.; Levine, J.D. PI3Kγ/AKT Signaling in High Molecular Weight Hyaluronan (HMWH)-Induced Anti-Hyperalgesia and Reversal of Nociceptor Sensitization. J. Neurosci. 2021, 41, 8414–8426. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage Plasticity, Polarization, and Function in Health and Disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-Based Network Analysis Reveals a Spectrum Model of Human Macrophage Activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef]
- Giannakis, N.; Sansbury, B.E.; Patsalos, A.; Hays, T.T.; Riley, C.O.; Han, X.; Spite, M.; Nagy, L. Dynamic Changes to Lipid Mediators Support Transitions among Macrophage Subtypes during Muscle Regeneration. Nat. Immunol. 2019, 20, 626–636. [Google Scholar] [CrossRef]
- Van Dierendonck, X.A.M.H.; Vrieling, F.; Smeehuijzen, L.; Deng, L.; Boogaard, J.P.; Croes, C.-A.; Temmerman, L.; Wetzels, S.; Biessen, E.; Kersten, S.; et al. Triglyceride Breakdown from Lipid Droplets Regulates the Inflammatory Response in Macrophages. Proc. Natl. Acad. Sci. USA 2022, 119, e2114739119. [Google Scholar] [CrossRef]
- MacKenzie, K.F.; Clark, K.; Naqvi, S.; McGuire, V.A.; Nöehren, G.; Kristariyanto, Y.; Van Den Bosch, M.; Mudaliar, M.; McCarthy, P.C.; Pattison, M.J.; et al. PGE2 Induces Macrophage IL-10 Production and a Regulatory-like Phenotype via a Protein Kinase A–SIK–CRTC3 Pathway. J. Immunol. 2013, 190, 565–577. [Google Scholar] [CrossRef]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted Roles of PGE2 in Inflammation and Cancer. Semin. Immunopathol. 2013, 35, 123–137. [Google Scholar] [CrossRef]
- Kalinski, P. Regulation of Immune Responses by Prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef]
- McKee, C.M.; Penno, M.B.; Cowman, M.; Burdick, M.D.; Strieter, R.M.; Bao, C.; Noble, P.W. Hyaluronan (HA) Fragments Induce Chemokine Gene Expression in Alveolar Macrophages. The Role of HA Size and CD44. J. Clin. Investig. 1996, 98, 2403–2413. [Google Scholar] [CrossRef]
- Petrey, A.C.; de la Motte, C.A. Hyaluronan, a Crucial Regulator of Inflammation. Front. Immunol. 2014, 5, 101. [Google Scholar] [CrossRef]
- McKee, C.M.; Lowenstein, C.J.; Horton, M.R.; Wu, J.; Bao, C.; Chin, B.Y.; Choi, A.M.K.; Noble, P.W. Hyaluronan Fragments Induce Nitric-Oxide Synthase in Murine Macrophages through a Nuclear Factor ΚB-Dependent Mechanism. J. Biol. Chem. 1997, 272, 8013–8018. [Google Scholar] [CrossRef]
- Yamawaki, H.; Hirohata, S.; Miyoshi, T.; Takahashi, K.; Ogawa, H.; Shinohata, R.; Demircan, K.; Kusachi, S.; Yamamoto, K.; Ninomiya, Y. Hyaluronan Receptors Involved in Cytokine Induction in Monocytes. Glycobiology 2008, 19, 83–92. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an Immune Regulator in Human Diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef]
- Alivernini, S.; MacDonald, L.; Elmesmari, A.; Finlay, S.; Tolusso, B.; Gigante, M.R.; Petricca, L.; Di Mario, C.; Bui, L.; Perniola, S.; et al. Distinct Synovial Tissue Macrophage Subsets Regulate Inflammation and Remission in Rheumatoid Arthritis. Nat. Med. 2020, 26, 1295–1306. [Google Scholar] [CrossRef]
- Kieu, T.Q.; Tazawa, K.; Kawashima, N.; Noda, S.; Fujii, M.; Nara, K.; Hashimoto, K.; Han, P.; Okiji, T. Kinetics of LYVE-1-Positive M2-like Macrophages in Developing and Repairing Dental Pulp in Vivo and Their pro-Angiogenic Activity in Vitro. Sci. Rep. 2022, 12, 5176. [Google Scholar] [CrossRef]
- Van Der Windt, G.J.W.; Van ′T Veer, C.; Florquin, S.; Van Der Poll, T. CD44 Deficiency Is Associated with Enhanced Escherichia Coli -Induced Proinflammatory Cytokine and Chemokine Release by Peritoneal Macrophages. Infect. Immun. 2010, 78, 115–124. [Google Scholar] [CrossRef]
- Qadri, M.; Almadani, S.; Jay, G.D.; Elsaid, K.A. Role of CD44 in Regulating TLR2 Activation of Human Macrophages and Downstream Expression of Proinflammatory Cytokines. J. Immunol. 2018, 200, 758–767. [Google Scholar] [CrossRef]
- Johnson, L.A.; Jackson, D.G. Hyaluronan and Its Receptors: Key Mediators of Immune Cell Entry and Trafficking in the Lymphatic System. Cells 2021, 10, 2061. [Google Scholar] [CrossRef]


| Target | Forward | Reverse |
|---|---|---|
| CD44 | 5′-ACG AGG AGG AGG TGT GAT GT-3′ | 5′-TCG CTT GTG AAA GCA CCA AC-3′ |
| TBP | 5′-CTG ACC ACT GCA CCG TTG CCA-3′ | 5′-GAC TGC AGC AAA TCG CTT GGG A-3′ |
| TNF | 5′-CTG AAC TTC GGG GTG ATC GG-3′ | 5′-GGC TTG TCA CTC GAA TTT TGA GA-3′ |
| EP1 | 5′-CAT GGT CTT CTT CGG CCT GT-3′ | 5′-GAT CAG TGG CTG CGT GAC A-3′ |
| EP2 | 5′-GGA GAC GGA CCA CCT CAT TC-3′ | 5′-TCC ATG TAG GCA AAG ATT GTG AA-3′ |
| EP3 | 5′-TAA TTG CAG TTC GCC TGG CT-3′ | 5′-GGT TGT TCA TCA TCT GGC AGA AC-3′ |
| EP4 | 5′-ACC TGA CTG AAA GCA GCC TC-3′ | 5′-AAG TTC TCA GCG AGG TGG TG-3′ |
| IL10 | 5′-GCT CTT ACT GAC TGG CAT GAG-3′ | 5′-CGC AGC TCT AGG AGC ATG TG-3′ |
| LYVE1 | 5′-CAG CAC ACT AGC CTG GTG TTA-3′ | 5′-CGC CCA TGA TTC TGC ATG TAG A-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hog, P.; Kuntschar, S.; Rappl, P.; Huard, A.; Weigert, A.; Brüne, B.; Schmid, T. Prostaglandin E2 Boosts the Hyaluronan-Mediated Increase in Inflammatory Response to Lipopolysaccharide by Enhancing Lyve1 Expression. Biology 2023, 12, 1441. https://doi.org/10.3390/biology12111441
Hog P, Kuntschar S, Rappl P, Huard A, Weigert A, Brüne B, Schmid T. Prostaglandin E2 Boosts the Hyaluronan-Mediated Increase in Inflammatory Response to Lipopolysaccharide by Enhancing Lyve1 Expression. Biology. 2023; 12(11):1441. https://doi.org/10.3390/biology12111441
Chicago/Turabian StyleHog, Pauline, Silvia Kuntschar, Peter Rappl, Arnaud Huard, Andreas Weigert, Bernhard Brüne, and Tobias Schmid. 2023. "Prostaglandin E2 Boosts the Hyaluronan-Mediated Increase in Inflammatory Response to Lipopolysaccharide by Enhancing Lyve1 Expression" Biology 12, no. 11: 1441. https://doi.org/10.3390/biology12111441





