Promising Biomolecules with High Antioxidant Capacity Derived from Cryptophyte Algae Grown under Different Light Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Setup
2.2. Biomass Production
2.3. Phycoerythrin Content
2.4. Phenol Content
2.5. Exopolysaccharide Content
2.6. Antioxidant Activity
2.7. Statistics
3. Results
3.1. Biomass Production
3.2. Biochemical Compositions
3.2.1. Phycoerythrin Extraction Yield
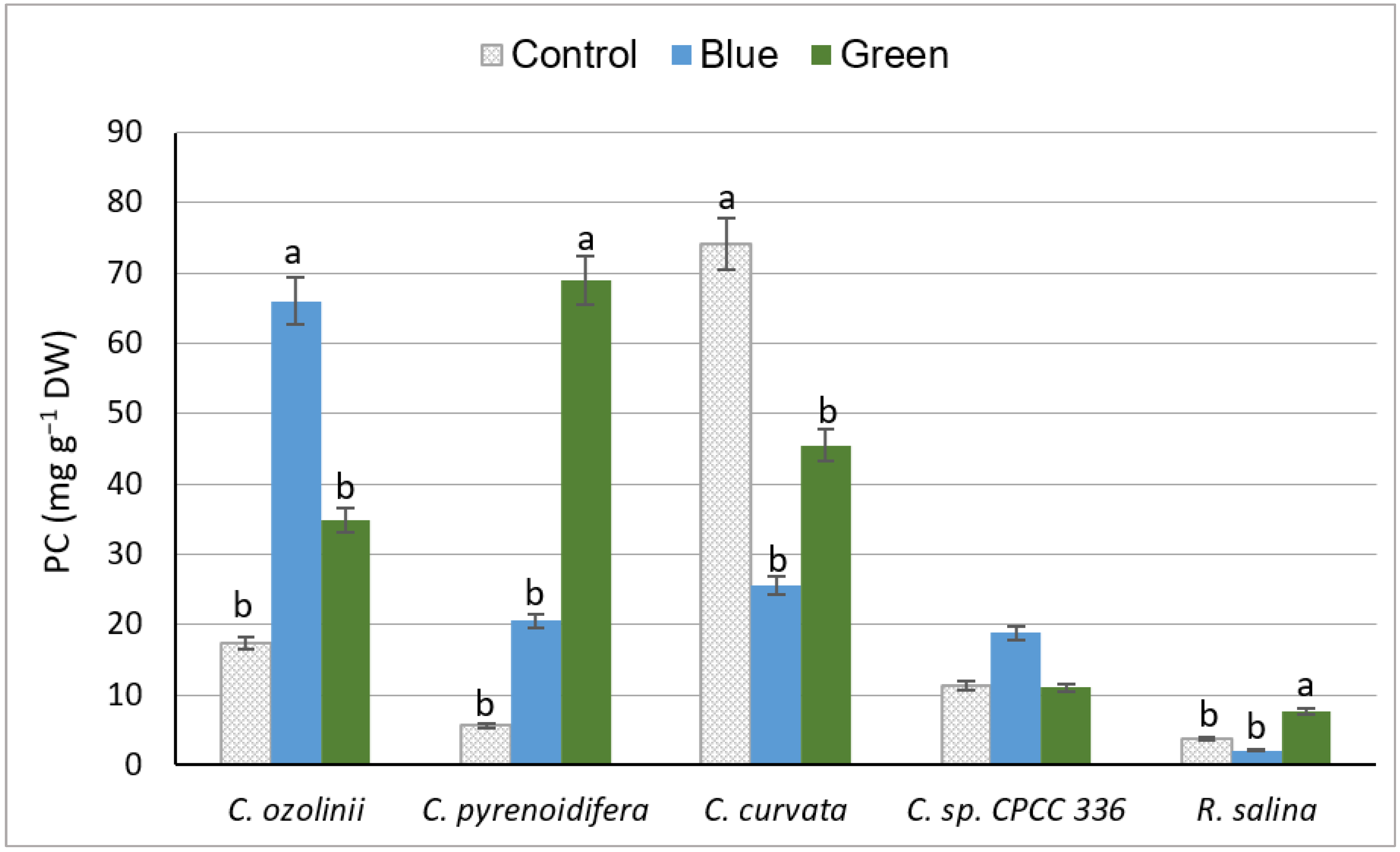
3.2.2. Phenol Content
3.2.3. Exopolysaccharide Content
3.3. Antioxidant Activity of Derived Bioactive Compounds
4. Discussion
4.1. Effect of Light Quality on Biomass Production
4.2. Effect of Light Quality on Bioactive Compounds Content
4.2.1. Phycoerythrin
4.2.2. Phenolic Content
4.2.3. Exopolysaccharides
4.3. The Effect of Light Quality on Antioxidant Activity
4.4. Commercial Perspective
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coulombier, N.; Nicolau, E.; Le Déan, L.; Antheaume, C.; Jauffrais, T.; Lebouvier, N. Impact of Light Intensity on Antioxidant Activity of Tropical Microalgae. Mar. Drugs 2020, 18, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razi Parjikolaei, B.; Kloster, L.; Bruhn, A.; Bo Rasmussen, M.; Frotté, C.X.; Christensen, V.K. Effect of light quality and nitrogen availability on the biomass production and pigment content of Palmaria palmate (Rhodophyta). Chem. Eng. Trans. 2013, 32, 967–972. [Google Scholar]
- Fu, W.; Nelson, D.; Yi, Z.; Xu, M.; Khraiwesh, B.; Jijakli, K.; Chaiboonchoe, A.; Alzahmi, A.; Al-Khairy, D.; Brynjolfsson, S.; et al. Bioactive Compounds from Microalgae: Current Development and Prospects. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 54, pp. 199–225. [Google Scholar]
- Sun, S.; Shumei, W.; Chen, L.; Gong, X. Promising fluorescent probes from phycobiliproteins. IEEE J. Sel. Top. Quantum Electron 2003, 9, 177–188. [Google Scholar] [CrossRef]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Martillanes, S.; Rocha-Pimienta, J.; Cabrera-Banegil, M.; Martin-Vertedor, D.; Delgado-Adamez, J. Application of phenolic compounds for food preservation: Food additive and active packaging. In Phenolic Compound-Biological Activity; Soto-Hernandez, M., Palma-Tenango, M., del Rosario Garcia-Mateos, M., Eds.; InTech: London, UK, 2017. [Google Scholar]
- Liu, Z.; Zhang, Z.; Qiu, L.; Zhang, F.; Xu, X.; Wei, H.; Tao, X. Characterization and bioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04. J. Dairy Sci. 2017, 100, 6895–6905. [Google Scholar] [CrossRef]
- Czaczyk, K.; Myszka, K. Biosynthesis of extracellular polymeric substances (EPS) and its role in microbial biofilm formation. Pol. J. Environ. Stud. 2007, 16, 799–806. [Google Scholar]
- Feldmane, J.; Semjonovs, P.; Ciprovica, I. Potential of exopolysaccharides in yoghurt production. Food Sci. Nutr. 2013, 7, 767–770. [Google Scholar]
- Hemlata, V.; Sumbul, A.; Tasneem, F. Extraction, purification and characterization of phycoerythrin from Michrochaete and its biological activities. Biocatal. Agri. Biotechnol. 2018, 13, 84–89. [Google Scholar] [CrossRef]
- Korzeniowska, K.; Leska, B.; Wieczorek, P.P. Isolation and determination of phenolic compounds from freshwater Cladophora glomerata. Algal Res. 2020, 48, 101912. [Google Scholar] [CrossRef]
- Cruz, D.; Vasconcelos, V.; Pierre, G.; Michaud, P.; Delattre, C. Exopolysaccharides from Cyanobacteria: Strategies for Bioprocess Development. Appl. Sci. 2020, 10, 3763. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brett, M.T.; Müller-Navarra, D.C.; Persson, J. Crustacean zooplankton fatty acid composition. In Lipids in Aquatic Ecosystems; Springer: New York, NY, USA, 2009; pp. 115–146. [Google Scholar] [CrossRef]
- Martin-Creuzburg, D.; Elert, E.V. Good food versus bad food: The role of sterols and polyunsaturated fatty acids in determining growth and reproduction of Daphnia magna. Aquat. Ecol. 2009, 43, 943–950. [Google Scholar] [CrossRef] [Green Version]
- Peltomaa, E.; Johnson, M.D.; Taipale, S.J. Marine Cryptophytes Are Great Sources of EPA and DHA. Mar. Drugs 2017, 16, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clay, B.L. Cryptomonads. In Freshwater algae of North America, 2nd ed.; Wehr, J.D., Sheath, R.G., Kociolek., P., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 1–11. [Google Scholar]
- Scholz, M.J.; Weiss, T.L.; Jinkerson, R.E.; Jing, J.; Roth, R.; Goodenough, U.; Posewitz, M.C.; Gerken, H.G. Ultrastructure and Composition of the Nannochloropsis gaditana Cell Wall. Eukaryot. Cell 2014, 13, 1450–1464. [Google Scholar] [CrossRef] [Green Version]
- Sidler, W.A. Phycobilisome and phycobiliprotein structures. In The Molecular Biology of Cyanobacteria; Bryant, D.A., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 139–216. [Google Scholar]
- Hill, D.R.A.; Rowan, K.S. The biliproteins of the Cryptophyceae. Phycologia 1989, 28, 455–463. [Google Scholar] [CrossRef]
- Mercier, L.; Peltomaa, E.; Ojala, A. Comparative analysis of phycoerythrin production in cryptophytes. J. Appl. Phycol. 2022, 34, 789–797. [Google Scholar] [CrossRef]
- Telford, W.G.; Moss, M.W.; Morseman, J.P.; Allnutt, F.C.T. Cryptomonad algal phycobiliproteins as fluorochromes for extracellular and intracellular antigen detection by flow cytometry. Cytometry 2001, 44, 16–23. [Google Scholar] [CrossRef]
- Arashiro, L.T.; Boto-Ordóñez, M.; Van Hulle, S.W.; Ferrer, I.; Garfí, M.; Rousseau, D.P. Natural pigments from microalgae grown in industrial wastewater. Bioresour. Technol. 2020, 303, 122894. [Google Scholar] [CrossRef] [Green Version]
- Latsos, C.; van Houcke, J.; Blommaert, L.; Verbeeke, G.P.; Kromkamp, J.; Timmermans, K.R. Effect of light quality and quantity on productivity and phycoerythrin concentration in the cryptophyte Rhodomonas sp. J. Appl. Phycol. 2021, 33, 729–741. [Google Scholar] [CrossRef]
- Coulombier, N.; Jauffrais, T.; Lebouvier, N. Antioxidant Compounds from Microalgae: A Review. Mar. Drugs 2021, 19, 549. [Google Scholar] [CrossRef]
- Abidizadegan, M.; Peltomaa, E.; Blomster, J. The Potential of Cryptophyte Algae in Biomedical and Pharmaceutical Applications. Front. Pharmacol. 2021, 11, 618836. [Google Scholar] [CrossRef] [PubMed]
- Jayshree, A.; Jayashree, S.; Thangaraju, N. Chlorella vulgaris and Chlamydomonas reinhardtii: Effective Antioxidant, Antibacterial and Anticancer Mediators. Indian J. Pharm. Sci. 2016, 78, 575–581. [Google Scholar] [CrossRef] [Green Version]
- Del Mondo, A.; Smerilli, A.; Ambrosino, L.; Albini, A.; Noonan, D.M.; Sansone, C.; Brunet, C. Insights into phenolic compounds from microalgae: Structural variety and complex beneficial activities from health to nutraceutics. Crit. Rev. Biotechnol. 2021, 41, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Freitas, M.; Fernandes, E.; Lima, J.L. Biological Activities of 2-Styrylchromones. Mini-Rev. Med. Chem. 2010, 10, 1–7. [Google Scholar] [CrossRef]
- Paulsen, B.S.; Vieira, A.A.H.; Klaveness, D. Structure of extracellular polysaccharides produced by a soil cryptomonas sp. (cryptophyceae)1. J. Phycol. 1992, 28, 61–63. [Google Scholar] [CrossRef]
- Giroldo, D.; Vieira, A.A. An extracellular sulfated fucose-rich polysaccharide produced by a tropical strain of Cryptomonas obovata (Cryptophyceae). J. Appl. Phycol. 2002, 14, 185–191. [Google Scholar] [CrossRef]
- Gris, B.; Sforza, E.; Morosinotto, T.; Bertucco, A.; La Rocca, N. Influence of light and temperature on growth and high-value molecules productivity from Cyanobacterium aponinum. J. Appl. Phycol. 2017, 29, 1781–1790. [Google Scholar] [CrossRef]
- Chentir, I.; Hamdi, M.; Doumandji, A.; HadjSadok, A.; Ouada, H.B.; Nasri, M.; Jridi, M. Enhancement of extracellular polymeric substances (EPS) production in Spirulina (Arthrospira sp.) by two-step cultivation process and partial characterization of their polysaccharidic moiety. Int. J. Biol. Macromol. 2017, 105, 1412–1420. [Google Scholar] [CrossRef]
- Ge, H.; Xia, L.; Zhou, X.; Zhang, D.; Hu, C. Effects of light intensity on components and topographical structures of extracellular polysaccharides from the cyanobacteria Nostoc sp. J. Microbiol. 2014, 52, 179–183. [Google Scholar] [CrossRef]
- Giroldo, D.; Vieira, A.A.; Paulsen, B.S. Extracellular polysaccharides produced by a tropical cryptophyte as a carbon source for natural bacterial populations. Eur. J. Phycol. 2005, 40, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Benavente-Valdes, J.R.; Aguilar, C.; Contreras-Esquivel, J.C.; Mendez-Zavala, A.; Montanez, J. Strategies to enhance the production of photosynthetic pigments and lipids in chlorophyte species. Biotechnol. Rep. 2016, 10, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Wang, X.; Shi, X.; Wang, B.; Zheng, X.; Wang, H.; Liu, F. Red and Blue Lights Significantly Affect Photosynthetic Properties and Ultrastructure of Mesophyll Cells in Senescing Grape Leaves. Hortic. Plant. J. 2016, 2, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Liu, X.; Mei, Z.; Lin, J.; Aaron, S.; Du, H. Effects of Various Light-Emitting Diode (LEd) Wavelengths on the Growth of Scenedesmus Obliquus Fachb-12 and Accumulation of Astaxanthin. Phyton 2019, 88, 335–348. [Google Scholar] [CrossRef]
- Wu, B.-S.; Rufyikiri, A.-S.; Orsat, V.; Lefsrud, M.G. Re-interpreting the photosynthetically action radiation (PAR) curve in plants. Plant Sci. 2019, 289, 110272. [Google Scholar] [CrossRef]
- Li, C.-X.; Xu, Z.-G.; Dong, R.-Q.; Chang, S.-X.; Wang, L.-Z.; Khalil-Ur-Rehman, M.; Tao, J.-M. An RNA-Seq Analysis of Grape Plantlets Grown in vitro Reveals Different Responses to Blue, Green, Red LED Light, and White Fluorescent Light. Front. Plant Sci. 2017, 8, 78. [Google Scholar] [CrossRef] [Green Version]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.-N.; Yoshihara, T. Blue Light-emitting Diode Light Irradiation of Seedlings Improves Seedling Quality and Growth after Transplanting in Red Leaf Lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Wang, Y.; Liu, M.; Xu, J.; Xu, Z. Effect of green and red lights on the growth and morphogenesis of potato (Solanum tuberosum L.) plantlets in vitro. Sci. Hortic. 2015, 190, 104–109. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Lorenzen, C.J. Yellow green algae with chlorophyllide. J. Phycol. 1972, 8, 10–14. [Google Scholar]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana hustedt and Detonula confervacea Cleve. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Pruvost, J.; Van Vooren, G.; Le Gouic, B.; Couzinet-Mossion, A.; Legrand, J. Systematic investigation of biomass and lipid productivity by microalgae in photobioreactors for biodiesel application. Bioresour. Technol. 2011, 102, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Lawrenz, E.; Fedewa, E.J.; Richardson, T.L. Extraction protocols for the qualification of phycobilins in aqueous phytoplankton extracts. J. Appl. Phycol. 2011, 23, 865–871, Erratum in J. Appl. Phycol. 2013, 25, 1269. [Google Scholar] [CrossRef]
- Román, R.B.; Alvárez-Pez, J.; Fernández, F.A.; Grima, E.M. Recovery of pure B-phycoerythrin from the microalga Porphyridium cruentum. J. Biotechnol. 2002, 93, 73–85. [Google Scholar] [CrossRef]
- Punampalam, R.; Khoo, K.S.; Sit, N.W. Evaluation of antioxidant properties of phycobiliproteins and phenolic compounds extracted from Bangia atropurpurea. Malays. J. Fundam. Appl. Sci. 2018, 14, 289–297. [Google Scholar] [CrossRef]
- Irondi, A.E.; Oboh, G.; Akinrunde, J.K. Comparative and synergistic antioxidant properties of Carica papaya and Azadarichta indica leaves. Int. J. Pharm. Sci. Res. 2012, 3, 4773–4779. [Google Scholar]
- Strieth, D.; Stiefelmaier, J.; Wrabl, B.; Schwing, J.; Schmeckebier, A.; Nonno, S.D.; Muffler, K.; Ulber, R. A new strategy for a combined isolation of EPS and pigments from cyanobacteria. J. Appl. Phycol. 2020, 32, 1729–1740. [Google Scholar] [CrossRef]
- Chang, S.-P.; Sheu, H.-L.; Lee, Y.-C. Comparison of EPS Extraction Efficiences from Spirogyra fluviatilis by Chemical and Physical Extraction Methods. Int. J. Biosci. Biochem. Bioinform. 2019, 9, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Mei, Z.-P.; Legendre, L.; Gratton, Y.; Tremblay, J.; Leblanc, B.; Klein, B.; Gosselin, M. Phytoplankton production in the North Water Polynya: Size-fractions and carbon fluxes, April to July 1998. Mar. Ecol. Prog. Ser. 2003, 256, 13–27. [Google Scholar] [CrossRef]
- Peltomaa, E.; Ojala, A. Size-related photosynthesis of algae in a strongly stratified humic lake. J. Plankton Res. 2009, 32, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Javornický, P. Light microscopical observations of the sigmoid species of the genus Cryptomonas Ehrenberg (Cryptophyceae) and their eventual pyrenoids. Acta Musei Sil. Sci. Nat. 2014, 63, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Sharmila, D.; Suresh, A.; Indhumathi, J.; Gowtham, K.; Velmurugan, N. Impact of various color filtered LED lights on microalgae growth, pigments and lipid production. European. J. Biotechnol. Biosci. 2018, 6, 1–7. [Google Scholar]
- Heidenreich, K.M.; Richardson, T.L. Photopigment, Absorption, and Growth Responses of Marine Cryptophytes to Varying Spectral Irradiance. J. Phycol. 2019, 56, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Ojala, A. Effects of light and tempreture on the cell size and some biochemical components in two freshwater cryptophytes. Nor. J. Bot. 1993, 13, 697–705. [Google Scholar] [CrossRef]
- Lafarga-Da la Cruz, F.; Valenzuela-Espinoza, E.; Millan-Nunez, R.; Trees, C.C.; Santamara-del-Angel, E.; Nunez-Cebrero, F. Nutrient uptake, chlorophyll a and carbon fixation by Rhodomonas sp. (Cryptophyceae) cultured at different irradiance and nutri-ent concentrations. Aquac. Eng. 2006, 35, 51–60. [Google Scholar] [CrossRef]
- Vesk, M.; Jeffrey, S.W. Effect of blue-green light on photosynthetic pigment and chloroplast structure in unicellular marine algae from six classes. J. Phycol. 1977, 13, 280–288. [Google Scholar] [CrossRef]
- Borlongan, I.A.; Suzuki, S.; Nishihara, G.N.; Kozono, J.; Terada, R. Effects of light quality and temperature on the photosynthesis and pigment content of a subtidal edible red alga Meristotheca papulose (Solieriaceae, Gigartinales) from Japan. J. Appl. Phycol. 2020, 32, 1329–1340. [Google Scholar] [CrossRef]
- Thinh, L.V. Effect of irradiance on the physiology and ultrastructure of the marine cryptomonads, Cryptomonas strain Lis (Cryptophyceae). Phycologia 1983, 22, 2–11. [Google Scholar] [CrossRef]
- Chaloub, R.M.; Motta, N.M.S.; de Araujo, S.P.; de Aguiar, P.F.; da Silva, A. Combined effects of irradiance, tempreture and nitrate concentration on phycoerythrin content in the microalga Rhodomonas sp. (Cryptophyceae). Algal Res. 2015, 8, 89–94. [Google Scholar] [CrossRef]
- Rito-Palomares, M.; Nuñez, L.; Amador, D. Practical application of aqueous two-phase systems for the development of a prototype process for c-phycocyanin recovery from Spirulina maxima. J. Chem. Technol. Biotechnol. 2001, 76, 1273–1280. [Google Scholar] [CrossRef]
- Ghosh, T.; Mishra, S. Studies on Extraction and Stability of C-Phycoerythrin from a Marine Cyanobacterium. Front. Sustain. Food Syst. 2020, 4, 102. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, J.; Ju, B.; Li, W.; Wen, S.; Pu, Y.; Qin, S. One-step chromatographic procedure for purification of B-phycoerythrin from Porphyridium cruentum. Protein Expr. Purif. 2016, 123, 70–74. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Q.; Hou, Y. Efficient Purification of R-phycoerythrin from Marine Algae (Porphyra yezoensis) Based on a Deep Eutectic Solvents Aqueous Two-Phase System. Mar. Drugs 2020, 18, 618. [Google Scholar] [CrossRef] [PubMed]
- Rossano, R.; Ungaro, N.; D’Ambrosio, A.; Liuzzi, G.M.; Riccio, P. Extracting and purifying R-phycoerythrin from Mediterranean re algae Corallina elongata Ellis & Solander. Biotechnol. J. 2003, 20, 289–293. [Google Scholar]
- Sathuvan, M.; Sakthivel, M.; Gopal, V.B.; Palani, P.; Rengasamy, R. Qualitative and quantitative determination of R-phycoerythrin from Halymenia floresia (Clemente) G. Agardh by polyacrylamide gel using electrophoretic elution technique. J. Chromatogr. A 2016, 1454, 120–126. [Google Scholar]
- Sudhakar, M.P. Extraction, purification and application study of R-phycoerythrin from Gracilaria corticata (J. Agardh) J. Agardh var. corticata. Indian J. Nat. Prod. Resour. 2014, 5, 371–374. [Google Scholar]
- Newsted, J.L. Effect of light, temperature, and pH on the accumulation of phenol by Selenastrum capricornutum, a green alga. Ecotoxicol. Environ. Saf. 2003, 59, 237–243. [Google Scholar] [CrossRef]
- da Costa, F.; Le Grand, F.; Quéré, C.; Bougaran, G.; Cadoret, J.-P.; Robert, R.; Soudant, P. Effects of growth phase and nitrogen limitation on biochemical composition of two strains of Tisochrysis lutea. Algal Res. 2017, 27, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Lian, T.T.; Cha, S.-Y.; Moe, M.M.; Kim, Y.J.; Bang, K.S. Effects of Different Colored LEDs on the Enhancement of Biologically Active Ingredients in Callus Cultures of Gynura procumbens (Lour.) Merr. Molecules 2019, 24, 4336. [Google Scholar] [CrossRef] [Green Version]
- Chung, I.M.; Paudel, N.; Kim, S.H.; Yu, C.Y.; Ghimire, B.K. The influence of light wavelength on growth and antioxidant capacity in Pachyrhizus erosus (L.) urban. Plant Growth Regul. 2020, 39, 296–312. [Google Scholar] [CrossRef]
- Engelsma, G. On the mechanism of the changes in phenylalanine ammonial-lyase activity induced by ultraviolet and blue light in gherkin hypocotyls. J. Plant Physiol. 1974, 54, 702–705. [Google Scholar] [CrossRef] [Green Version]
- Battistoni, B.; Amoros, A.; Tapia, M.L.; Escalona, V. Effect of LED lights on the antioxidant properties of baby spinach leaves (Spinacia oleracea L.). Rev. FCA UNCuyo. 2021, 53, 98–108. [Google Scholar] [CrossRef]
- Penna, A.; Berluti, S.; Penna, N.; Magnani, M. Influence of nutrient ratios on the in vitro extracellular polysaccharide production by marine diatoms from the Adriatic Sea. J. Plankton Res. 1999, 21, 1681–1690. [Google Scholar] [CrossRef] [Green Version]
- You, T.; Barnett, S.M. Effect of light quality on production of extracellular polysaccharides and growth rate of Porphyridium cruentum. Biochem. Eng. J. 2004, 19, 251–258. [Google Scholar] [CrossRef]
- Han, P.P.; Sun, Y.; Wu, X.Y.; Yuan, Y.J.; Dai, Y.J.; Jia, S.R. Emulsifying, flocculating, and physiochemical properties of exopoly-saccharide produced by cyanobacterium Nostoc flagelliforme. Appl. Biochem. Biotechnol. 2014, 172, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Medina-Cabrera, E.V.; Rühmann, B.; Schmid, J.; Sieber, V. Optimization of growth and EPS production in two Porphyridum strains. Bioresour. Technol. Rep. 2020, 11, 100486. [Google Scholar] [CrossRef]
- Singh, S.; Das, S. Screening, production, optimization and characterization of cyanobacterial polysaccharide. World J. Microbiol. Biotechnol. 2011, 27, 1971–1980. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Lei, S. Effects of light regime on extracellular polysaccharide production by Porphyridium cruentum cultured in flat plate photo bioreactors. In Proceedings of the 2008 2nd International Conference on Bioinformatics and Biomedical Engineering, Shanghai, China, 16–18 May 2008; pp. 1488–1491. [Google Scholar]
- Babiak, W.; Krzemińska, I. Extracellular Polymeric Substances (EPS) as Microalgal Bioproducts: A Review of Factors Affecting EPS Synthesis and Application in Flocculation Processes. Energies 2021, 14, 4007. [Google Scholar] [CrossRef]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-Regulated Plant Growth and Development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar]
- Reynolds, C.S. Variability in the provision and function of mucilage in phytoplankton: Facultative responses to the environment. Hydrobiologia 2007, 578, 37–45. [Google Scholar] [CrossRef]
- Otero, A.; Vincenzini, M. Extracellular polysaccharide synthesis by Nostoc starins as affected by N source and light intensity. Biotechnol. J. 2003, 102, 143–152. [Google Scholar] [CrossRef]
- Trabelsi, L.; Ouada, H.B.; Bacha, H.; Ghoul, M. Combined effect of tempreture and light intensity on growth and extracellular polymeric substance production by the cyanobacterium Arthrospira platensis. J. Appl. Phycol. 2009, 21, 405–412. [Google Scholar] [CrossRef]
- Han, P.-P.; Shen, S.-G.; Guo, R.-J.; Zhao, D.-X.; Lin, Y.-H.; Jia, S.-R.; Yan, R.-R.; Wu, Y.-K. ROS Is a Factor Regulating the Increased Polysaccharide Production by Light Quality in the Edible Cyanobacterium Nostoc flagelliforme. J. Agric. Food Chem. 2019, 67, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Jun, M.; Fu, H.Y.; Hong, J.; Wan, X.; Yang, C.s.; Ho, C.T. Comparison of antioxidant activities of isoflavonoids from kudzu root (Pueraria lobate Ohwi). J. Food Sci. 2006, 68, 2117–2122. [Google Scholar] [CrossRef]
- Levy, O.; Achituv, Y.; Yacobi, Y.; Stambler, N.; Dubinsky, Z. The impact of spectral composition and light periodicity on the activity of two antioxidant enzymes (SOD and CAT) in the coral Favia favus. J. Exp. Mar. Biol. Ecol. 2006, 328, 35–46. [Google Scholar] [CrossRef]
- Bermejo, R.; Talavera, E.M.; Alvarez-Pez, J.M. Chromatographic purification and characterization of B-phycoerythrin from Porphyridium cruentum semipreparative high-performance liquid chromatographic separation and characterization of its subu-nits. J. Chromatogr. A 2001, 917, 135–145. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Espinosa, C.; Paredes, A.; Palma, J.; Jaime, C.; Vílchez, C.; Cerezal, P. Determining the Potential of Haematococcus pluvialis Oleoresin as a Rich Source of Antioxidants. Molecules 2019, 24, 4073. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, S.S.M. Microalgal biotechnology: Prospects and applications. In Plant Science; Dhal, N.B., Sahu, S.C., Eds.; InTech: Croatia, Balkans, 2012; pp. 275–305. [Google Scholar]
- Custódio, L.; Justo, T.; Silvestre, L.; Barradas, A.; Duarte, C.V.; Pereira, H.; Barreira, L.; Rauter, A.P.; Alberício, F.; Varela, J. Microalgae of different phyla display antioxidant, metal chelating and acetylcholinesterase inhibitory activities. Food Chem. 2011, 131, 134–140. [Google Scholar] [CrossRef]
- Li, S.; Ji, L.; Shi, Q.; Wu, H.; Fan, J. Advances in the production of bioactive substances from marine unicellular microalgae Porphyridium spp. Bioresour. Technol. 2019, 292, 122048. [Google Scholar] [CrossRef]
- Lewin, R.A. Extracellular Polysaccharides of Green Algae. Can. J. Microbiol. 1956, 2, 665–672. [Google Scholar] [CrossRef]
- Putri, R.A.E.; Winasti, N.M.S.; Dharmawan, M.T.T.; Suyono, E.A. Concentrations differences of microalgal extracellular polymeric substances as edible coating in shelf-life extension of Fragaria spp. AIP Conf. Proc. 2020, 2260, 090003. [Google Scholar] [CrossRef]



| C. ozolinii | C. pyrenoidifera | C. curvata | C. sp. CPCC 336 | R. salina | |
|---|---|---|---|---|---|
| LED Lights | PI | PI | PI | PI | PI |
| control | 2.7 b | 5.4 | 0.2 b | 4 | 3.1 |
| Blue | 2.2 b | 8.6 | 3.9 a | 5.7 | 2.4 |
| Green | 9.5 a | 13 | 3 a | 6.4 | 3.5 |
| C. ozolinii | C. pyrenoidifera | C. curvata | C. sp. CPCC 336 | R. salina | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 | IC50 | IC50 | IC50 | IC50 | |||||||||||
| LED Lights | PE | PC | EPS | PE | PC | EPS | PE | PC | EPS | PE | PC | EPS | PE | PC | EPS |
| Control | 35.5 c | 148 c | 9.7 c | 70 b | 13 a | 12.2 a | 10 | 1.1 a | 10 b | 97 b | 30.5 c | 18.6 b | 140 b | 8.9 b | 35 c |
| Blue | 25 b | 7.3 a | 6 b | 30 a | 15.7 a | 17.7 b | 14 | 3.3 b | 6.3 a | 41 a | 12 b | 18.7 c | 18 a | 4.3 a | 27 b |
| Green | 14.5 a | 33 b | 3.5 a | 26 a | 19 b | 11 a | 13.5 | 0.93 a | 6.3 a | 40 a | 7 a | 15 a | 17 a | 10 b | 20 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abidizadegan, M.; Blomster, J.; Fewer, D.; Peltomaa, E. Promising Biomolecules with High Antioxidant Capacity Derived from Cryptophyte Algae Grown under Different Light Conditions. Biology 2022, 11, 1112. https://doi.org/10.3390/biology11081112
Abidizadegan M, Blomster J, Fewer D, Peltomaa E. Promising Biomolecules with High Antioxidant Capacity Derived from Cryptophyte Algae Grown under Different Light Conditions. Biology. 2022; 11(8):1112. https://doi.org/10.3390/biology11081112
Chicago/Turabian StyleAbidizadegan, Maryam, Jaanika Blomster, David Fewer, and Elina Peltomaa. 2022. "Promising Biomolecules with High Antioxidant Capacity Derived from Cryptophyte Algae Grown under Different Light Conditions" Biology 11, no. 8: 1112. https://doi.org/10.3390/biology11081112






