Pattern of β-Diversity and Plant Species Richness along Vertical Gradient in Northwest Himalaya, India
Abstract
:Simple Summary
Abstract
1. Introduction
- (a)
- assess the floristic diversity within the study area;
- (b)
- calculate the total species diversity at each elevation belt;
- (c)
- explore the deviation in plant species composition among the elevation belts;
- (d)
- calculate the beta diversity as well as its underlying components among the elevation belts;
- (e)
- and study the influence of elevation on species richness and diversity.
2. Materials and Methods
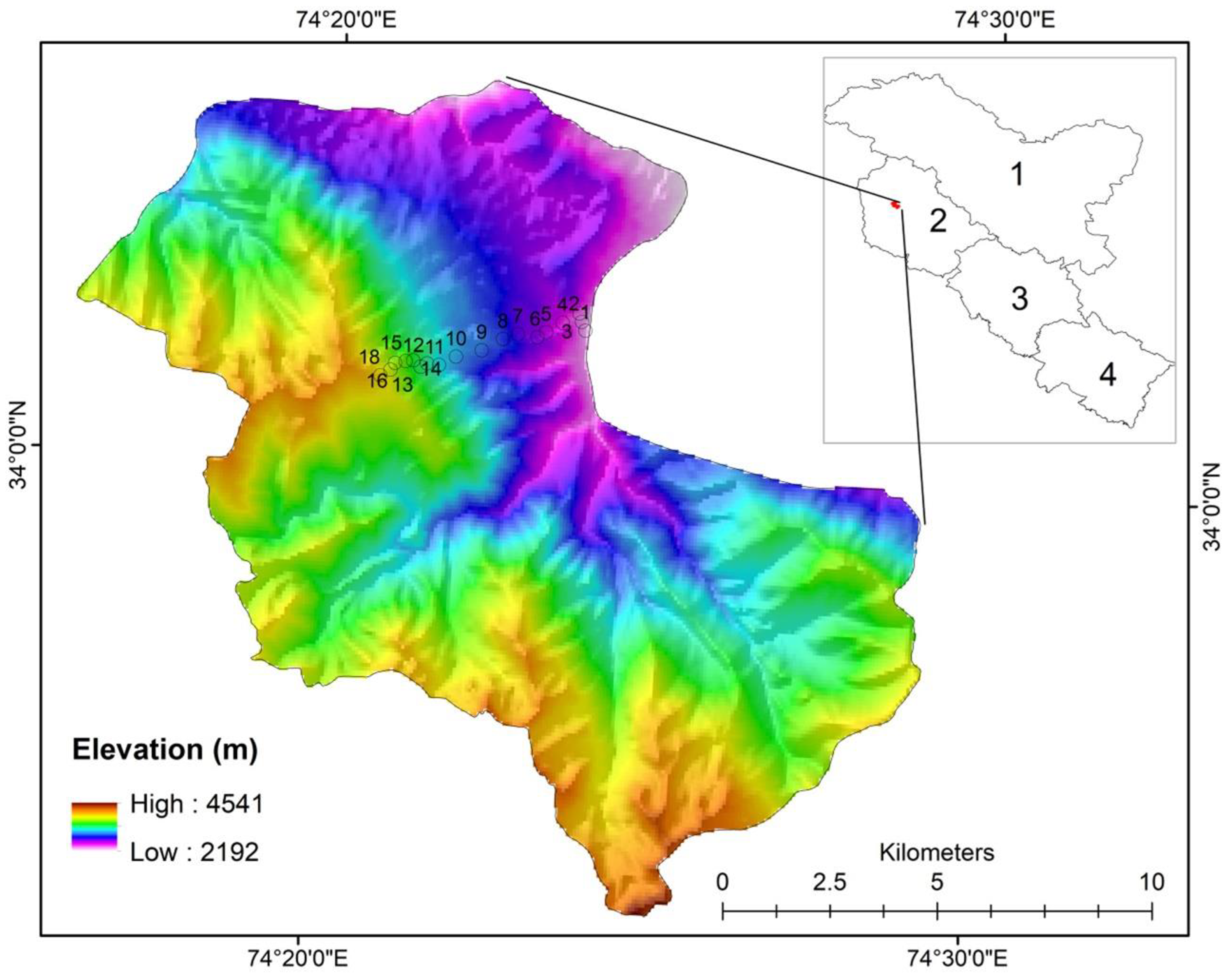
2.1. Study Area
2.2. Site Selection and Data Collection
2.3. Data Analysis
3. Results
3.1. Floristic Diversity
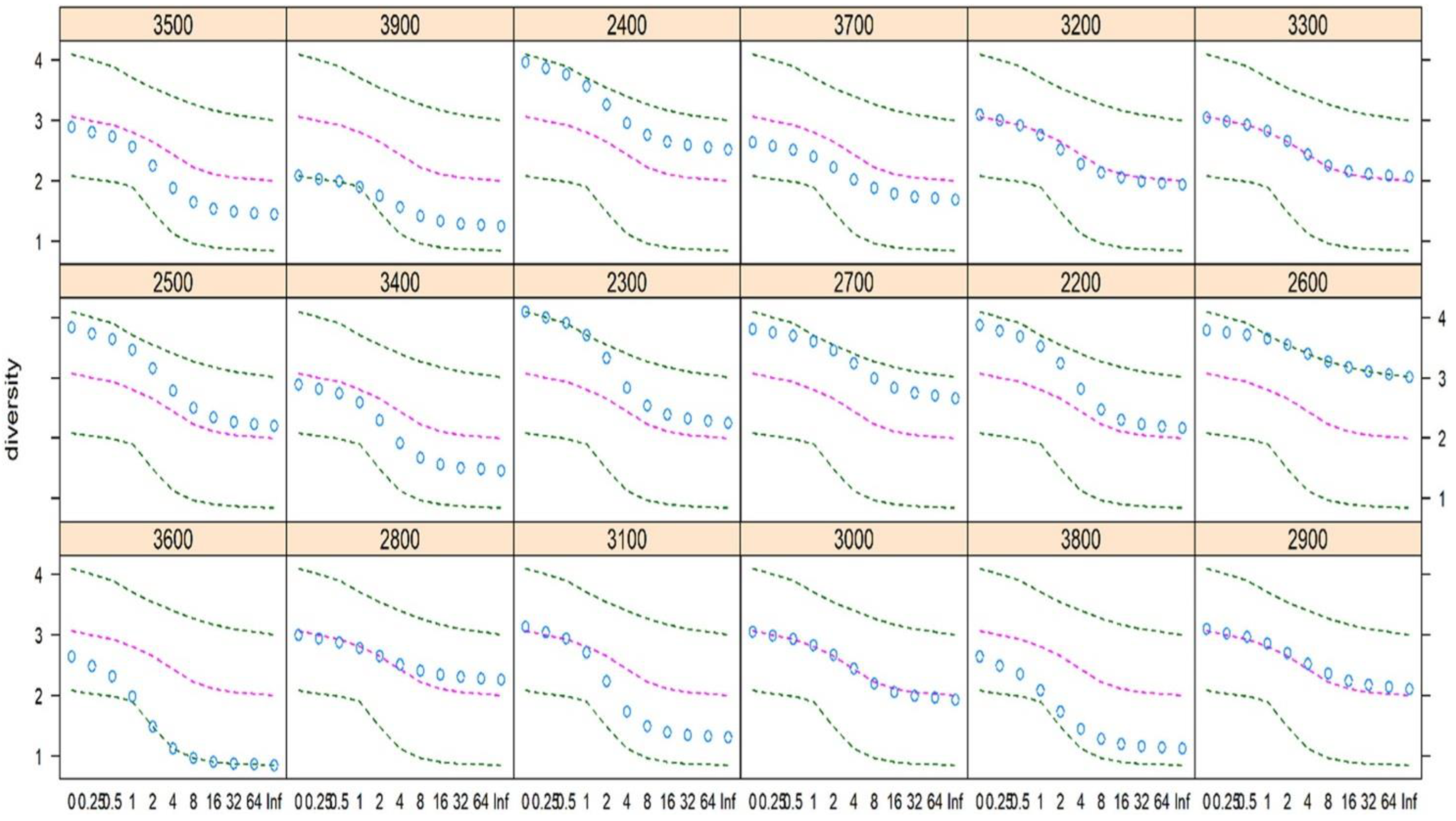
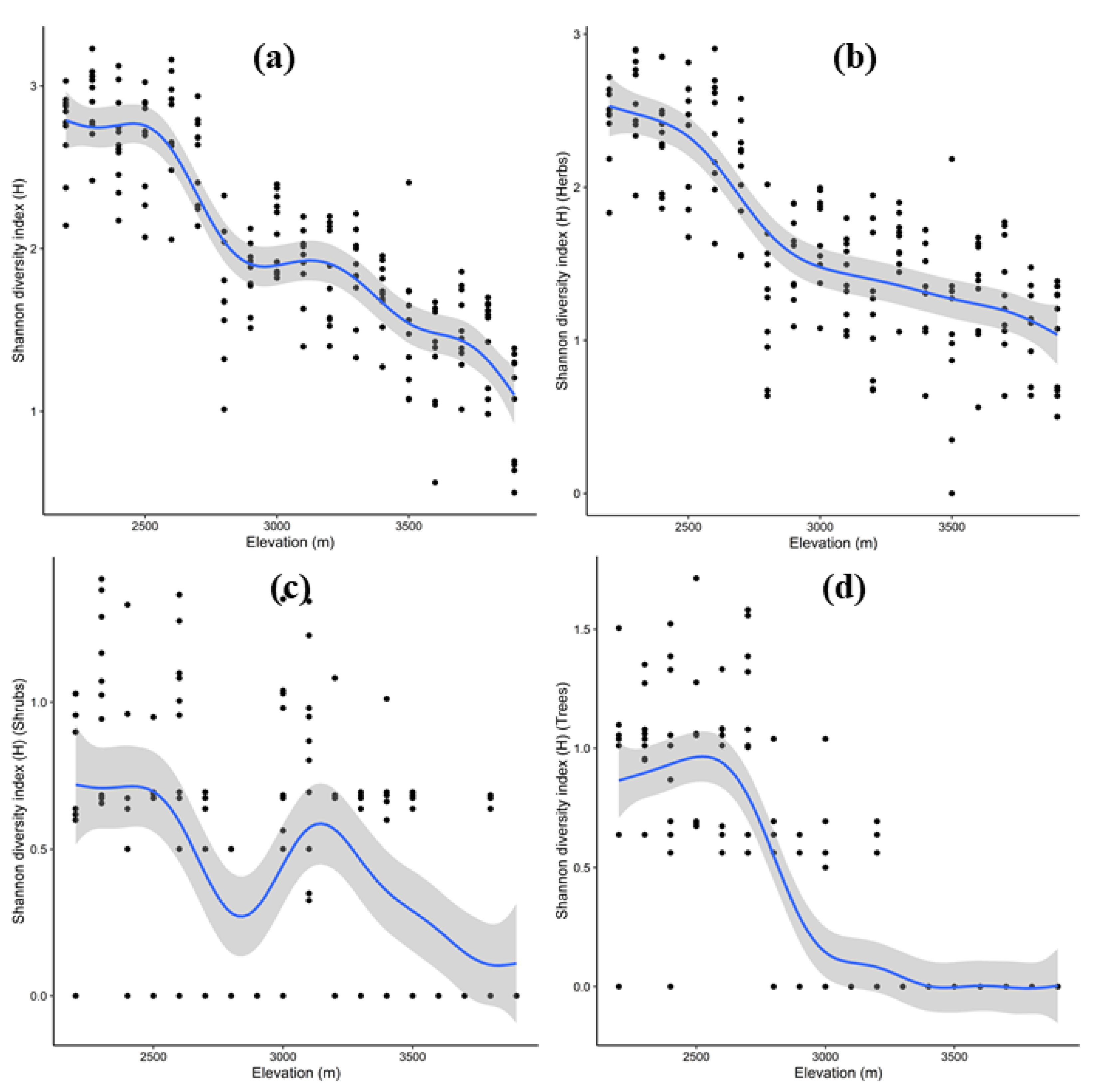
3.2. Species Diversity at Each Elevation Belt
3.3. Deviation in Species Composition among Elevation Belts
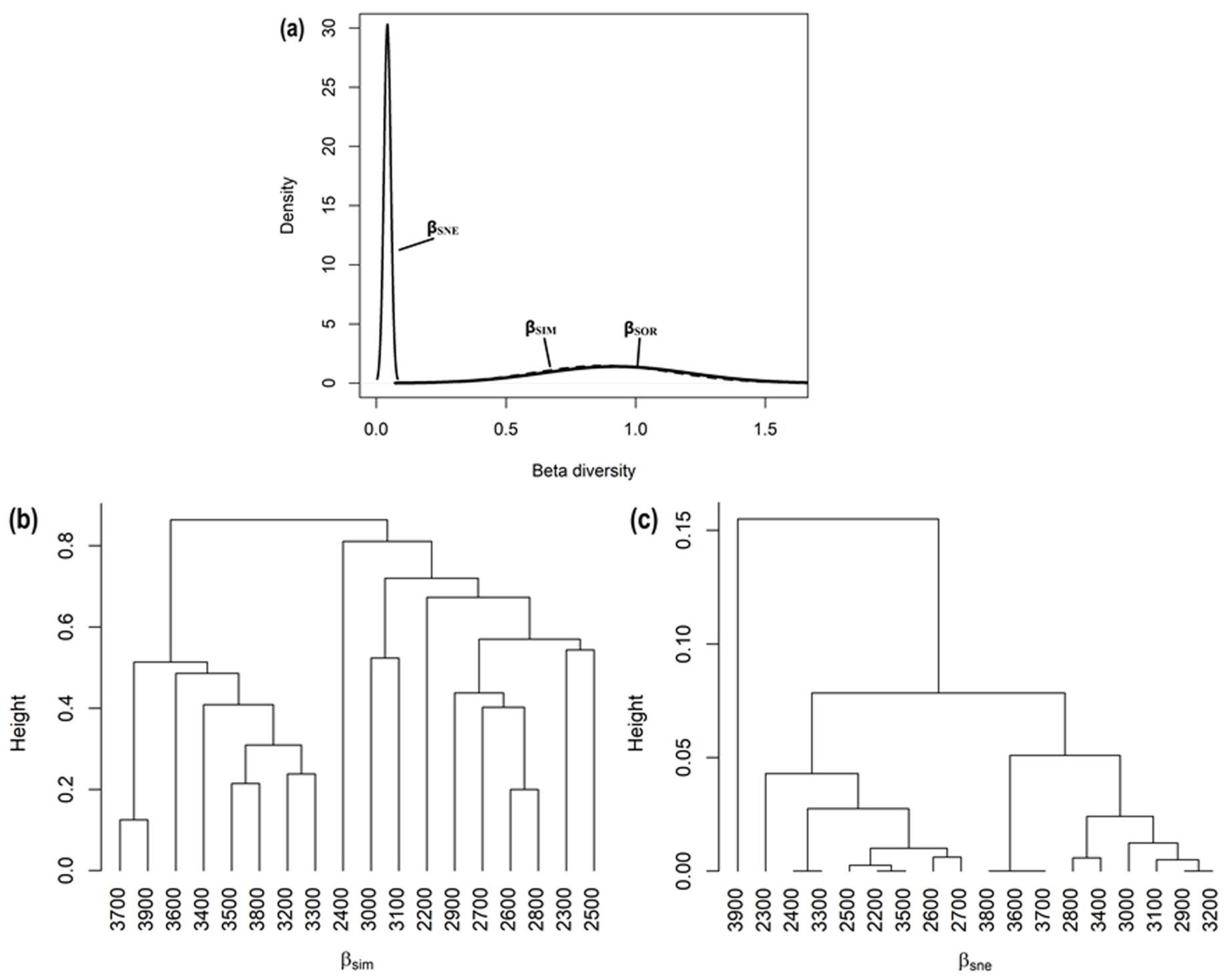
3.4. Spatial β-Diversity Patterns among Elevation Belts
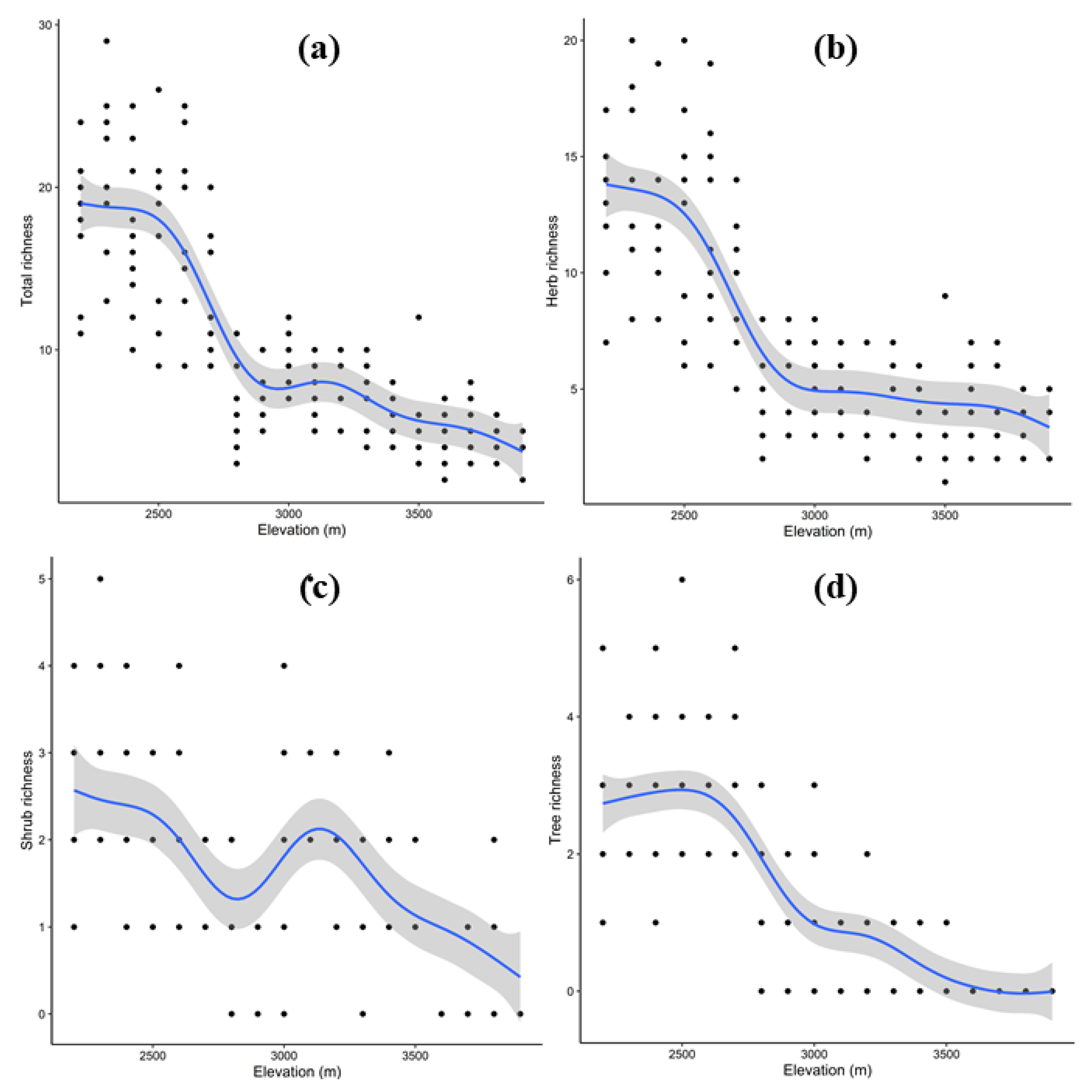
3.5. Pattern of Species Richness and Diversity along Elevation Gradient
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grytnes, J.A.; Heegaard, E.; Ihlen, P.G. Species richness of vascular plants, bryophytes, and lichens along an altitudinal gradient in western Norway. Acta Oecol. 2006, 29, 241–246. [Google Scholar] [CrossRef]
- Sharma, N.; Behera, M.D.; Das, A.P.; Panda, R.M. Plant species richness in an elevation gradient in the Eastern Himalaya. Biodivers. Conserv. 2019. [Google Scholar] [CrossRef]
- Korner, C. Mountain biodiversity, its causes and function. Ambio 2004, 13, 11–17. [Google Scholar] [CrossRef]
- Saikia, P.; Deka, J.; Bharali, S.; Kumar, A.; Tripathi, O.P.; Singha, L.B.; Khan, M.L. Plant diversity patterns and conservation status of eastern Himalayan forests in Arunachal Pradesh, Northeast India. For. Ecosyst. 2017, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Shooner, S.; Davies, T.J.; Saikia, P.; Deka, J.; Bharali, S.; Tripathi, O.P.; Dayanandan, S. Phylogenetic diversity patterns in Himalayan forests reveal evidence for environmental fltering of distinct lineages. Ecosphere 2018, 9, e02157. [Google Scholar] [CrossRef]
- Sinha, S.; Badola, H.K.; Chhetri, B.; Gaira, K.S.; Lepcha, J.; Dhyani, P.P. Effect of altitude and climate in shaping the forest compositions of Singalila National Park in Khangchendzonga landscape, eastern Himalaya, India. J. Asia Pac. Biodivers. 2018, 11, 267–275. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Vetaas, O.R. Variation in plant species richness of different life forms along a subtropical elevation gradient in the Himalayas, east Nepal. Glo Eco Biogeog. 2003, 12, 327–340. [Google Scholar] [CrossRef]
- Moradi, H.; Fattorini, S.; Oldeland, J. Influence of elevation on the species-area relationship. J. Biogeogr. 2020, 47, 2029–2041. [Google Scholar] [CrossRef]
- Carpenter, C. The environmental control of plant species density on a Himalayan elevation gradient. J. Biogeogr. 2005, 32, 999–1018. [Google Scholar] [CrossRef]
- Acharya, K.P.; Vetaas, O.R.; Birks, H.J.B. Orchid species richness along himalayan elevational gradients. J. Biogeogr. 2011, 38, 1821–1833. [Google Scholar] [CrossRef]
- Manish, K.; Pandit, M.K. Phylogenetic diversity, structure and diversifcation patterns of endemic plants along the elevational gradient in the Eastern Himalaya. Plant Ecol Divers. 2018, 11, 501–513. [Google Scholar] [CrossRef]
- Sun, L.; Luo, J.; Qian, L.; Deng, T.; Sun, H. The relationship between elevation and seed-plant species richness in the Mt. Namjagbarwa region (Eastern Himalayas) and its underlying determinants. Glob. Ecol. Conserv. 2020, 23, e01053. [Google Scholar] [CrossRef]
- Semwal, R.L.; Nautiyal, S.; Sen, K.K.; Rana, U.; Maikhuri, R.K.; Rao, K.S.; Saxena, K.G. Patterns and ecological implications of agricultural land-use changes: A case study from central Himalaya, India. Agric. Ecosyst. Environ. 2004, 102, 81–92. [Google Scholar] [CrossRef]
- Pandit, M.K. Life in the Himalaya—An Ecosystem at Risk; Harvard University Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Liang, J.; Ding, Z.; Lie, G.; Zhou, Z.; Singh, P.K.; Zhang, Z.; Hu, H. Species richness patterns of vascular plants and their drivers along an elevational gradient in the central Himalayas. Glob. Ecol. Conserv. 2020, 24, e01279. [Google Scholar] [CrossRef]
- Gairola, S.; Rawal, R.S.; Todaria, N.P. Forest vegetation pattern along an altitudinal gradient in sub-alpine zone of west Himalaya, India. Afr. J. Plant Sci. 2008, 2, 42–48. [Google Scholar] [CrossRef]
- Shaheen, H.; Ullah, Z.; Khan, S.M.; Harper, D.M. Species composition and community structure of western Himalayan moist temperate forests in Kashmir. For. Ecol. Manag. 2012, 278, 138–145. [Google Scholar] [CrossRef]
- Bisht, A.S.; Bhat, A.B. Vegetation structure and plant diversity relation in a sub-alpine region of Garhwal Himalaya, Uttarakhand, India. Afr. J. Plant Sci. 2013, 7, 401–406. [Google Scholar] [CrossRef] [Green Version]
- Lelli, C.; Bruun, H.H.; Chiarucci, A.; Donati, D.; Frascaroli, F.; Fritz, O.; Heilmann-Clausen, J. Biodiversity response to forest structure and management: Comparing species richness, conservation relevant species and functional diversity as metrics in forest conservation. For. Ecol. Manag. 2019, 432, 707–717. [Google Scholar] [CrossRef]
- Schmidt, I.; Zebre, S.; Betzin, J.; Weckesser, M. An approach to the identification of indicators for forest biodiversity-The solling Mountains (NW Germany) as an example. Restor. Ecol. 2006, 14, 123–136. [Google Scholar] [CrossRef]
- Sharma, C.M.; Ghildiyal, S.K.; Gairola, S.; Suyal, S. Vegetation structure, composition and diversity in relation to the soil characteristics of temperate mixed broad-leaved forest along an altitudinal gradient in Garhwal Himalaya. Indian J. Sci. Technol. 2009, 2, 39–45. [Google Scholar] [CrossRef]
- Dar, J.A.; Sundarapandian, S. Patterns of plant diversity in seven temperate forest types of Western Himalaya, India. J. Asia Pac. Biodivers. 2016, 9, 280–292. [Google Scholar] [CrossRef] [Green Version]
- McVicar, T.R.; Korner, C. On the use of elevation, altitude and height in the ecological and climatological literature. Oecologia 2013, 171, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, P.M.; Oxley, E.R.B. The growth form composition of plant communities in the Ecuadorian paramos. Plant Ecol. 1997, 131, 173–192. [Google Scholar] [CrossRef]
- Raina, A.K.; Sharma, N. Species composition and diversity of tree species along an altitudinal gradient in Sewa catchment of north-western Himalayas, Jammu and Kashmir, India. Environ. Conserv. J. 2012, 13, 173–179. [Google Scholar]
- Grytnes, J.A.; Vetaas, O.R. Species richness and altitude: A comparison between null models and interpolated plant species richness along the Himalayan altitudinal gradient, Nepal. Am. Nat. 2002, 159, 294–304. [Google Scholar] [CrossRef]
- Virtanen, R.; Luoto, M.; Rama, T.; Mikkola, K.; Hjort, J.; Grytnes, J.A.; Birks, H.J.B. Recent vegetation changes at the high-latitude tree line ecotone are controlled by geomorphological disturbance, productivity and diversity. Glob. Ecol. Biogeogr. 2010, 19, 810–821. [Google Scholar] [CrossRef]
- Boscutti, F.; Casolo, V.; Beraldo, P.; Braidot, E.; Zancani, M.; Rixen, C. Shrub growth and plant diversity along an elevation gradient: Evidence of indirect effects of climate on alpine ecosystems. PLoS ONE 2018, 13, e0196653. [Google Scholar] [CrossRef] [Green Version]
- Spehn, E.M.; Rudmann-Maurer, K.; Korner, C. Mountain biodiversity. Plant Ecol. Divers. 2011, 4, 301–302. [Google Scholar] [CrossRef]
- Pathak, R.; Negi, V.S.; Rawal, R.S.; Bhatt, I.D. Alien plant invasion in the Indian Himalayan region; state of knowledge and research priorities. Biodivers. Conserv. 2021, 28, 2073–3102. [Google Scholar] [CrossRef]
- Chawla, A.; Rajkumar, S.; Singh, K.N.; Lal, B.; Singh, R.D. Plant Species Diversity along an Altitudinal Gradient of Bhabha Valley in Western Himalaya. J. Mt. Sci. 2008, 5, 157–177. [Google Scholar] [CrossRef]
- Sang, W. Plant diversity patterns and their relationships with soil and climatic factors along an altitudinal gradient in the middle Tianshan Mountain area, Xinjiang, China. Ecol. Res. 2009, 24, 303–314. [Google Scholar] [CrossRef]
- Shimono, A.; Zhou, H.; Shen, H.; Hirota, M.; Ohtsuka, T.; Tang, Y. Patterns of plant diversity at high altitudes on the Qinghai-Tibetan Plateau. J. Plant Ecol. 2010, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kessler, M.; Kluge, J.; Hemp, A.; Ohlemüller, R. A global comparative analysis of elevational species richness patterns of ferns. Glob. Ecol. Biogeogr. 2011, 20, 868–880. [Google Scholar] [CrossRef]
- Vibhuti; Bargali, K.; Bargali, S.S. Effects of homegarden size on floristic composition and diversity along an altitudinal gradient in Central Himalaya, India. Curr. Sci. 2018, 114, 2494–2503. [Google Scholar] [CrossRef]
- Thakur, D.; Chawla, A. Functional diversity along elevational gradients in the high-altitude vegetation of the western Himalaya. Biodivers. Conserv. 2019, 28, 1977–1996. [Google Scholar] [CrossRef]
- Hussain, M.S.; Sultana, A.; Khan, J.A.; Khan, A. Species composition and community structure of forest stands in Kumaon Himalaya, Uttarakhand, India. Trop. Ecol. 2008, 49, 167. [Google Scholar]
- Bano, S.; Khan, S.M.; Alam, J.; Alqarawi, A.A.; AbdAllah, E.F.; Ahmad, Z.; Rahman, I.U.; Ahmad, H.; Aldubise, A. Eco-Floristic studies of the Beer Hills along the Indus River in the districts Haripur and Abbottabad, Pakistan. Saudi J. Biol. Sci. 2017, 25, 801–810. [Google Scholar] [CrossRef]
- Sharma, N.; Kala, C.P. Patterns in distribution, population density and uses of medicinal plants along the altitudinal gradient in Dhauladhar mountain range of Indian Himalayas. Curr. Sci. 2018, 114, 2323–2328. [Google Scholar] [CrossRef]
- Bhatta, K.P.; Chaudhary, R.P.; Vetaas, O.R. A comparison of systematic versus stratified-random sampling design for gradient analyses: A case study in subalpine Himalaya, Nepal. Phytocoenologia 2012, 42, 191–202. [Google Scholar] [CrossRef]
- Bisht, M.; Sekar, K.C.; Mukherjee, S.; Thapliyal, N.; Bahukhandi, A.; Singh, D.; Dey, D. Influence of Anthropogenic Pressure on the Plant Species Richness and Diversity Along the Elevation Gradients of Indian Himalayan High-Altitude Protected Areas. Front. Ecol. Evol. 2022, 10, 751989. [Google Scholar] [CrossRef]
- Joshi, H.C. Assessment of Habitat Diversity, Forest Vegetation and Human Dependence in the Buffer Zone of Nanda Devi Biosphere Reserve of west Himalaya. Ph.D. Thesis, Kumaun University, Nainital, India, 2002. [Google Scholar]
- Rawat, B.; Gairola, S.; Sekar, K.C.; Rawal, R.S. Community structure, regeneration potential and future dynamics of natural forest site in part of Nanda Devi Biosphere Reserve, Uttarakhand, India. Afr. J. Plant Sci. 2014, 8, 380–391. [Google Scholar]
- Rawat, B.; Negi, A.S. 2021. Plant diversity patterns along environmental gradients in Nanda Devi Biosphere Reserve, West Himalaya. Trop. Ecol. 2021, 62, 61–70. [Google Scholar] [CrossRef]
- Ahmad, M.; Uniyal, S.K.; Batish, D.R.; Singh, H.P.; Jaryan, V.; Rathee, S.; Sharma, P.; Kohli, R.K. Patterns of plant communities along vertical gradient in Dhauladhar Mountains in Lesser Himalayas in North-Western India. Sci. Total Environ. 2020, 716, 136919. [Google Scholar] [CrossRef]
- Bellhouse, D. Systematic Sampling Methods. Encyclopedia of Biostatistics; Wiley Online Library: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Kachroo, P.; Singh, G. Forest Flora of Srinagar and Neighborhood; Bishen Singh Pal Singh: Dehradun, India, 1976. [Google Scholar]
- Navchoo, I.A.; Kachroo, P. Flora of Pulwama, Kashmir; Bishen Singh and Mahendra Pal Singh: Dehradun, India, 1995. [Google Scholar]
- Singh, N.P.; Singh, D.K.; Uniyal, B.P. Flora of Jammu and Kashmir; Botanical Survey of India: Calcutta, India, 2002. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 14 July 2021).
- Kindt, R.; Coe, R. Tree Diversity Analysis. A Manual and Software for Common Statistical Methods for Ecological and Biodiversity Studies; World Agroforestry Centre (ICRAF): Nairobi, Kenya, 2005. [Google Scholar]
- Ahmad, R.; Khuroo, A.A.; Hamid, M.; Malik, A.H.; Rashid, I. Scale and season determine the magnitude of invasion impacts on plant communities. Flora 2019, 260, 151481. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier Science: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Rawal, P.; Kittur, S.; Chatakonda, M.K.; Sundar, S.G. Winter bird abundance, species richness and functional guild composition at Delhi’s ponds: Does time of day and wetland extent matter? J. Urban Ecol. 2021, 7, juab001. [Google Scholar] [CrossRef]
- Shahabuddin, G.; Goswami, R.; Krishnadas, M.; Menon, T. Decline in forest bird species and guilds due to land use change in the Western Himalaya. Glob. Ecol. Conserv. 2021, 25, e01447. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, D.; Villeger, S.; De Bortoli, J.; Leprieur, F.; Logez, M. Betapart: Partitioning Beta Diversity into Turnover and Nestedness Components. R Package Version 1.5.4. 2021. Available online: https://CRAN.R-project.org/package=betapart (accessed on 14 December 2021).
- Baselga, A.; Orme, C.D.L. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Glob. Ecol. Biogeogr. 2012, 21, 1223–1232. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman and Hall: London, UK; CRC: Boca Raton, FL, USA, 2017. [Google Scholar]
- Khan, S.M.; Page, S.; Ahmad, H.A.; Shaheen, H.; Harper, D.M. Vegetation dynamics in the Western Himalayas, diversity indices and climate change. Sci. Technol. Dev. 2012, 31, 232–243. [Google Scholar]
- Dar, G.H.; Khuroo, A.A. Floristic diversity in the Kashmir Himalaya: Progress, problems and prospects. Sains Malays. 2013, 42, 1377–1386. [Google Scholar]
- Altaf, A.; Haq, S.M.; Shabnum, N.; Jan, H.A. Comparative assessment of Phyto diversity in Tangmarg Forest division in Kashmir Himalaya, India. Acta Ecol. Sin. 2021. [Google Scholar] [CrossRef]
- Koul, A.R.; Chauhan, D.S.; Todaria, N.P. Plant diversity and floristic composition of three Forest types in Mata Vaishno Devi Forest of Jammu and Kashmir, Western Himalaya, India. Glob. J. Bot. Sci. 2015, 3, 7–16. [Google Scholar]
- Kumar, M.; Sharma, C.M. Fuelwood consumption pattern at different altitudes in rural areas of Garhwal Himalaya. Biomass Bioenergy 2009, 33, 1413–1418. [Google Scholar] [CrossRef]
- Bhat, J.A.; Kumar, M.; Pala, N.A.; Shah, S.; Dayal, S.; Gunathilake, C.; Negi, A.K. Influence of altitude on the distribution pattern of flora in a protected area of Western Himalaya. Acta Ecol. Sin. 2020, 40, 30–43. [Google Scholar] [CrossRef]
- Rawat, D.S.; Bagri, A.S.; Parveen, M.; Nautiyal, M.; Tiwari, P.; Tiwari, J.K. Pattern of species richness and floristic spectrum along the elevation gradient: A case study from western Himalaya, India. Acta Ecol. Sin. 2021, 41, 545–551. [Google Scholar] [CrossRef]
- Rawat, D.S.; Tiwari, J.K.; Tiwari, P.; Singh, H. Floristic diversity of montane zone of western Ramganga valley, Uttarakhand, India. J. Econ. Taxon. Bot. 2016, 40, 104–125. [Google Scholar]
- Rawat, D.S.; Dash, S.S.; Sinha, B.K.; Kumar, V.; Banerjee, A.; Singh, P. Community structure and regeneration status of tree species in eastern Himalaya: A case study from Neora Valley National Park, West Bengal, India. Taiwania 2018, 63, 16–24. [Google Scholar] [CrossRef]
- Kharkwal, G.; Mehrotra, P.; Rawat, Y.S.; Pangtey, Y.P.S. Phytodiversity and growth form in relation to altitudinal gradient in the central Himalayan (Kumaun) region of India. Curr. Sci. 2005, 89, 873–878. [Google Scholar]
- Tiwari, S.C. Concepts of Modern Ecology; Bishen Singh Mahendra Pal Singh: Dehradun, India, 2005. [Google Scholar]
- Khuroo, A.A.; Weber, E.; Malik, A.H.; Reshi, Z.A.; Dar, G.H. Altitudinal distribution patterns of the native and alien woody flora in Kashmir Himalaya, India. Environ. Res. 2011, 111, 967–977. [Google Scholar] [CrossRef]
- Rawat, D.S.; Tiwari, J.K.; Tiwari, P.; Nautiyal, M.; Praveen, M.; Singh, N. Tree species richness, dominance and regeneration status in western Ramganga valley, Uttarakhand Himalaya, India. Indian For. 2018, 144, 595–603. [Google Scholar]
- Mishra, D.; Mishra, T.K.; Banerjee, S.K. Comparative phytosociological and soil physic-chemical aspects between managed and unmanaged lateritic land. Ann. For. 1997, 5, 16–25. [Google Scholar]
- Thakur, S.; Negi, V.S.; Dhyani, R.; Bhatt, I.D.; Yadava, A.K. Influence of environmental factors on species diversity and composition in the Indian Western Himalaya. For. Ecol. Manag. 2022, 503, 119746. [Google Scholar] [CrossRef]
- Das, D.S.; Rawat, D.S.; Maity, D.; Dash, S.S.; Sinha, B.K. Species richness patterns of different life-forms along altitudinal gradients in the Great Himalayan National Park, western Himalaya, India. Taiwania 2020, 65, 154–162. [Google Scholar] [CrossRef]
- Scheiner, S.M.; Chiarucci, A.; Fox, G.A.; Helmus, M.R.; McGlinn, D.J.; Willing, M.R. The underpinnings of the relationship of species richness with space & time. Ecol. Monogr. 2011, 81, 195–213. [Google Scholar] [CrossRef] [Green Version]
- Ziska, L.H. Evaluation of the growth response of six invasive species to past, present and future atmospheric carbon dioxide. J. Exp. Bot. 2003, 54, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Rawal, R.S.; Rawal, R.; Rawat, B.; Negi, V.S.; Pathak, R. Plant species diversity and rarity patterns along altitude range covering treeline ecotone in Uttarakhand; conservation implications. Trop. Ecol. 2018, 59, 225–239. [Google Scholar]
- Graham, C.H.; Carnaval, A.C.; Cadena, C.D.; Zamudio, K.R.; Roberts, T.E.; Parra, J.L.; McCain, C.M.; Bowie, R.C.; Moritz, C.; Baines, S.B.; et al. The origin and maintenance of montane diversity: Integrating evolutionary and ecological processes. Ecography 2014, 37, 711–719. [Google Scholar] [CrossRef]
- Lomolino, M.V. Elevation gradients of species-density: Historical and prospective views. Glob. Ecol. Biogeogr. 2008, 10, 3–13. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, G.; Yang, L.; Li, Z. Distribution, species diversity and life-form spectra of plant communities along an altitudinal gradient in the northern slopes of Qilianshan Mountains, Gansu, China. Plant Ecol. 2002, 165, 169–181. [Google Scholar] [CrossRef]
- Korner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef]
- Kraft, N.J.; Comita, L.S.; Chase, J.M.; Sanders, N.J.; Swenson, N.G.; Crist, T.O.; Stegen, J.C.; Vellend, M.; Boyle, B.; Anderson, M.J.; et al. Disentangling the drivers of β-diversity along latitudinal and elevational gradients. Science 2011, 333, 1755–1758. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.X.; Guo, H.; Wang, X.A.; Fan, W.Y.; Duan, R.Y. Multiple-scale assessment of understory herb species diversity in pine plantations after long-term restoration. Nord. J. Bot. 2014, 32, 680–688. [Google Scholar] [CrossRef]
- Coelho, M.S.; Carneiro, M.A.A.; Branco, C.A.; Borges, R.A.X.; Fernandes, G.W. Species turnover drives β-diversity patterns across multiple spatial scales of plant-galling interactions in mountaintop grasslands. PLoS ONE 2018, 13, e0195565. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Fang, J.; Chi, X.; Feng, J.; Liu, Y.; Shen, Z.; Wang, X.; Wang, Z.; Wu, X.; Zheng, C.; et al. Patterns of plant beta-diversity along elevational and latitudinal gradients in mountain forests of China. Ecography 2012, 35, 1083–1091. [Google Scholar] [CrossRef]
- Kluge, J.; Worm, S.; Lange, S.; Long, D.; Bohner, J.; Yangzom, R.; Miehe, G. Elevational seed plant richness patterns in Bhutan, Eastern Himalaya. J. Biogeogr. 2017, 44, 1711–1722. [Google Scholar] [CrossRef]
- Manish, K.; Pandit, M.K.; Telwala, Y.; Nautiyal, D.C.; Koh, L.P.; Tiwari, S. Elevational plant species richness patterns and their drivers across nonendemics, endemics and growth forms in the Eastern Himalaya. J. Plant Res. 2017, 130, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.K.; Hemp, A.; Appelhans, T.; Behler, C.; Classen, A.; Detsch, F.; Ensslin, A.; Ferger, S.W.; Frederiksen, S.B.; Gebert, F.; et al. Predictors of elevational biodiversity gradients change from single taxa to the multi-taxa community level. Nat. Commun. 2016, 7, 13736. [Google Scholar] [CrossRef] [Green Version]
- Nanda, S.A.; Reshi, Z.A.; Haq, M.; Lone, B.A.; Mir, S.A. Taxonomic and functional plant diversity patterns along an elevational gradient through treeline ecotone in Kashmir. Trop. Ecol. 2018, 59, 211–224. [Google Scholar]
- Stevens, G.C. The elevational gradient in altitudinal range: An extension of Rapoport’s latitudinal rule to altitude. Am. Nat. 1992, 140, 893–911. [Google Scholar] [CrossRef]
- Gomez-Diaz, J.A.; Kromer, T.; Kreft, H.; Gerold, G.; Carvajal-Hernandez, C.I.; Heitkamp, F. Diversity and composition of herbaceous angiosperms along gradients of elevation and forest-use intensity. PLoS ONE 2017, 12, e0182893. [Google Scholar] [CrossRef] [Green Version]
- McCain, C.M. Could temperature and water availability drive elevational species richness patterns? A global case study for bats. Glob. Ecol. Biogeogr. 2007, 16, 1–13. [Google Scholar] [CrossRef]
- Lee, C.B.; Chun, J.H.; Song, H.K.; Cho, H.J. Altitudinal patterns of plant species richness on the Baekdudaegan Mountains, South Korea: Mid-domain effect, area, climate, and Rapoport’s rule. Ecol. Res. 2013, 28, 67–79. [Google Scholar] [CrossRef]
- Negi, V.S.; Kewlani, P.; Pathak, R.; Bhatt, D.; Bhatt, I.D.; Rawal, R.S.; Sundriyal, R.C.; Nandi, S.K. Criteria and indicators for promoting cultivation and conservation of medicinal and aromatic plants in Western Himalaya, India. Ecol. Indic. 2018, 93, 434–446. [Google Scholar] [CrossRef]
- Kewlani, P.; Negi, V.S.; Bhatt, I.D.; Rawal, R.S.; Nandi, S.K. Soil nutrients concentration along altitudinal gradients in Indian Western Himalaya. Scand. J. For. Res. 2021, 36, 98–104. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Mian, R. Effects of elevation and disturbance gradients on forest diversity in the Wulingshan nature reserve, North China. Environ. Earth Sci. 2016, 75, 904. [Google Scholar] [CrossRef]
- Prescott, C.E.; Zabek, L.M.; Stanley, C.L.; Kabzems, R. Decomposition of broadleaf and needle litter in forests of British Columbia: Influences of litter type, forest type and litter mixtures. Can. J. For. Res. 2000, 30, 1742–1750. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, A.; Lopez-Mata, L. Plant species richness and diversity along an altitudinal gradient in the Sierra Nevada, Mexico. Divers. Dis. 2005, 11, 567–575. [Google Scholar] [CrossRef]
- Fabbro, T.; Korner, C. Altitudinal differences in flower traits and reproductive allocation. Flora 2004, 199, 70–81. [Google Scholar] [CrossRef] [Green Version]


| Altitude (m) | Most Frequent Species |
|---|---|
| 2200 | Pinus wallichiana; Capsella bursa-pastoris; Geum roylei; Leucanthemum vulgare |
| 2300 | Abies pindrow; Virburnum grandiflorum; Lamium album; Valeriana jatamansii |
| 2400 | Abies pindrow; Leucanthemum vulgare; Rumex nepalensis; Polyygonum aviculare |
| 2500 | Aesculus indica; Parrotiopsis jacquemontiana, Prunus cornuta |
| 2600 | Abies pindrow; Bistorta amplexicaulis; Skimmia anquetilia; Arisaema jacquemontii |
| 2700 | Abies pindrow; Polygonum aviculare; Rumex nepalensis; Epimedium elatum |
| 2800 | Bistorta amplexicaulis; Cirsium arvensis; Cirsium falconeri |
| 2900 | Cirsium arvensis; Euphorbia wallichi |
| 3000 | Bupleurum candollei; Iris hookeriana; Cirsium falconeri; Thymus linearis |
| 3100 | Abies pindrow; Rhododendron campanulatum; Salix denticulata; Euphorbia wallichii; Bistorta amplexicaulis |
| 3200 | Betula utilis; Salix denticulata; Aconitum heterophyllum; Bupleurum candollei |
| 3300 | Betula utilis; Bistorta amplexicaulis; Iris hookeriana |
| 3400 | Sibbaldia cuneata; Saussurea atkinsonii; Bergenia ciliata |
| 3500 | Juniperus squamata; Betula utilis; Salix denticulata; Salvia hians; Swertia petiolata; Phlomis bracteosa |
| 3600 | Juniperus squamata; Rhododendron anthopogon; Anaphalis roylei; Cordiofontis flexuosa; Thymus linearis |
| 3700 | Bisorta affinis; Taraxacum sect Taraxacum; Viola biflora |
| 3800 | Juniperus squamata; Rhododendron anthopogon; Bistorta affinis; Rheum webbianum |
| 3900 | Bistorta affinis; Bergenia ciliata |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wani, Z.A.; Khan, S.; Bhat, J.A.; Malik, A.H.; Alyas, T.; Pant, S.; Siddiqui, S.; Moustafa, M.; Ahmad, A.E. Pattern of β-Diversity and Plant Species Richness along Vertical Gradient in Northwest Himalaya, India. Biology 2022, 11, 1064. https://doi.org/10.3390/biology11071064
Wani ZA, Khan S, Bhat JA, Malik AH, Alyas T, Pant S, Siddiqui S, Moustafa M, Ahmad AE. Pattern of β-Diversity and Plant Species Richness along Vertical Gradient in Northwest Himalaya, India. Biology. 2022; 11(7):1064. https://doi.org/10.3390/biology11071064
Chicago/Turabian StyleWani, Zishan Ahmad, Sajid Khan, Jahangeer A. Bhat, Akhtar H. Malik, Tahira Alyas, Shreekar Pant, Sazada Siddiqui, Mahmoud Moustafa, and Ahmad Ezzat Ahmad. 2022. "Pattern of β-Diversity and Plant Species Richness along Vertical Gradient in Northwest Himalaya, India" Biology 11, no. 7: 1064. https://doi.org/10.3390/biology11071064







