Spatiotemporal Variability of Trace Elements Fingerprints in Otoliths of Japanese Eel (Anguilla japonica) and Its Use in Tracing Geographic Origin
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
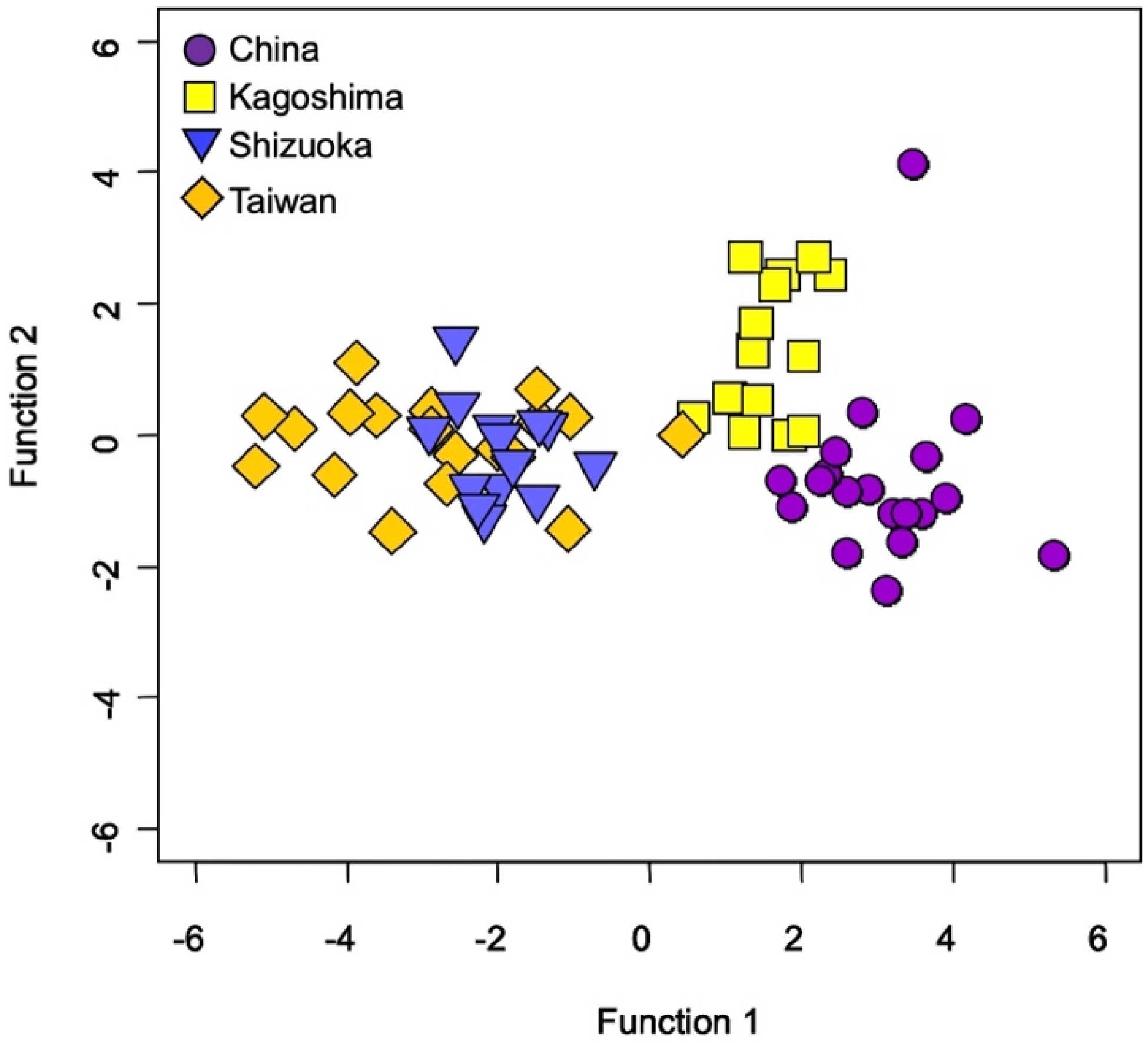
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marko, P.B.; Lee, S.C.; Rice, A.M.; Gramling, J.M.; Fitzhenry, T.M.; McAlister, J.S.; Harper, G.R.; Moran, A.M. Fisheries: Mislabelling of a depleted reef fish. Nature 2004, 430, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, J.L.; Pauly, D. Trade secrets: Renaming and mislabeling of seafood. Mar. Policy 2008, 32, 309–318. [Google Scholar] [CrossRef]
- Leal, M.C.; Pimentel, T.; Ricardo, F.; Rosa, R.; Calado, R. Seafood traceability: Current needs, available tools and biotechnological challenges for origin certification. Trends Biotechnol. 2015, 33, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Grahl-Nielsen, O.; Jacobsen, A.; Christophersen, G.; Magnesen, T. Fatty acid composition in adductor muscle of juvenile scallops (Pecten maximus) from five Norwegian populations reared in the same environment. Biochem. Syst. Ecol. 2010, 38, 478–488. [Google Scholar] [CrossRef]
- Haye, P.A.; Segovia, N.I.; Vera, R.; Gallardo, M.d.l.A.; Gallardo-Escárate, C. Authentication of commercialized crab-meat in Chile using DNA Barcoding. Food Control 2012, 25, 239–244. [Google Scholar] [CrossRef]
- Galimberti, A.; Mattia, F.D.; Losa, A.; Bruni, I.; Federici, S.; Casiraghi, M.; Martellos, S.; Labra, M. DNA barcoding as a new tool for food traceability. Food Res. Int. 2013, 50, 55–63. [Google Scholar] [CrossRef]
- Arai, T.; Amalina, R.; Bachok, Z. Similarity in the feeding ecology of parrotfish (Scaridae) in coral reef habitats of the Malaysian South China Sea, as revealed by fatty acid signatures. Biochem Syst. Ecol. 2015, 59, 85–90. [Google Scholar] [CrossRef]
- Yamane, K.; Shirai, K.; Nagakura, Y.; Yamaguchi, M.; Takita, A.; Horii, T.; Tanaka, N.; Yamane, S.; Arai, T.; Otake, T. Spatial variation in otolith elemental composition of the Pacific herring Clupea pallasii in northern Japan. Aquat. Biol. 2010, 10, 283–290. [Google Scholar] [CrossRef]
- Ricardo, F.; Pimentel, T.; Génio, L.; Calado, R. Spatio-temporal variability of trace elements fingerprints in cockle (Cerastoderma edule) shells and its relevance for tracing geographic origin. Sci. Rep. 2017, 7, 3475. [Google Scholar] [CrossRef]
- Vasconcelos, J.; Vieira, A.R.; Sequeira, V.; Gonzalez, J.A.; Kaufmann, M.; Gordo, L.S. Identifying populations of the blue jack mackerel (Trachurus picturatus) in the Northeast Atlantic by using geometric morphometrics and otolith shape analysis. Fish. Bull. 2018, 116, 81–92. [Google Scholar] [CrossRef]
- Campana, S.E. Chemistry and composition of fish otoliths: Pathways, mechanisms and applications. Mar. Ecol. Prog. Ser. 1999, 188, 263–297. [Google Scholar] [CrossRef] [Green Version]
- Arai, T. Migration ecology in the freshwater eels of the genus Anguilla Schrank, 1798. Trop. Ecol. 2022, 63, 155–170. [Google Scholar] [CrossRef]
- Arai, T. Ecology and evolution of migration in the freshwater eels of the genus Anguilla Schrank, 1798. Heliyon 2020, 6, e05176. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Chino, N. Diverse migration strategy between freshwater and seawater habitats in the freshwater eels genus Anguilla. J. Fish Biol. 2012, 81, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.M.; Limburg, K. Determination of River Herring Natal Origin using Otolith Chemical Markers: Accuracy as a Function of Spatial Scale and Choice of Markers. Trans. Am. Fish. Soc. 2014, 143, 1530–1543. [Google Scholar] [CrossRef]
- Garcez, R.C.; Humston, R.; Harbor, D.; Freitas, C.E.C. Otolith geochemistry in young-of-the-year peacock bass Cichla temensis for investigating natal dispersal in the Rio Negro (Amazon–Brazil) river system. Ecol. Freshw. Fish 2015, 24, 242–251. [Google Scholar] [CrossRef]
- Tripp, A.; Murphy, H.M.; Davoren, G.K. Otolith Chemistry reveals natal region of larval capelin in coastal Newfoundland, Canada. Front. Mar. Sci. 2020, 7, 258. [Google Scholar] [CrossRef]
- Arai, T. How have spawning ground investigations of the Japanese eel Anguilla japonica contributed to the stock enhancement? Rev. Fish Biol. Fish. 2014, 24, 75–88. [Google Scholar] [CrossRef]
- Arai, T. Do we protect freshwater eels or do we drive them to extinction? SpringerPlus 2014, 3, 534. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Davenport, E.R.; Wang, D.; Clark, A.G. Lack of spatial and temporal genetic structure of Japanese eel (Anguilla japonica) populations. Conserv. Genet. 2019, 20, 467–475. [Google Scholar] [CrossRef]
- Arai, T. Sustainable management of tropical anguillid eels in Southeast Asia. In Natural Resources Conservation and Advances for Sustainability; Jhariya, M.K., Meena, R.S., Banerjee, A., Meena, S.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 461–480. [Google Scholar]
- Arai, T.; Hirata, T.; Takagi, Y. Application of laser ablation ICPMS to trace the environmental history of chum salmon Oncorhynchus keta. Mar. Environ. Res. 2007, 63, 55–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, T.; Ohji, M.; Hirata, T. Trace metal deposition in teleost fish otolith as an environmental indicator. Water Air Soil Pollut. 2007, 179, 255–263. [Google Scholar] [CrossRef]
- Arai, T. Effect of salinity on strontium:calcium ratios in the otoliths of Sakhalin taimen, Hucho perryi. Fish. Sci. 2010, 76, 451–455. [Google Scholar] [CrossRef]
- Arai, T.; Chino, N. Influence of water salinity on the strontium:calcium ratios in otoliths of the giant mottled eel, Anguilla marmorata. Environ. Biol. Fish. 2017, 100, 281–286. [Google Scholar] [CrossRef]
- Arai, T.; Otake, T.; Tsukamoto, K. Drastic changes in otolith microstructure and microchemistry accompanying the onset of metamorphosis in the Japanese eel Anguilla japonica. Mar. Ecol. Prog. Ser. 1997, 161, 17–22. [Google Scholar] [CrossRef]
- Kotake, A.; Okamura, A.; Yamada, Y.; Utoh, T.; Arai, T.; Miller, M.J.; Oka, H.P.; Tsukamoto, K. Seasonal variation in migratory history of the Japanese eel, Anguilla japonica, in Mikawa Bay, Japan. Mar. Ecol. Prog. Ser. 2005, 293, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Arai, T.; Kotake, A.; Ohji, M. Variation in migratory history of Japanese eels, Anguilla japonica, collected in the northernmost part of its distribution. J. Mar. Biol. Assoc. UK 2008, 88, 1075–1080. [Google Scholar] [CrossRef]
- Chino, N.; Arai, T. Relative contribution of migratory type on the reproduction of migrating silver eels Anguilla japonica, collected off Shikoku Island, Japan. Mar. Biol. 2009, 156, 661–668. [Google Scholar] [CrossRef]
- Chino, N.; Arai, T. Migratory history of the giant mottled eel (Anguilla marmorata) in the Bonin Islands of Japan. Ecol. Freshw. Fish. 2010, 19, 19–25. [Google Scholar] [CrossRef]
- Arai, T.; Chino, N. Opportunistic migration and habitat use of the giant mottled eel Anguilla marmorata (Teleostei: Elopomorpha). Sci. Rep. 2018, 8, 5666. [Google Scholar] [CrossRef]
- Arai, T.; Kotake, A.; Harrod, C.; Morrissey, M.; Mccarthy, T.K. Ecological plasticity of the European eel Anguilla anguilla in a tidal Atlantic lake system in Ireland. J. Mar. Biol. Assoc. UK 2019, 99, 1189–1195. [Google Scholar] [CrossRef]
- Arai, T.; Chino, N. Contribution of migratory types to the reproduction of migrating silver eels in a tropical eel, Anguilla bicolor bicolor. Heliyon 2022, 8, e09491. [Google Scholar] [CrossRef] [PubMed]
- Tunheng, A.; Hirata, T. Development of signal smoothing device for laser ablation–ICP-mass spectrometer. J. Anal. At. Spectrom. 2004, 19, 932–934. [Google Scholar] [CrossRef]
- Elsdon, T.S.; Gillanders, B.M. Reconstructing migratory patterns of fish based on environmental influences on otolith chemistry. Rev. Fish Biol. Fish. 2003, 13, 217–235. [Google Scholar] [CrossRef]
- Gaetani, G.A.; Cohen, A.L. Element partitioning during precipitation of aragonite from seawater: A framework for understanding paleoproxies. Geochim. Cosmochim. Acta 2006, 70, 4617–4634. [Google Scholar] [CrossRef]
- Walther, B.D.; Kingsford, M.J.; O’Callaghan, M.D.; McCulloch, M.T. Interactive effects of ontogeny, food ration and temperature on elemental incorporation in otoliths of a coral reef fish. Environ. Biol. Fish. 2010, 89, 441–451. [Google Scholar] [CrossRef]
- Sturrock, A.M.; Trueman, C.N.; Milton, J.A.; Waring, C.P.; Cooper, M.J.; Hunter, E. Physiological influences can outweigh environmental signals in otolith microchemistry research. Mar. Ecol. Prog. Ser. 2014, 500, 245–264. [Google Scholar] [CrossRef] [Green Version]
- Stanley, R.R.E.; Bradbury, I.R.; DiBacco, C.; Snelgrove, P.V.R.; Thorrold, S.R.; Killen, S.S. Environmentally mediated trends in otolith composition of juvenile Atlantic cod (Gadus morhua). ICES J. Mar. Sci. 2015, 72, 2350–2363. [Google Scholar]
- Mazloumi, N.; Doubleday, Z.A.; Gillanders, B.M. The effects of temperature and salinity on otolith chemistry of King George whiting. Fish. Res. 2017, 196, 66–74. [Google Scholar] [CrossRef]
- Walsh, C.T.; Gillanders, B.M. Extrinsic factors affecting otolith chemistry—Implications for interpreting migration patterns in a diadromous fish. Environ. Biol. Fish. 2018, 101, 905–916. [Google Scholar] [CrossRef]
- Lin, S.H.; Chang, C.H.; Iizuka, Y.; Tzeng, W.N. Salinities, not diets, affect strontium/calcium ratios in otoliths of Anguilla japonica. J. Exp. Mar. Biol. Ecol. 2007, 341, 254–263. [Google Scholar] [CrossRef]
- Arai, T.; Hirata, T. Differences in the trace element deposition in otoliths between marine- and freshwater-resident Japanese eels, Anguilla japonica, as determined by laser ablation ICPMS. Environ. Biol. Fish. 2006, 75, 173–182. [Google Scholar] [CrossRef]
- Liao, C.; Hsu, Y.K.; Lee, W.C. Technical Innovations in Eel Culture Systems. Rev. Fish. Sci. 2002, 10, 433–450. [Google Scholar] [CrossRef]
- Radigan, W.J.; Carlson, A.K.; Fincel, M.J.; Graeb, B.D.S. Otolith chemistry as a fisheries management tool after flooding: The case of Missouri River gizzard shad. River Res. Appl. 2018, 34, 270–278. [Google Scholar] [CrossRef]
- Taddese, F.; Reid, M.R.; Closs, G.P. Direct relationship between water and otolith chemistry in juvenile estuarine triplefin Forsterygion nigripenne. Fish. Res. 2019, 211, 32–39. [Google Scholar] [CrossRef]
- Brand, U.; Morrison, J.O.; Campbell, I.T. Strontium in sedimentary rocks. In Geochemistry. Encyclopedia of Earth Science; Marshall, C.P., Fairbridge, R.W., Eds.; Springer: Dordrecht, The Netherlands, 1998; pp. 600–603. [Google Scholar]




| Location | Sample Size | Mean ± SD | Range |
|---|---|---|---|
| Wild habitat | |||
| Oi River | |||
| 2007 | 30 | 508 ± 84.9 | 371–741 |
| 2008 | 20 | 572 ± 111 | 373–765 |
| 2009 | 20 | 516 ± 111 | 364–752 |
| Aquaculture pond | |||
| China | 19 | 495 ± 19.1 | 466–551 |
| Japan, Kagoshima | 14 | 496 ± 22.7 | 462–545 |
| Japan, Shizuoka | 14 | 504 ± 20.2 | 458–528 |
| Taiwan (Republic of China) | 20 | 501 ± 20.9 | 463–539 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arai, T.; Kimura, S. Spatiotemporal Variability of Trace Elements Fingerprints in Otoliths of Japanese Eel (Anguilla japonica) and Its Use in Tracing Geographic Origin. Biology 2022, 11, 1733. https://doi.org/10.3390/biology11121733
Arai T, Kimura S. Spatiotemporal Variability of Trace Elements Fingerprints in Otoliths of Japanese Eel (Anguilla japonica) and Its Use in Tracing Geographic Origin. Biology. 2022; 11(12):1733. https://doi.org/10.3390/biology11121733
Chicago/Turabian StyleArai, Takaomi, and Shogo Kimura. 2022. "Spatiotemporal Variability of Trace Elements Fingerprints in Otoliths of Japanese Eel (Anguilla japonica) and Its Use in Tracing Geographic Origin" Biology 11, no. 12: 1733. https://doi.org/10.3390/biology11121733






