Teucrium polium: Potential Drug Source for Type 2 Diabetes Mellitus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Teucrium polium
2.1. Antidiabetic Effect of Tp
2.2. Identified Bioactive Constituents in Tp
Bioactivity and Bioavailability of the Key Flavonoids Found in Tp
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41 (Suppl. 1), S13–S27. [Google Scholar] [CrossRef] [Green Version]
- World Health Organisation. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation. 2006. Available online: https://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf (accessed on 19 December 2021).
- International Diabetes Federation. Diabetes is “a pandemic of unprecedented magnitude” now affecting one in 10 adults worldwide. Diabetes Res. Clin. Pract. 2021, 181, 109133. [Google Scholar] [CrossRef] [PubMed]
- Parohan, M.; Yaghoubi, S.; Seraji, A.; Javanbakht, M.H.; Sarraf, P.; Djalali, M. Risk factors for mortality in patients with Coronavirus disease 2019 (COVID-19) infection: A systematic review and meta-analysis of observational studies. Aging Male 2020, 23, 1416–1424. [Google Scholar] [CrossRef]
- Apicella, M.; Campopiano, M.C.; Mantuano, M.; Mazoni, L.; Coppelli, A.; Del Prato, S. COVID-19 in people with diabetes: Understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020, 8, 782–792. [Google Scholar] [CrossRef]
- Erener, S. Diabetes, infection risk and COVID-19. Mol. Metab. 2020, 39, 101044. [Google Scholar] [CrossRef]
- Ghosal, S.; Arora, B.; Dutta, K.; Ghosh, A.; Sinha, B.; Misra, A. Increase in the risk of type 2 diabetes during lockdown for the COVID19 pandemic in India: A cohort analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 949–952. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Z.F. Impact of the COVID-19 pandemic on mental health and quality of life among local residents in Liaoning Province, China: A cross-sectional study. Int. J. Environ. Res. Public Health 2020, 17, 2381. [Google Scholar] [CrossRef] [Green Version]
- Mendis, S.; Bettcher, D.; Branca, F. World Health Organization Global Status Report on Noncommunicable Diseases. 2014. Available online: http://apps.who.int/iris/bitstream/handle/10665/148114/9789241564854_eng.pdf;jsessionid=39235CE69F7E48BF856DAFA5E81A8D0B?sequence=1 (accessed on 19 December 2021).
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- World Health Organizations. Global Report on Diabetes: Global Burden of Diabetes. 2016. Available online: http://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf?sequence=1 (accessed on 19 December 2021).
- Udler, M.S. Type 2 Diabetes: Multiple Genes, Multiple Diseases. Curr. Diabetes Rep. 2019, 19, 55. [Google Scholar] [CrossRef] [Green Version]
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Pathgenesis and treatment. Lancet 2008, 371, 2153–2156. [Google Scholar] [CrossRef]
- Ali, O. Genetics of type 2 diabetes. World J. Diabetes 2013, 4, 114–123. [Google Scholar] [CrossRef]
- Rudenski, A.S.; Hadden, D.R.; Atkinson, A.B.; Kennedy, L.; Matthews, D.R.; Merrett, J.D.; Pockaj, B.; Turner, R.C. Natural history of pancreatic islet B-cell function in type 2 diabetes mellitus studied over six years by homeostasis model assessment. Diabet. Med. 1988, 5, 36–41. [Google Scholar] [CrossRef]
- Cnop, M.; Welsh, N.; Jonas, J.C.; Jorns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes 2005, 54 (Suppl. 2), S97–S107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. β-Cell Deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, A.C.; Ambale-Venkatesh, B.; Turkbey, E.; Donekal, S.; Chamera, E.; Backlund, J.Y.; Cleary, P.; Lachin, J.; Bluemke, D.A.; Lima, J.A.C.; et al. Association of cardiovascular risk factors and myocardial fibrosis with early cardiac dysfunction in type 1 diabetes: The diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2017, 40, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, R.; Rajagopalan, R. Diabetes and insulin resistance associated disorders: Disease and the therapy. Curr. Sci. 2002, 83, 1533–1538. [Google Scholar]
- Miller, E.; Aguilar, R.B.; Herman, M.E.; Schwartz, S.S. Type 2 diabetes: Evolving concepts and treatment. Clevel. Clin. J. Med. 2019, 86, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Gerich, J.E. Control of glycaemia. Baillieres Clin. Endocrinol. Metab. 1993, 7, 551–586. [Google Scholar] [CrossRef]
- Greiner, E.F.; Guppy, M.; Brand, K. Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. J. Biol. Chem. 1994, 269, 31484–31490. [Google Scholar] [CrossRef]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef]
- Doyle-Delgado, K.; Chamberlain, J.J.; Shubrook, J.H.; Skolnik, N.; Trujillo, J. Pharmacologic approaches to glycemic treatment of type 2 diabetes: Synopsis of the 2020 American Diabetes Association’s Standards of Medical Care in Diabetes clinical guideline. Ann. Intern. Med. 2020, 173, 813–821. [Google Scholar] [CrossRef]
- Peña, A.S.; Curran, J.A.; Fuery, M.; George, C.; Jefferies, C.A.; Lobley, K.; Ludwig, K.; Maguire, A.M.; Papadimos, E.; Peters, A.; et al. Screening, assessment and management of type 2 diabetes mellitus in children and adolescents: Australasian Paediatric Endocrine Group guidelines. Med. J. Aust. 2020, 213, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Cantello, B.C.C.; Cawthorne, M.A.; Cottam, G.P.; Duff, P.T.; Haigh, D.; Hindley, R.M.; Lister, C.A.; Smith, S.A.; Thurlby, P.L. [[omega-(Heterocyclylamino)alkoxy]benzyl]-2,4-thiazolidinediones as potent antihyperglycemic agents. J. Med. Chem. 1994, 37, 3977–3985. [Google Scholar] [CrossRef]
- Drucker, D.J. Glucagon-like peptides. Diabetes 1998, 47, 159–169. [Google Scholar] [CrossRef]
- Porksen, N.K.; Munn, S.R.; Steers, J.L.; Schmitz, O.; Veldhuis, J.D.; Butler, P.C. Mechanisms of sulfonylurea’s stimulation of insulin secretion in vivo: Selective amplification of insulin secretory burst mass. Diabetes 1996, 45, 1792–1797. [Google Scholar] [CrossRef]
- Read, P.A.; Khan, F.Z.; Heck, P.M.; Hoole, S.P.; Dutka, D.P. DPP-4 inhibition by sitagliptin improves the myocardial response to dobutamine stress and mitigates stunning in a pilot study of patients with coronary artery disease. Circ. Cardiovasc. Imaging 2010, 3, 195–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Laar, F.A.; Lucassen, P.L.; Akkermans, R.P.; Van de Lisdonk, E.H.; Rutten, G.E.; Van Weel, C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2005, 2009, CD003639. [Google Scholar] [CrossRef] [Green Version]
- Bailey, C.J.; Turner, R.C. Metformin. N. Engl. J. Med. 1996, 334, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Grunberger, G. Should side effects influence the selection of antidiabetic therapies in type 2 diabetes? Curr. Diabetes Rep. 2017, 17, 21. [Google Scholar] [CrossRef]
- Scheen, A.J.; Paquot, N. Metformin revisited: A critical review of the benefit-risk balance in at-risk patients with type 2 diabetes. Diabetes Metab. 2013, 39, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Gheissari, A.; Hemmatzadeh, S.; Merrikhi, A.; Fadaei Tehrani, S.; Madihi, Y. Chronic kidney disease in children: A report from a tertiary care center over 11 years. J. Nephropathol. 2012, 1, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Davoren, P. Safe prescribing of metformin in diabetes. Aust. Prescr. 2014, 37, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.; Tripathi, P.; Pandey, R.; Srivatava, R.; Goswami, S. Alternative therapies useful in the management of diabetes: A systematic review. J. Pharm. Bioallied Sci. 2011, 3, 504–512. [Google Scholar] [PubMed]
- Alam, F.; Islam, M.A.; Kamal, M.A.; Gan, S.H. Updates on managing type 2 diabetes mellitus with natural products: Towards antidiabetic drug development. Curr. Med. Chem. 2018, 25, 5395–5431. [Google Scholar] [CrossRef] [PubMed]
- Saad, B.; Zaid, H.; Shanak, S.; Kadan, S. Anti-Diabetes and Anti-Obesity Medicinal Plants and Phytochemicals; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Hawash, M.; Jaradat, N.; Elaraj, J.; Hamdan, A.; Abu Lebdeh, S.; Halawa, T. Evaluation of the hypoglycemic effect of seven wild folkloric edible plants from Palestine: Antidiabetic effect of seven plants from Palestine. J. Complement. Integr. Med. 2019, 17, 20190032. [Google Scholar] [CrossRef]
- Perla, V.; Jayanty, S.S. Biguanide related compounds in traditional antidiabetic functional foods. Food Chem. 2013, 138, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Nyenwe, E.A.; Jerkins, T.W.; Umpierrez, G.E.; Kitabchi, A.E. Management of type 2 diabetes: Evolving strategies for the treatment of patients with type 2 diabetes. Metabolism 2011, 60, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saad, B.; Said, O. Greco-Arab and Islamic Herbal Medicine: Traditional System, Ethics, Safety, Efficacy, and Regulatory Issues; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Arumugam, G.; Manjula, P.; Paari, N. A review: Anti diabetic medicinal plants used for diabetes mellitus. J. Acute Dis. 2013, 2, 196–200. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.K.; Prasad, S.K.; Kumar, R.; Hemalatha, S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012, 2, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Asghari, A.A.; Mokhtari-Zaer, A.; Niazmand, S.; Mc Entee, K.; Mahmoudabady, M. Anti-diabetic properities and bioactive compounds of Teucrium polium L. Asian Pac. J. Trop. Biomed. 2020, 10, 433–441. [Google Scholar] [CrossRef]
- Kasabri, V.; Abu-Dahab, R.; Afifi, F.U.; Naffa, R.; Majdalawi, L. In vitro modulation of pancreatic MIN6 insulin secretion and proliferation and extrapancreatic glucose absorption by paronechia argentea, Rheum ribes and Teucrium polium extracts. Jordan J. Pharm. Sci. 2012, 5, 203–219. [Google Scholar]
- Mannan, A. Molecular Signaling Pathways Involved in the Glucose-Lowering Effect of Teucrium Polium; Curtin University: Perth, WA, Australia, 2017. [Google Scholar]
- Mirghazanfari, S.M.; Keshavarz, M.; Nabavizadeh, F.; Soltani, N.; Kamalinejad, M. The Effect of Teucrium polium L. extracts on insulin release from in situ isolated perfused rat pancreas in a newly modified isolation method: The role of Ca2+ and K+ Channels. Iran. Biomed. J. 2010, 14, 178–185. [Google Scholar] [PubMed]
- Stefkov, G.; Kulevanova, S.; Miova, B.; Dinevska-Kjovkarovska, S.; Mølgaard, P.; Jäger, A.K.; Josefsen, K. Effects of Teucrium polium spp. capitatum flavonoids on the lipid and carbohydrate metabolism in rats. Pharm. Biol. 2011, 49, 885–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, M.; Takei, I.; Umezawa, K. Antidiabetic effect of orally administered conophylline-containing plant extract on streptozotocin-treated and Goto-Kakizaki rats. Biomed. Pharmacother. 2009, 63, 710–716. [Google Scholar] [CrossRef]
- Ramkumar, K.M.; Lee, A.S.; Krishnamurthi, K.; Devi, S.S.; Chakrabarti, T.; Kang, K.P.; Lee, S.; Kim, W.; Park, S.K.; Lee, N.H.; et al. Gymnema montanum H. protects against alloxan-induced oxidative stress and apoptosis in pancreatic β-cells. Cell. Physiol. Biochem. 2009, 24, 429–440. [Google Scholar] [CrossRef]
- Yahaya, N.; Mohd Dom, N.S.; Adam, Z.; Hamid, M. Insulinotropic activity of standardized methanolic extracts of Ficus deltoidea from seven varieties. Evid. Based Complement. Altern. Med. 2018, 2018, 3769874. [Google Scholar] [CrossRef] [Green Version]
- Chien, S.-C.; Young, P.H.; Hsu, Y.-J.; Chen, C.-H.; Tien, Y.-J.; Shiu, S.-Y.; Li, T.-H.; Yang, C.-W.; Marimuthu, P.; Tsai, L.F.-L.; et al. Anti-diabetic properties of three common Bidens pilosa variants in Taiwan. Phytochemistry 2009, 70, 1246–1254. [Google Scholar] [CrossRef]
- Ireng, A.; Helmerhorst, E.; Parsons, R.; Caccetta, R. Teucrium polium significantly lowers blood glucose levels acutely in normoglycemic male Wistar rats: A comparative to insulin and metformin. Adv. Med. Plant. Res. 2016, 4, 1–10. [Google Scholar]
- Frezza, C.; Venditti, A.; Serafini, M.; Bianco, A. Chapter 4: Phytochemistry, Chemotaxonomy, Ethnopharmacology, and Nutraceutics of Lamiaceae. In Studies in Natural Products Chemistry; Atta-ur-Rahma, Ed.; Elsevier: Amestrdam, The Netherlands, 2019; Volume 62, pp. 125–178. [Google Scholar]
- Venditti, A.; Frezza, C.; Zadeh, S.M.M.; Foddai, S.; Serafini, M.; Bianco, A. Secondary metabolites from Teucrium polium L. collected in Southern Iran. AJMAP 2017, 3, 108–123. [Google Scholar]
- Hasani-Ranjbar, S.; Nayebi, N.; Larijani, B.; Abdollahi, M. A systematic review of the efficacy and safety of Teucrium species; from anti-oxidant to anti-diabetic effects. Int. J. Pharmacol. 2010, 6, 315–325. [Google Scholar] [CrossRef] [Green Version]
- Mazokopakis, E.; Lazaridou, S.; Tzardi, M.; Mixaki, J.; Diamantis, I.; Ganotakis, E. Acute cholestatic hepatitis caused by Teucrium polium L. Phytomedicine 2004, 11, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.I.; Igoli, J.O.; Edrada-Ebel, R. Natural products isolation in modern drug discovery programs. Methods Mol. Biol. 2012, 864, 515–534. [Google Scholar]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st Century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [Green Version]
- Yaniv, Z.; Dafni, A.; Friedman, J.; Palevitch, D. Plants used for the treatment of diabetes in Israel. J. Ethnopharmacol. 1987, 19, 145–151. [Google Scholar] [CrossRef]
- Ardestani, A.; Yazdanparast, R.; Jamshidi, S. Therapeutic effects of Teucrium polium extract on oxidative stress in pancreas of streptozotocin-induced diabetic rats. J. Med. Food 2008, 11, 525–532. [Google Scholar] [CrossRef]
- Yazdanparas, R.; Esmaeili, M.A.; Ashrafi Helan, J. Teucrium polium extract effects pancreatic function of streptozotocin diabetic rats: A histopathological examination. Iran. Biomed. J. 2005, 9, 81–85. [Google Scholar]
- Gharaibeh, M.N.; Elayan, H.H.; Salhab, A.S. Hypoglycemic effects of Teucrium polium. J. Ethnopharmacol. 1988, 24, 93–99. [Google Scholar] [CrossRef]
- Vessal, M.; Hemmati, M.; Vasei, M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003, 135, 357–364. [Google Scholar] [CrossRef]
- Stankovic, M.S.; Niciforovic, N.; Mihailovic, V.; Topuzovic, M.; Solujic, S. Antioxidant activity, total phenolic content and flavonoid concentrations of different plant parts of Teucrium polium L. subsp. polium. Acta Soc. Bot. Pol. 2012, 81, 117–122. [Google Scholar] [CrossRef]
- Sharififar, F.; Dehghn-Nudeh, G.; Mirtajaldini, M. Major flavonoids with antioxidant activity from Teucrium polium L. Food Chem. 2009, 112, 885–888. [Google Scholar] [CrossRef]
- Pacifico, S.; D’Abrosca, B.; Scognamiglio, M.; D’Angelo, G.; Gallicchio, M.; Galasso, S.; Monaco, P.; Fiorentino, A. NMR-based metabolic profiling and in vitro antioxidant and hepatotoxic assessment of partially purified fractions from Golden germander (Teucrium polium L.) methanolic extract. Food Chem. 2012, 135, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Farahmandfar, R.; Asnaashari, M.; Bakhshandeh, T. Influence of ultrasound-assist and classical extractions on total phenolic, tannin, flavonoids, tocopherol and antioxidant characteristics of Teucrium polium aerial parts. J. Food Meas. Charact. 2019, 13, 1357–1363. [Google Scholar] [CrossRef]
- Esmaeili, M.A.; Zohari, F.; Sadeghi, H. Antioxidant and protective effects of major flavonoids from Teucrium polium on β-cell destruction in a model of streptozotocin-induced diabetes. Planta Med. 2009, 75, 1418–1420. [Google Scholar] [CrossRef]
- Rauter, A.P.; Martins, A.; Borges, C.; Mota-Filipe, H.; Pinto, R.M.; Sepodes, B.; Justino, J. Antihyperglycaemic and protective effects of flavonoids on streptozotocin–induced diabetic rats. Phytother. Res. 2010, 24 (Suppl. 2), S133–S138. [Google Scholar] [CrossRef] [Green Version]
- Osigwe, C.C.; Akah, P.A.; Nworu, C.S.; Okoye, F.B. Apigenin: A methanol fraction component of Newbouldia laevis leaf, as a potential antidiabetic agent. J. Phytopharm. 2017, 6, 38–44. [Google Scholar] [CrossRef]
- Bansal, P.; Paul, P.; Mudgal, J.; Nayak, P.G.; Pannakal, S.T.; Priyadarsini, K.; Unnikrishnan, M. Antidiabetic, antihyperlipidemic and antioxidant effects of the flavonoid rich fraction of Pilea microphylla (L.) in high fat diet/streptozotocin-induced diabetes in mice. Exp. Toxicol. Pathol. 2012, 64, 651–658. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Rodriguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-mediated gut microbiota modulation: Toward prebiotics and further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Ali, F.; Rahul Naz, F.; Jyoti, S.; Siddique, Y.H. Health functionality of apigenin: A review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- D’Abrosca, B.; Pacifico, S.; Scognamiglio, M.; D’Angelo, G.; Galasso, S.; Monaco, P.; Fiorentino, A. A new acylated flavone glycoside with antioxidant and radical scavenging activities from Teucrium polium leaves. Nat. Prod. Res. 2013, 27, 356–363. [Google Scholar] [CrossRef]
- Kawashty, S.; El-Din, E.G.; Saleh, N. The flavonoid chemosystematics of two Teucrium species from Southern Sinai, Egypt. Biochem. Syst. Ecol. 1999, 27, 657–660. [Google Scholar] [CrossRef]
- Esmaeili, M.A.; Sadeghi, H. Pancreatic Β-cell protective effect of rutin and apigenin isolated from Teucrium polium. Pharmacologyonline 2009, 2, 341–353. [Google Scholar]
- Hossain, C.M.; Ghosh, M.K.; Satapathy, B.S.; Dey, N.S.; Mukherjee, B. Apigenin causes biochemical modulation, GLUT4 and CD38 alterations to improve diabetes and to protect damages of some vital organs in experimental diabetes. Am. J. Pharmacol. Toxicol. 2014, 9, 39–52. [Google Scholar] [CrossRef]
- Alam, M.M.; Meerza, D.; Naseem, I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014, 109, 8. [Google Scholar] [CrossRef] [PubMed]
- Kamalakkannan, N.; Prince, P.S.M. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin. Pharmacol. Toxicol. 2006, 98, 97–103. [Google Scholar] [CrossRef]
- Youl, E.; Bardy, G.; Magous, R.; Cros, G.; Sejalon, F.; Virsolvy, A.; Richard, J.; Quignard, J.F.; Gross, R.; Petit, P.; et al. Quercetin potentiates insulin secretion and protects INS-1 pancreatic β-cells against oxidative damage via the ERK1/2 pathway. Br. J. Pharmacol. 2010, 161, 799–814. [Google Scholar] [CrossRef] [Green Version]
- Eid, H.M.; Nachar, A.; Thong, F.; Sweeney, G.; Haddad, P.S. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn. Mag. 2015, 11, 74–81. [Google Scholar]
- Walle, T.; Browning, A.M.; Steed, L.L.; Reed, S.G.; Walle, U.K. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. J. Nutr. 2005, 135, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Day, A.J.; DuPont, M.S.; Ridley, S.; Rhodes, M.; Rhodes, M.J.; Morgan, M.R.; Williamson, G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett. 1998, 436, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Spencer, J.P.; Chowrimootoo, G.; Choudhury, R.; Debnam, E.S.; Srai, S.K.; Rice-Evans, C. The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Lett. 1999, 458, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Lin, H.; Hu, M. Metabolism of flavonoids via enteric recycling: Role of intestinal disposition. J. Pharmacol. Exp. Ther. 2003, 304, 1228–1235. [Google Scholar] [CrossRef]
- Gradolatto, A.; Canivenc-Lavier, M.C.; Basly, J.P.; Siess, M.H.; Teyssier, C. Metabolism of apigenin by rat liver phase I and phase ii enzymes and by isolated perfused rat liver. Drug Metab. Dispos. 2004, 32, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Morand, C.; Crespy, V.; Demigné, C.; Texier, O.; Régérat, F.; Rémésy, C. Quercetin is recovered in human plasma as conjugated derivatives which retain antioxidant properties. FEBS Lett. 1998, 426, 331–336. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Ebeler, S.E.; Zweigenbaum, J.A.; Mitchell, A.E. UHPLC-(ESI) QTOF MS/MS profiling of quercetin metabolites in human plasma postconsumption of apple sauce enriched with apple peel and onion. J. Agric. Food Chem. 2012, 60, 8510–8520. [Google Scholar] [CrossRef]
- Bell, J.R.; Donovan, J.L.; Wong, R.; Waterhouse, A.L.; German, J.B.; Walzem, R.L.; Kasim-Karakas, S.E. (+)-Catechin in human plasma after ingestion of a single serving of reconstituted red wine. Am. J. Clin. Nutr. 2000, 71, 103–108. [Google Scholar] [CrossRef]

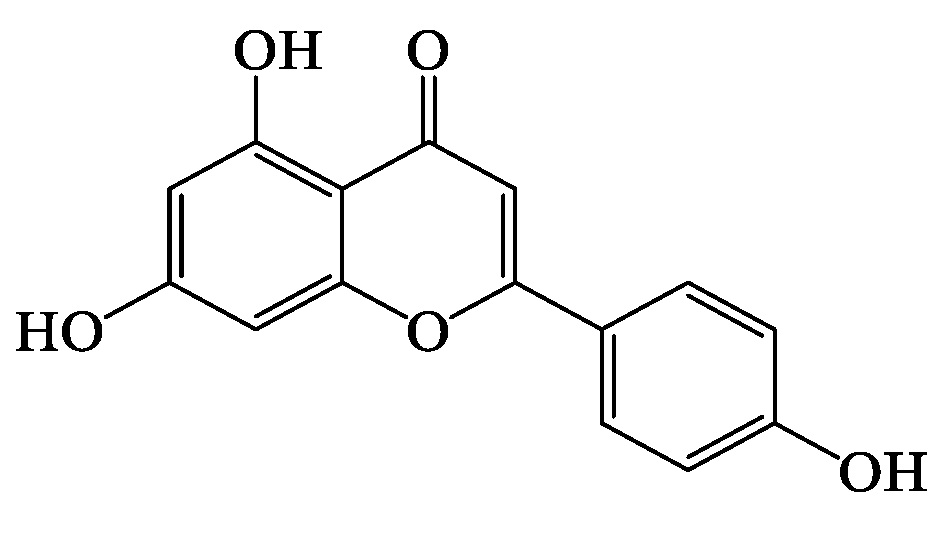
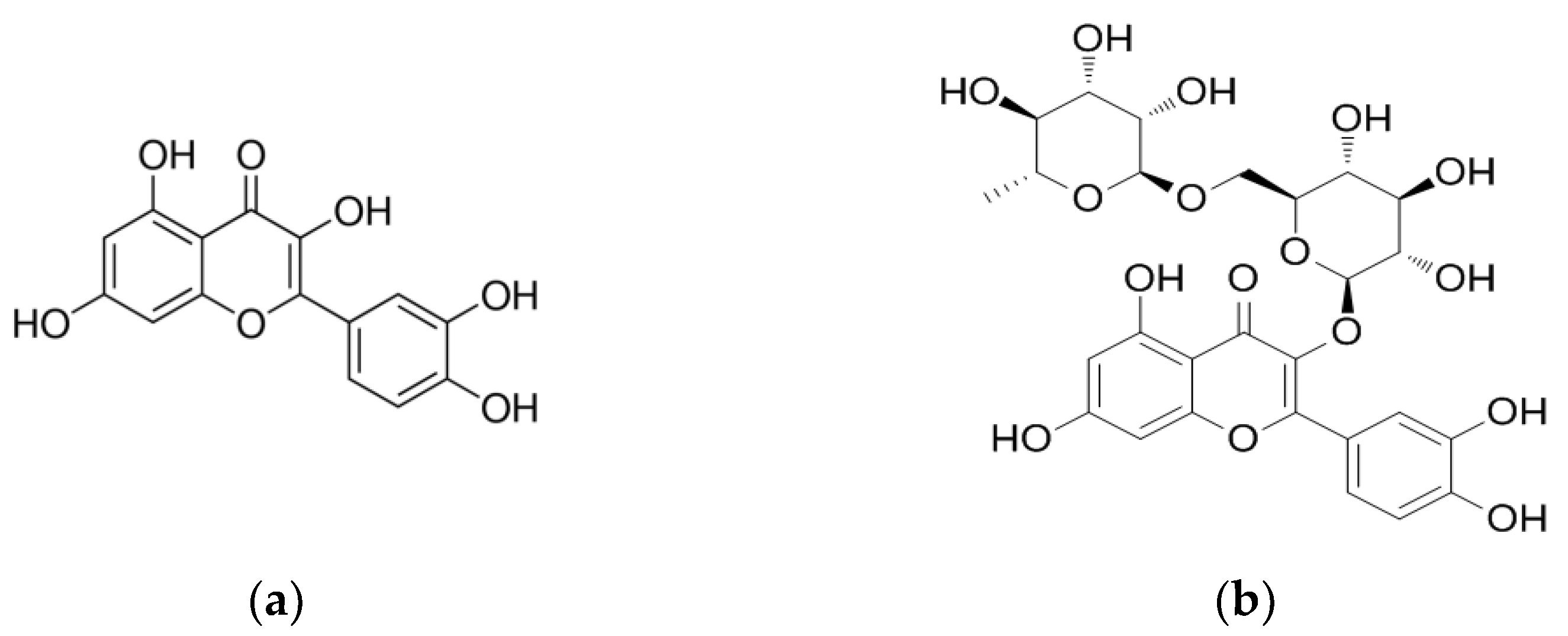
| References | Plant Name | Antidiabetic Activity |
|---|---|---|
| [46,47,48,49,50] | Teucrium polium | Increase insulin secretion |
| [51] | Tabernaemontana divaricata | Increase blood insulin and promote pancreatic β-cells regeneration in mice |
| [52] | Gymnema sylvestre | Increase insulin and pancreatic β-cells regeneration in rodent |
| [53] | Ficus deltoidea | Increase insulin secretion |
| [54] | Bidens pilosa | Increase insulin secretion |
| Reference | Design | Target | Extract | Treatment | Possible Mechanistic Role |
|---|---|---|---|---|---|
| [47] | In vitro | Pancreatic β-cells MIN6 | Aqueous | Tp at 10, 100, 1000, 10K & 25K μg/mL in 5.6 mmol/L glucose) and L-alanin (10 mmol/L) as a positive control | ↑ GSIS (25K μg/mL was most effective), mainly via Ca 2+ influx as this effect reduced when tested in calcium-free-KRH buffer (−2.5 mmol/L CaCl2) |
| [48] | In vitro | Pancreatic β-cells BRIN-BD11 | Methanol | Tp at 62, 125, 250 & 500 μg/mL in 5.5 mmol/L glucose) and Gliclazide (5, 500 μmol/L in 5.5 mmol/L glucose) as a positive control | ↑ GSIS, and glucose uptake (p < 0.05) through: ↑ GLUT2 expression, ↑ Glucokinase, ↑ ATP production, ↑ Ca 2+ influx |
| [49] | In situ | Isolated perfused rat pancreas | Methanol | Tp at 1000 μg/mL in 2.8 or 16.1 mmol/L glucose | ↑ GSIS, mainly via Ca 2+ and K + channel as this effect reduced when tested in the presence of diazoxide and verapamil. Notably, apigenin was the only bioactive constituent detected by GCMS analysis |
| [50] | In vitro | Pancreatic β-cells INS-1E | Ethanol | Tp at 5, 50, 250, 500 & 1000 μg/mL in 20 mmol/L glucose | ↑ GSIS in a dose-dependent manner (500 μg/mL was most effective; however, at 1000 μg/mL this effect was decreased |
| Reference | Target | Study Design | Dose | Positive Control | Duration | Outcome |
|---|---|---|---|---|---|---|
| [50] | Pancreatic β-cells INS-1E | Group 1: Glucose only at (20 mmol/L) Group 2: Apigenin-7-glycoside | (500 μg/mL in 20 mmol/L glucose) | - | 30 min | Significant (p < 0.05) increase in GSIS compared to 20 mmol/L glucose |
| [72] | STZ-induced (40 mg/kg i.p) diabetic rats (150–250 g) | Group 1: Normal control received saline + 5% Ethanol n = 6 Group 1: Diabetic control received saline + 5% Ethanol n = 6 Group 2: Diabetic + Apigenin n = 6 Group 3: Diabetic + Apigenin-7-glycoside n = 6 Group 4: Normal group received no treatment n = 6 | Apigenin i.p at (4 mg/kg/day) | - | 7 days | On day 7, apigenin significantly (p < 0.01) reduced blood glucose levels compared with diabetic control. No significant effect with apigenin-7-glycoside. |
| [73] | Alloxan-induced (65 mg/kg i.v) albino diabetic rats (150–250 g) | Group 1: (3% Tween 80, 5 mL/kg) as diabetic control n = 6 Groups 2–4: Apigenin n = 6 each Group 5: Glibenclamide at (5 mg/kg) n = 6 | Apigenin orally at (25, 50 and 100 mg/kg) | Glibenclamide at (5 mg/kg) | 1 day | Significant (p < 0.05) reduction in mean glucose level compared to diabetic control. At 25 mg/kg, hypoglycaemic effect of apigenin was comparable to that of Glibenclamide (p < 0.05) |
| [73] | Normoglycemic rats (150–250 g) | Fasted normoglycemic rats treated as: Group 1: (3% Tween 80, 5 mL/kg) as normal control n = 6 Group 2: Apigenin at (25 mg/kg) n = 6 Group 3: Apigenin at (50 mg/kg) n = 6 Group 4: Glibenclamide (5 mg/kg) n = 6 | Apigenin orally (25 and 50 mg/kg) Adrenaline (0.8 mg/kg i.p) injected after 2 h of treatment | Glibenclamide at (5 mg/kg) | 4 h | Significant dose-related decrease in hyperglycemia response to adrenaline compared to that of normal control (p < 0.05). The effect of apigenin at 25 mg/kg was comparable to that of Glibenclamide. A blood sample was taken at: 30, 60, 90, 120, 180 and 240 min |
| [73] | Normal and alloxan-induced (65 mg/kg i.v) albino diabetic rats (150–250 g) | Glycogen content (from skeletal muscle and liver tissues) Group 1: Normal non-diabetics rats (3% Tween 80, 5 mL/kg/day) as normal control n = not given Groups 2: Alloxan-induced diabetic rats + 3% Tween 80, 5 mL/kg/day) as diabetic controls n = 6 Group 3: Alloxan-induced diabetic rats + Apigenin at (50 mg/kg/day) n = 6 Group 4: Alloxan-induced diabetic rats + Glibenclamide (5 mg/kg/day) n = 6 | Apigenin orally at 50 mg/kg/day | Glibenclamide at (5 mg/kg) | 7 days | Apigenin gave a significant (p < 0.05) reduction in fasted glucose level compared to diabetic control. Liver and muscle glycogen content significantly (p < 0.05) increased with apigenin. |
| [81] | Isolated islets from normal and STZ-induced diabetic rats | Group 1: Normal control Group 2: Normal + Apigenin Group 3: Diabetic control Group 4: Diabetic + Apigenin | Apigenin at 50 or 75 μg/mL in 5 or 11.1 mmol/L glucose, respectively | - | 30 min to 3.5 h | Significant (p < 0.05) increase in GSIS in islets from apigenin treated STZ-diabetic rats compared to the STZ-diabetic controls. |
| [82] | STZ-induced (40 mg/kg i.p) diabetic male rats and normoglycemic rats (110–130 g) | Group 1: Normal control (0.1% v/v DMSO via i.p every other day) Group 2: STZ-diabetic control Group 3: STZ- diabetic + Apigenin Group 4: Apigenin control Group 5: Glipizide control Group 6: STZ-diabetic + Glipizide | Apigenin i.p (1.5 mg/kg) every alternate day and Glipizide orally (5 mg/kg) daily, for 28 days; starting from day 15 after the STZ injected | Glipizide orally (5 mg/kg) daily | 43 days | FBG levels measured every 7 days. Apigenin significantly (p < 0.05) decreased blood glucose levels. enhanced GLUT4 translocation, decreased CD38 expression and preserved β-cell distruction. |
| Reference | Target | Study Design | Dose | Duration | Outcome |
|---|---|---|---|---|---|
| [50] | Pancreatic β-cells INS-1E | Group 1: Glucose only (20 mmol/L) Group 2: Rutin Group 3: Quercetin | Rutin or Quercetin (500 μg/mL in 20 mmol/L glucose). | 30 min | Significant increase in GSIS compared to 20 mmol/L glucose (p < 0.05). |
| [72] | STZ-induced diabetic rat (150–250 g) at (40 mg/kg i.p) | Group 1: Diabetic control (Saline + 5% Ethanol)—n = 6 Group 2: Diabetic + Rutin—n = 6 Group 3: Normal control—n = 6 | Rutin i.p at (4 mg/kg) | 7 days | Rutin significantly (p < 0.01) reduced blood glucose levels (oral glucose tolerance test) compared to the diabetic control. |
| [81] | Isolated islets from normal and STZ-induced diabetic rats | Group 1: Normal control Group 2: Normal + Rutin Group 3: Diabetic control Group 4: Diabetic + Rutin | (50 and 75 μg/mL in 5 or 11.1 mmol/L glucose) | 30 min to 3.5 h | Significant (p < 0.05) increase in insulin secretion in rutin treated islets from STZ-induced diabetic rats compared to STZ-induced diabetic control. |
| [83] | Swiss albino mice Alloxan-induced diabetic (150 mg/kg i.p) and normal rats | Group 1: Normal (saline) control (n = 6) Group 2: Alloxan-diabetic rats (n = 6) Group 3: Alloxan-diabetic + quercetin (n = 6) For GLUT4 (Serum and tissue homogenates adipocytes and skeletal muscles) | Orally (quercetin (20 mg/kg/day) | 3 weeks | ↓ FBG (p < 0.05) ↑ Hexokinase (p < 0.05) ↓ FBPase (p < 0.05) ↓ G6Pase (p < 0.05) ↑ GLUT4 (p < 0.05) |
| [84] | STZ-induced male rats (150–180 g) at (50 mg/kg i.p) and healthy rats | Group 1: Normal control (n = 8) Group 2–4: Normal + Rutin (n = 8 each) Group 5: Diabetic control (n = 8) Group 6–8: Diabetic + Rutin (n = 8 each) | Orally (rutin at 25, 50 and 100 mg/kg), (1 mL/rat) | 45 days | Significantly decreased the plasma glucose levels by the different doses (44.36%, 50.92% and 62.73% respectively) compared to diabetic control (p < 0.05). At 100 mg/kg, rutin significantly increased plasma insulin level by 58.49% (p < 0.05). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albadr, Y.; Crowe, A.; Caccetta, R. Teucrium polium: Potential Drug Source for Type 2 Diabetes Mellitus. Biology 2022, 11, 128. https://doi.org/10.3390/biology11010128
Albadr Y, Crowe A, Caccetta R. Teucrium polium: Potential Drug Source for Type 2 Diabetes Mellitus. Biology. 2022; 11(1):128. https://doi.org/10.3390/biology11010128
Chicago/Turabian StyleAlbadr, Yaser, Andrew Crowe, and Rima Caccetta. 2022. "Teucrium polium: Potential Drug Source for Type 2 Diabetes Mellitus" Biology 11, no. 1: 128. https://doi.org/10.3390/biology11010128
APA StyleAlbadr, Y., Crowe, A., & Caccetta, R. (2022). Teucrium polium: Potential Drug Source for Type 2 Diabetes Mellitus. Biology, 11(1), 128. https://doi.org/10.3390/biology11010128







