Modeling Post-Scratching Locomotion with Two Rhythm Generators and a Shared Pattern Formation
Abstract
:Simple Summary
Abstract
1. Introduction
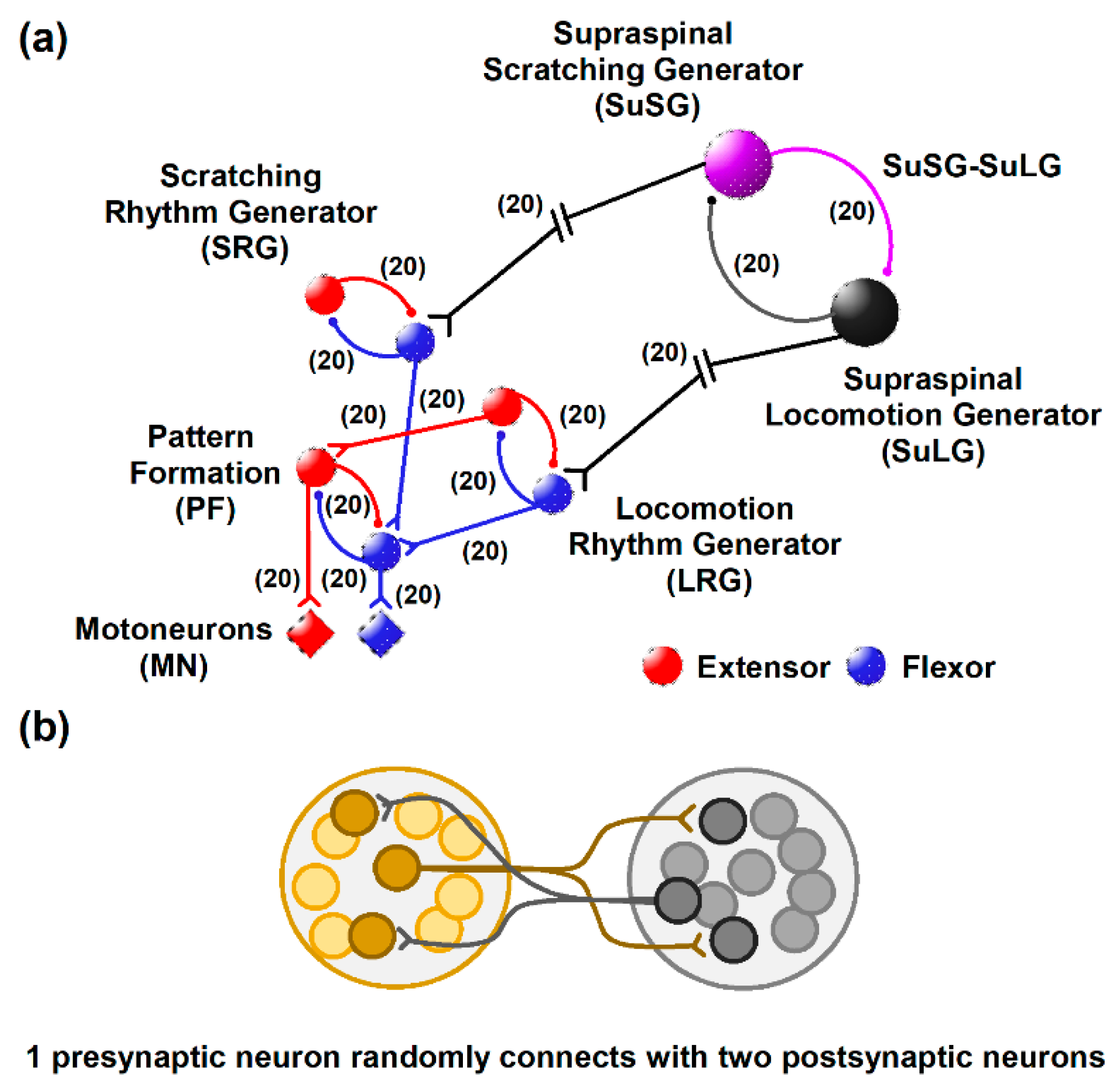
2. Materials and Methods
Model Equations
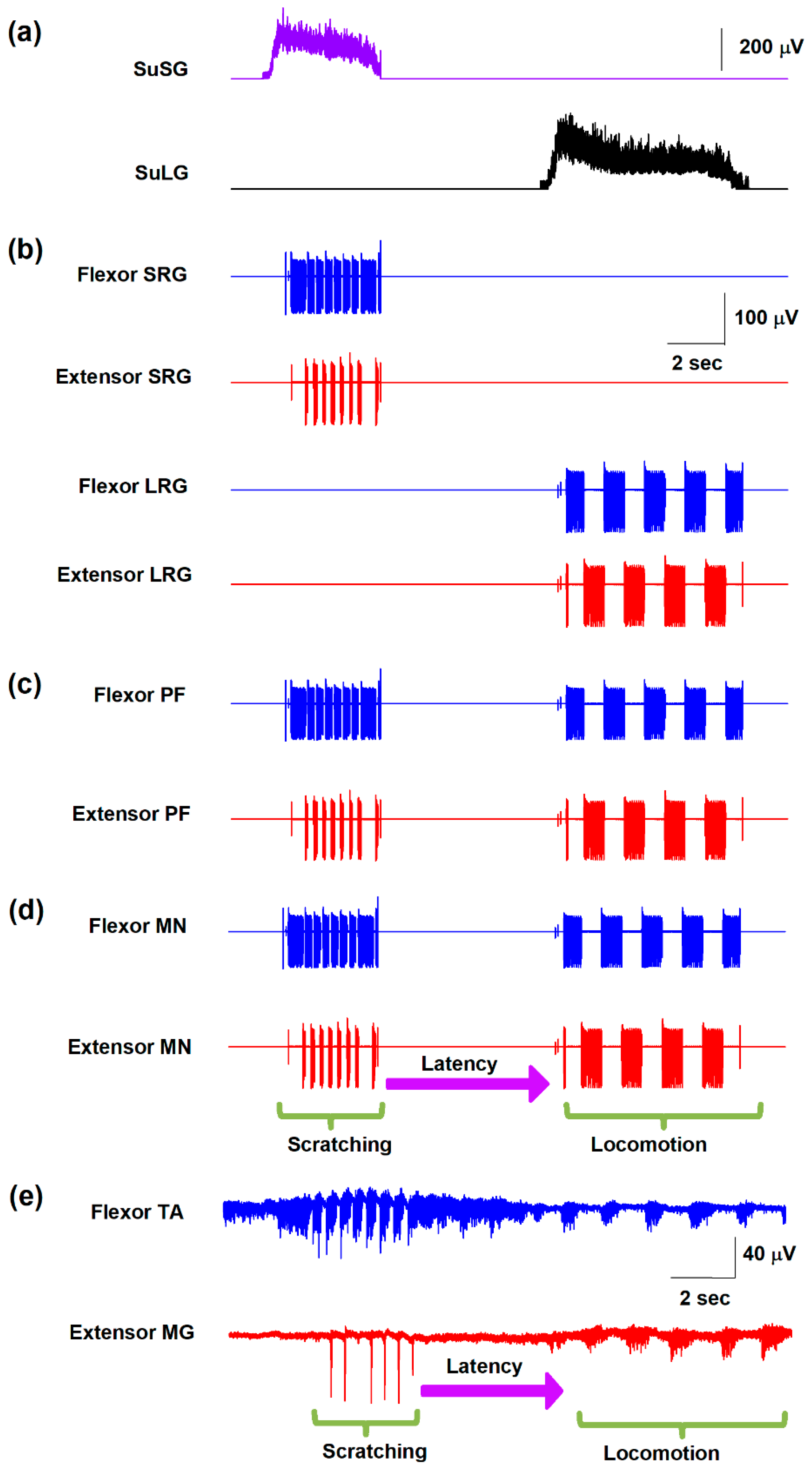
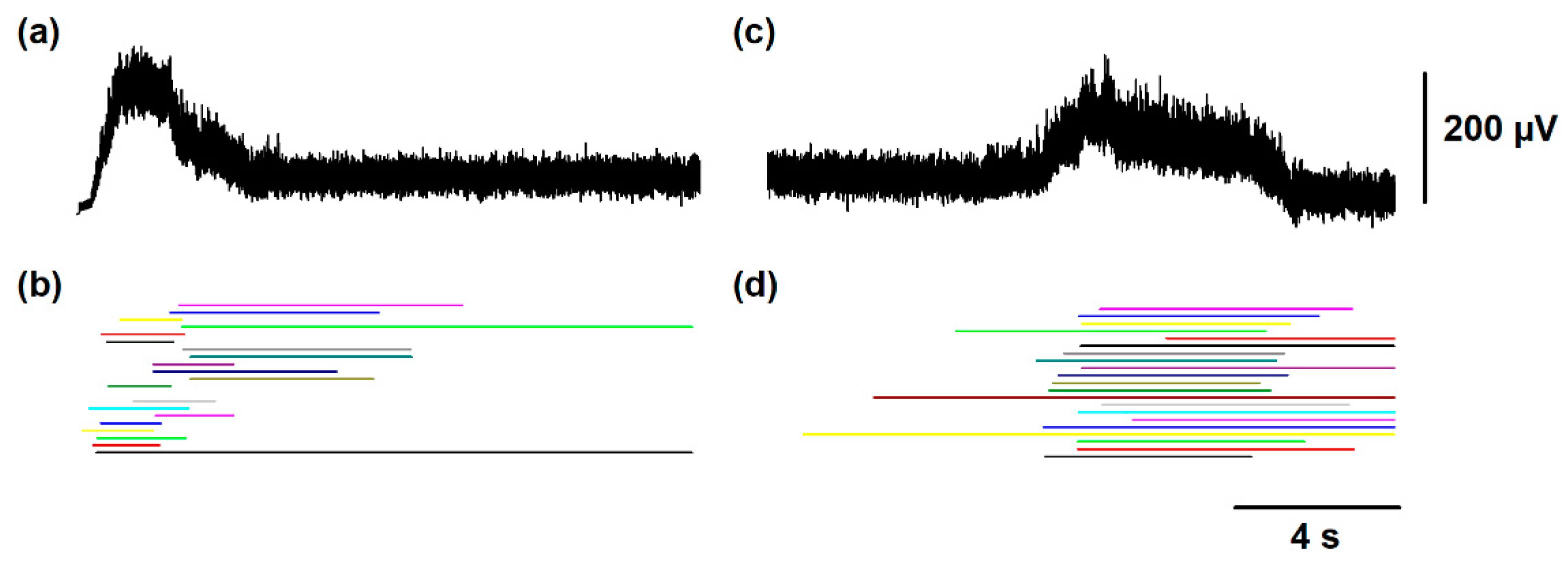
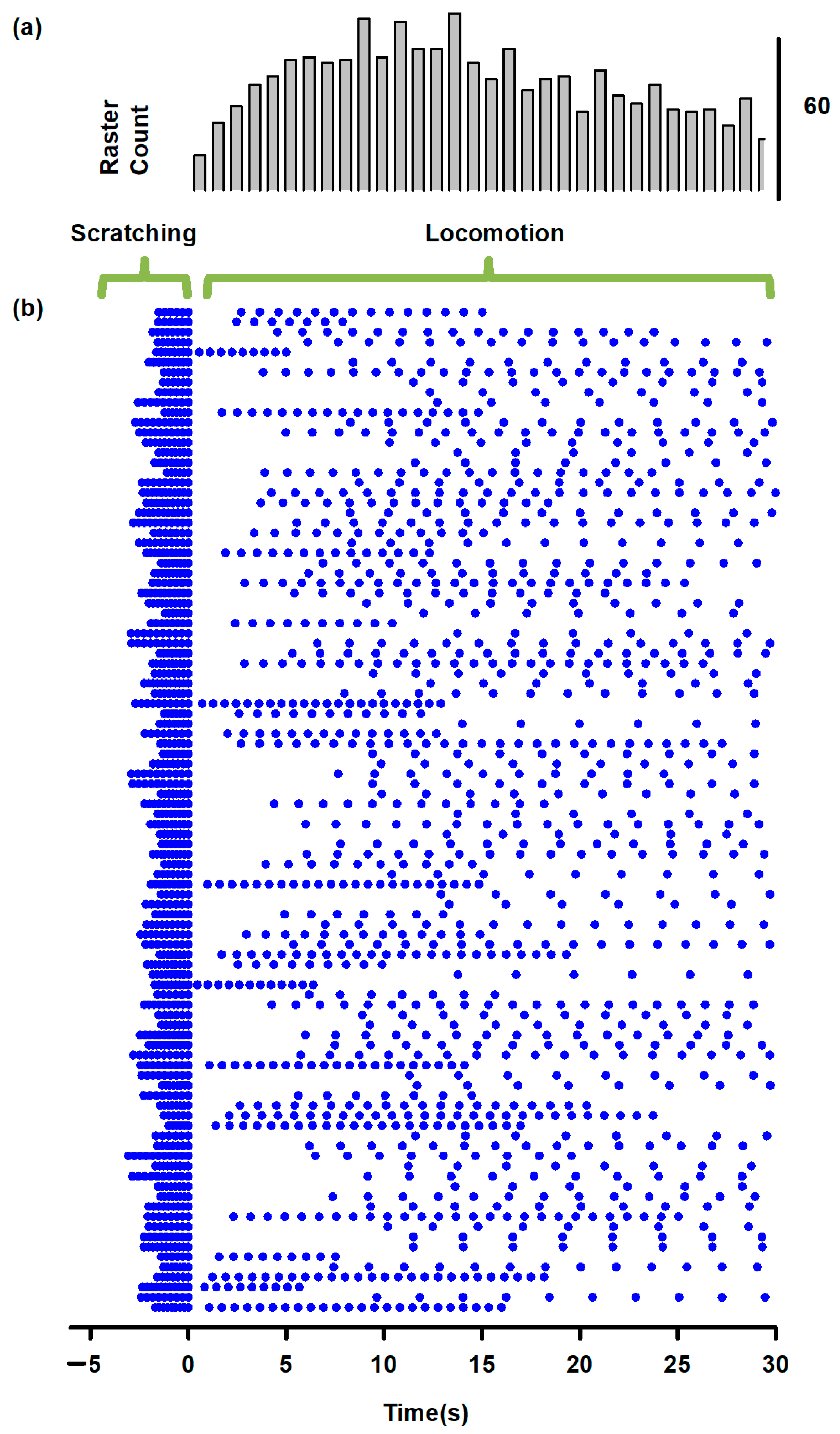
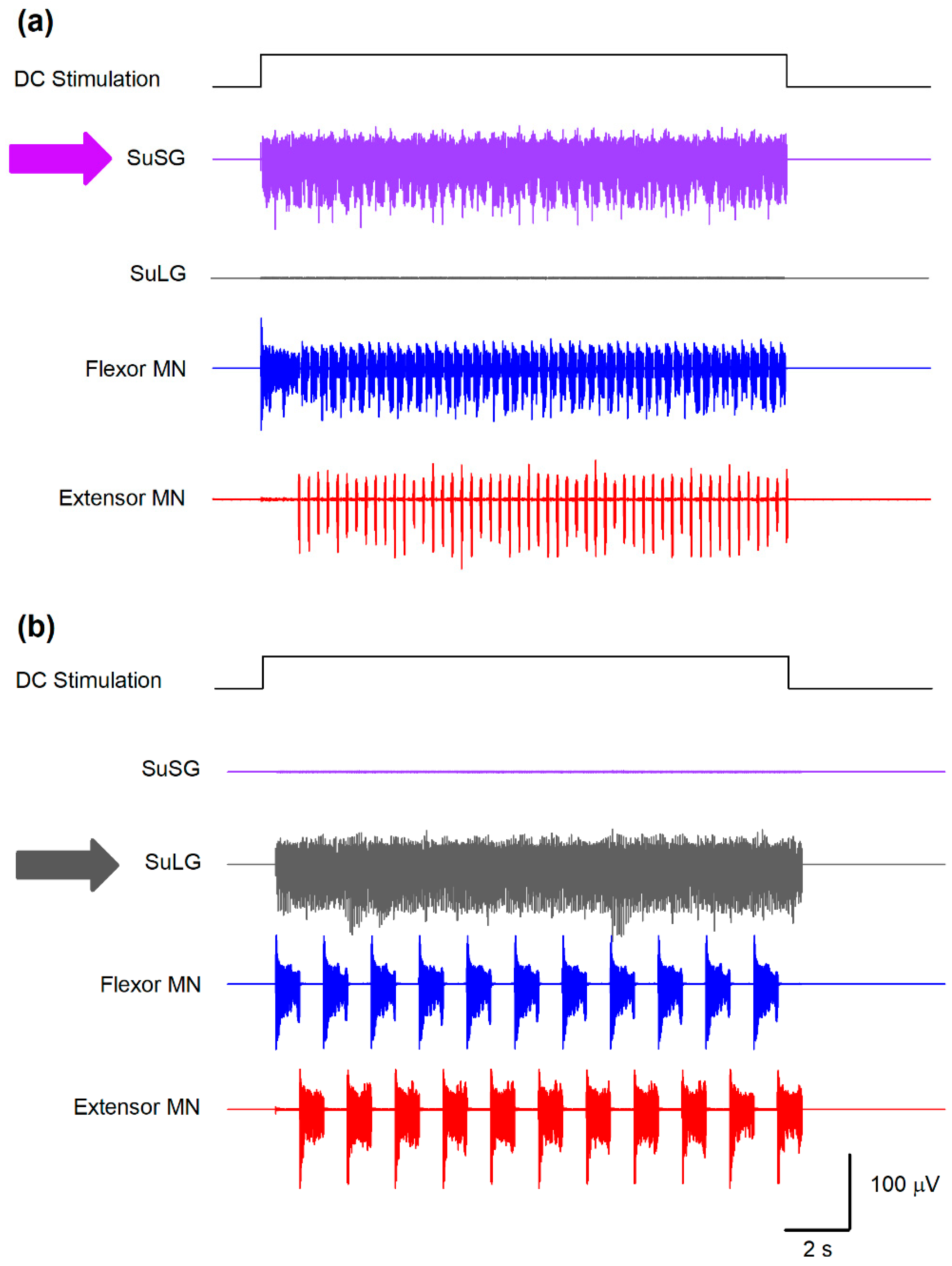
3. Results
4. Discussion
4.1. Descending Drives to Produce Different Motor Behaviors
4.2. Multifunctional Interneurons
4.3. Neuronal Topology
4.4. Predictions
4.5. Lateralization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cordo, P.; Schieppati, M.; Bevan, L.; Carlton, L.G.; Carlton, M.J. Central and Peripheral Coordination in Movement Sequences. Psychol. Res. 1993, 55, 124–130. [Google Scholar] [CrossRef]
- Rhodes, B.J.; Bullock, D.; Verwey, W.B.; Averbeck, B.B.; Page, M.P.A. Learning and Production of Movement Sequences: Behavioral, Neurophysiological, and Modeling Perspectives. Hum. Mov. Sci. 2004, 23, 699–746. [Google Scholar] [CrossRef] [Green Version]
- Kadmon Harpaz, N.; Ungarish, D.; Hatsopoulos, N.G.; Flash, T. Movement Decomposition in the Primary Motor Cortex. Cereb. Cortex 2019, 29, 1619–1633. [Google Scholar] [CrossRef] [PubMed]
- Tanneberg, D.; Ploeger, K.; Rueckert, E.; Peters, J. SKID RAW: Skill Discovery from Raw Trajectories. IEEE Robot. Autom. Lett. 2021, 6, 4696–4703. [Google Scholar] [CrossRef]
- McCrea, D.A.; Rybak, I.A. Organization of Mammalian Locomotor Rhythm and Pattern Generation. Brain Res. Rev. 2008, 57, 134–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkowitz, A.; Hao, Z.-Z. Partly Shared Spinal Cord Networks for Locomotion and Scratching. Integr. Comp. Biol. 2011, 51, 890–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkowitz, A. Both Shared and Specialized Spinal Circuitry for Scratching and Swimming in Turtles. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2002, 188, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, A. Physiology and Morphology Indicate That Individual Spinal Interneurons Contribute to Diverse Limb Movements. J. Neurophysiol. 2005, 94, 4455–4470. [Google Scholar] [CrossRef] [Green Version]
- Berkowitz, A. Physiology and Morphology of Shared and Specialized Spinal Interneurons for Locomotion and Scratching. J. Neurophysiol. 2008, 99, 2887–2901. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, A. Multifunctional and Specialized Spinal Interneurons for Turtle Limb Movements: Multifunctional and Specialized Spinal Interneurons. Ann. N. Y. Acad. Sci. 2010, 1198, 119–132. [Google Scholar] [CrossRef]
- Hao, Z.-Z.; Berkowitz, A. Shared Components of Rhythm Generation for Locomotion and Scratching Exist Prior to Motoneurons. Front. Neural Circuits 2017, 11, 54. [Google Scholar] [CrossRef] [Green Version]
- Geertsen, S.S.; Stecina, K.; Meehan, C.F.; Nielsen, J.B.; Hultborn, H. Reciprocal Ia Inhibition Contributes to Motoneuronal Hyperpolarisation during the Inactive Phase of Locomotion and Scratching in the Cat. J. Physiol. 2011, 589, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Trejo, A.; Tapia, J.A.; De la Torre Valdovinos, B.; Huidobro, N.; Flores, G.; Flores-Hernandez, J.; Flores, A.; Manjarrez, E. Transition of Pattern Generation: The Phenomenon of Post-Scratching Locomotion. Neuroscience 2015, 288, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Rinzel, J.; Ermentrout, G.B. Analysis of neural excitability and oscillations. In Methods in Neuronal Modelling: From Ions to Networks; Koch, C., Segev, I., Eds.; MIT: Cambridge, MA, USA, 1998; pp. 251–291. [Google Scholar]
- Pérez, T.; Tapia, J.A.; Mirasso, C.R.; García-Ojalvo, J.; Quevedo, J.; Cuellar, C.A.; Manjarrez, E. An Intersegmental Neuronal Architecture for Spinal Wave Propagation under Deletions. J. Neurosci. 2009, 29, 10254–10263. [Google Scholar] [CrossRef] [Green Version]
- Destexhe, A.; Mainen, Z.F.; Sejnowski, T.J. An Efficient Method for Computing Synaptic Conductances Based on a Kinetic Model of Receptor Binding. Neural Comput. 1994, 6, 14–18. [Google Scholar] [CrossRef]
- Rybak, I.A.; Shevtsova, N.A.; Lafreniere-Roula, M.; McCrea, D.A. Modelling Spinal Circuitry Involved in Locomotor Pattern Generation: Insights from Deletions during Fictive Locomotion. J. Physiol. 2006, 577, 617–639. [Google Scholar] [CrossRef] [PubMed]
- Rybak, I.A.; Stecina, K.; Shevtsova, N.A.; McCrea, D.A. Modelling Spinal Circuitry Involved in Locomotor Pattern Generation: Insights from the Effects of Afferent Stimulation: Modelling Afferent Control of Locomotor Pattern Generation. J. Physiol. 2006, 577, 641–658. [Google Scholar] [CrossRef]
- Li, W.-C.; Sautois, B.; Roberts, A.; Soffe, S.R. Reconfiguration of a Vertebrate Motor Network: Specific Neuron Recruitment and Context-Dependent Synaptic Plasticity. J. Neurosci. 2007, 27, 12267–12276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.-C.; Soffe, S.R.; Wolf, E.; Roberts, A. Persistent Responses to Brief Stimuli: Feedback Excitation among Brainstem Neurons. J. Neurosci. 2006, 26, 4026–4035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shik, M.L.; Severin, F.V.; Orlovsky, G.N. Control of Walking and Running by Means of Electrical Stimulation of the Mesencephalon. Electroencephalogr. Clin. Neurophysiol. 1969, 26, 549. [Google Scholar]
- Noga, B.R.; Kriellaars, D.J.; Jordan, L.M. The Effect of Selective Brainstem or Spinal Cord Lesions on Treadmill Locomotion Evoked by Stimulation of the Mesencephalic or Pontomedullary Locomotor Regions. J. Neurosci. 1991, 11, 1691–1700. [Google Scholar] [CrossRef] [Green Version]
- Tapia, J.A.; Trejo, A.; Linares, P.; Alva, J.M.; Kristeva, R.; Manjarrez, E. Reticular Activating System of a Central Pattern Generator: Premovement Electrical Potentials. Physiol. Rep. 2013, 1, e00129. [Google Scholar] [CrossRef]
- Cabelguen, J.-M.; Bourcier-Lucas, C.; Dubuc, R. Bimodal Locomotion Elicited by Electrical Stimulation of the Midbrain in the Salamander Notophthalmus Viridescens. J. Neurosci. 2003, 23, 2434–2439. [Google Scholar] [CrossRef] [Green Version]
- Frigon, A. Reconfiguration of the Spinal Interneuronal Network during Locomotion in Vertebrates. J. Neurophysiol. 2009, 101, 2201–2203. [Google Scholar] [CrossRef] [PubMed]
- Ijspeert, A.J.; Crespi, A.; Ryczko, D.; Cabelguen, J.-M. From Swimming to Walking with a Salamander Robot Driven by a Spinal Cord Model. Science 2007, 315, 1416–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, J.M.; Pearson, K.G. Generation of Motor Patterns for Walking and Flight in Motoneurons Supplying Bifunctional Muscles in the Locust. J. Neurobiol. 1988, 19, 257–282. [Google Scholar] [CrossRef] [PubMed]
- Ritter, D.A.; Bhatt, D.H.; Fetcho, J.R. In Vivo Imaging of Zebrafish Reveals Differences in the Spinal Networks for Escape and Swimming Movements. J. Neurosci. 2001, 21, 8956–8965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briggman, K.L.; Kristan, W.B., Jr. Imaging Dedicated and Multifunctional Neural Circuits Generating Distinct Behaviors. J. Neurosci. 2006, 26, 10925–10933. [Google Scholar] [CrossRef]
- Weimann, J.M.; Marder, E. Switching Neurons Are Integral Members of Multiple Oscillatory Networks. Curr. Biol. 1994, 4, 896–902. [Google Scholar] [CrossRef]
- Meyrand, P.; Simmers, J.; Moulins, M. Construction of a Pattern-Generating Circuit with Neurons of Different Networks. Nature 1991, 351, 60–63. [Google Scholar] [CrossRef]
- Jing, J.; Weiss, K.R. Interneuronal Basis of the Generation of Related but Distinct Motor Programs in Aplysia: Implications for Current Neuronal Models of Vertebrate Intralimb Coordination. J. Neurosci. 2002, 22, 6228–6238. [Google Scholar] [CrossRef] [Green Version]
- Popescu, I.R.; Frost, W.N. Highly Dissimilar Behaviors Mediated by a Multifunctional Network in the Marine Mollusk Tritonia Diomedea. J. Neurosci. 2002, 22, 1985–1993. [Google Scholar] [CrossRef] [Green Version]
- Mortin, L.I.; Keifer, J.; Stein, P.S. Three Forms of the Scratch Reflex in the Spinal Turtle: Movement Analyses. J. Neurophysiol. 1985, 53, 1501–1516. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, A. Broadly Tuned Spinal Neurons for Each Form of Fictive Scratching in Spinal Turtles. J. Neurophysiol. 2001, 86, 1017–1025. [Google Scholar] [CrossRef]
- Frigon, A.; Gossard, J.-P. Evidence for Specialized Rhythm-Generating Mechanisms in the Adult Mammalian Spinal Cord. J. Neurosci. 2010, 30, 7061–7071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perreault, M.C.; Enriquez-Denton, M.; Hultborn, H. Proprioceptive Control of Extensor Activity during Fictive Scratching and Weight Support Compared to Fictive Locomotion. J. Neurosci. 1999, 19, 10966–10976. [Google Scholar] [CrossRef] [PubMed]
- Radosevic, M.; Willumsen, A.; Petersen, P.C.; Lindén, H.; Vestergaard, M.; Berg, R.W. Decoupling of timescales reveals sparse convergent CPG network in the adult spinal cord. Nat Commun. 2019, 10, 2937. [Google Scholar] [CrossRef] [Green Version]
- Vallortigara, G.; Chiandetti, C.; Sovrano, V.A. Brain asymmetry (animal). Wiley Interdiscip. Rev. Cogn. Sci. 2011, 2, 146–157. [Google Scholar] [CrossRef]
- Romano, D.; Canale, A.; Benelli, G. Do right-biased boxers do it better? Population-level asymmetry of aggressive displays enhances fighting success in blowflies. Behav. Process. 2015, 113, 159–162. [Google Scholar] [CrossRef]
- Ghirlanda, S.; Frasnelli, E.; Vallortigara, G. Intraspecific competition and coordination in the evolution of lateralization. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 861–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Parameter | Value | Parameter | Value | ||
|---|---|---|---|---|---|
| gCa | 4.0 mS/cm2 | ε | SuSG-SuLG | 1.75 × 10–2 s−1 | |
| gK | 8.0 mS/cm2 | LRG | 4 × 10–4 s−1 | ||
| gL | 2.0 mS/cm2 | SRG | 1.75 × 10–3 s−1 | ||
| gKCa | 0.25 mS/cm2 | PF | 4 × 10–4 s−1 | ||
| VCa | 120 mV | MN | 4 × 10–4 s−1 | ||
| VK | −84.0 mV | µ | All other neurons | 0.2 ± 0.05 | |
| VL | −60.0 ± 6 mV | SRG_Flexor | 0.1 ± 0.01 | ||
| V1 | 1.2 mV | SRG_Extensor | 0.178 ± 0.02 | ||
| V2 | −18 mV | IApp | 45 mA/cm2 for SuSG and SuLG; 43.8 mA/cm2 for every other neuron | ||
| W1 | 12 mV | α | 0.1 ms−1 | ||
| W2 | 17.4 mV | β | 0.2 ms−1 | ||
| φ | SuSG SuLG | 0.0002 s−1 | ES | 0 mV (Excitatory); −80 mV (Inhibitory) | |
| LRG | 0.22 s−1 | gsyn | 0.1 ± 0.01 mS/cm2 | ||
| SRG | 0.23 s−1 | ||||
| All other neurons | 0.20 s−1 | Ca0 | 10 mM | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tapia, J.A.; Reid, A.; Reid, J.; Dominguez-Nicolas, S.M.; Manjarrez, E. Modeling Post-Scratching Locomotion with Two Rhythm Generators and a Shared Pattern Formation. Biology 2021, 10, 663. https://doi.org/10.3390/biology10070663
Tapia JA, Reid A, Reid J, Dominguez-Nicolas SM, Manjarrez E. Modeling Post-Scratching Locomotion with Two Rhythm Generators and a Shared Pattern Formation. Biology. 2021; 10(7):663. https://doi.org/10.3390/biology10070663
Chicago/Turabian StyleTapia, Jesus A., Argelia Reid, John Reid, Saul M. Dominguez-Nicolas, and Elias Manjarrez. 2021. "Modeling Post-Scratching Locomotion with Two Rhythm Generators and a Shared Pattern Formation" Biology 10, no. 7: 663. https://doi.org/10.3390/biology10070663






