Does an Invasive Bivalve Outperform Its Native Congener in a Heat Wave Scenario? A Laboratory Study Case with Ruditapes decussatus and R. philippinarum
Abstract
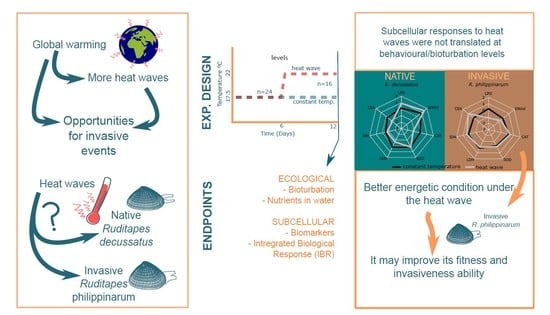
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Species and Aquaria Setup
2.2. Measurement of Particle Reworking
2.3. Determination of Biochemical Responses and Data Treatment
2.4. Statistical Analysis
3. Results
3.1. Environmental Variables
3.2. Behavioural and Ecological Responses
3.2.1. Sediment Reworking
3.2.2. Nutrients
3.3. Subcellular and Biochemical Responses
3.3.1. Oxidative Stress and Damage
3.3.2. Energy Metabolism Related Enzymes
3.3.3. Cellular Energy Allocation
3.3.4. Integrated Biological Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strayer, D.L.; Hillebrand, H. Eight questions about invasions and ecosystem functioning. Ecol. Lett. 2012, 15, 1199–1210. [Google Scholar] [CrossRef]
- Gallardo, B.; Clavero, M.; Sánchez, M.I.; Vilà, M. Global ecological impacts of invasive species in aquatic ecosystems. Glob. Chang. Biol. 2016, 22, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Zenetos, A.; Gofas, S.; Morri, C.; Rosso, A.; Violanti, D.; García Raso, J.E.; Çinar, M.E.; Almogi-Labin, A.; Ates, A.S.; Azzurro, E.; et al. Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Introduction trends and pathways. Mediterr. Mar. Sci. 2012, 13, 328. [Google Scholar] [CrossRef] [Green Version]
- Bielen, A.; Bošnjak, I.; Sepčić, K.; Jaklič, M.; Cvitanić, M.; Lušić, J.; Lajtner, J.; Simčič, T.; Hudina, S. Differences in tolerance to anthropogenic stress between invasive and native bivalves. Sci. Total Environ. 2016, 543, 449–459. [Google Scholar] [CrossRef]
- Ferreira-Rodríguez, N.; Fernández, I.; Cancela, M.L.; Pardo, I. Multibiomarker response shows how native and non-native freshwater bivalves differentially cope with heat-wave events. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 934–943. [Google Scholar] [CrossRef]
- Smale, D.A.; Wernberg, T.; Oliver, E.C.J.; Thomsen, M.; Harvey, B.P.; Straub, S.C.; Burrows, M.T.; Alexander, L.V.; Benthuysen, J.A.; Donat, M.G.; et al. Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Chang. 2019, 9, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Sorte, C.J.B.; Williams, S.L.; Zerebecki, R.A. Ocean warming increases threat of invasive species in a marine fouling community. Ecology 2010, 91, 2198–2204. [Google Scholar] [CrossRef]
- Lopes, M.L.; Rodrigues, J.P.; Crespo, D.; Dolbeth, M.; Calado, R.; Lillebø, A.I. Functional traits of a native and an invasive clam of the genus Ruditapes occurring in sympatry in a coastal lagoon. Sci. Rep. 2018, 8, 16901. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Hadly, E.A.; Bascompte, J.; Berlow, E.L.; Brown, J.H.; Fortelius, M.; Getz, W.M.; Harte, J.; Hastings, A.; Marquet, P.A.; et al. Approaching a state shift in Earth’s biosphere. Nature 2012, 486, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Strayer, D.L. Non-native species have multiple abundance–impact curves. Ecol. Evol. 2020, 10, 6833–6843. [Google Scholar] [CrossRef] [PubMed]
- Novoa, A.; Richardson, D.M.; Pyšek, P.; Meyerson, L.A.; Bacher, S.; Canavan, S.; Catford, J.A.; Čuda, J.; Essl, F.; Foxcroft, L.C.; et al. Invasion syndromes: A systematic approach for predicting biological invasions and facilitating effective management. Biol. Invasions 2020, 22, 1801–1820. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Sánchez-Benítez, A.; Barriopedro, D.; García-Herrera, R. Tracking Iberian heatwaves from a new perspective. Weather Clim. Extrem. 2020, 28, 100238. [Google Scholar] [CrossRef]
- Oliver, E.C.J.; Burrows, M.T.; Donat, M.G.; Sen Gupta, A.; Alexander, L.V.; Perkins-Kirkpatrick, S.E.; Benthuysen, J.A.; Hobday, A.J.; Holbrook, N.J.; Moore, P.J.; et al. Projected Marine Heatwaves in the 21st Century and the Potential for Ecological Impact. Front. Mar. Sci. 2019, 6, 734. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, P.G.; Raffaelli, D.; Pardal, M.A. The impact of extreme weather events on the seagrass Zostera noltii and related Hydrobia ulvae population. Mar. Pollut. Bull. 2008, 56, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Hobday, A.J.; Alexander, L.V.; Perkins, S.E.; Smale, D.A.; Straub, S.C.; Oliver, E.C.J.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Feng, M.; et al. A hierarchical approach to defining marine heatwaves. Prog. Oceanogr. 2016, 141, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Oliver, E.C.J.; Donat, M.G.; Burrows, M.T.; Moore, P.J.; Smale, D.A.; Alexander, L.V.; Benthuysen, J.A.; Feng, M.; Sen Gupta, A.; Hobday, A.J.; et al. Longer and more frequent marine heatwaves over the past century. Nat. Commun. 2018, 9, 1324. [Google Scholar] [CrossRef] [PubMed]
- Dolbeth, M.; Crespo, D.; Leston, S.; Solan, M. Realistic scenarios of environmental disturbance lead to functionally important changes in benthic species-environment interactions. Mar. Environ. Res. 2019, 150, 104770. [Google Scholar] [CrossRef]
- Zerebecki, R.A.; Sorte, C.J.B. Temperature tolerance and stress proteins as mechanisms of invasive species success. PLoS ONE 2011, 6, e014806. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, C.; Devin, S.; Mouneyrac, C.; Giambérini, L. Eco-physiological responses to salinity changes across the freshwater-marine continuum on two euryhaline bivalves: Corbicula fluminea and Scrobicularia plana. Ecol. Indic. 2017, 74, 334–342. [Google Scholar] [CrossRef]
- Lemos, M.F.L.; Soares, A.M.V.M.; Correia, A.C.; Esteves, A.C. Proteins in ecotoxicology—How, why and why not? Proteomics 2010, 10, 873–887. [Google Scholar] [CrossRef]
- Crespo, D.; Solan, M.; Leston, S.; Pardal, M.A.; Dolbeth, M. Ecological consequences of invasion across the freshwater-marine transition in a warming world. Ecol. Evol. 2018, 8, 1807–1817. [Google Scholar] [CrossRef] [Green Version]
- Carregosa, V.; Velez, C.; Soares, A.M.V.M.; Figueira, E.; Freitas, R. Physiological and biochemical responses of three Veneridae clams exposed to salinity changes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2014, 177–178, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Solan, M.; Ward, E.R.; White, E.L.; Hibberd, E.E.; Cassidy, C.; Schuster, J.M.; Hale, R.; Godbold, J.A. Worldwide measurements of bioturbation intensity, ventilation rate, and the mixing depth of marine sediments. Sci. Data 2019, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Queirós, A.M.; Hiddink, J.G.; Johnson, G.; Cabral, H.N.; Kaiser, M.J. Context dependence of marine ecosystem engineer invasion impacts on benthic ecosystem functioning. Biol. Invasions 2011, 13, 1059–1075. [Google Scholar] [CrossRef]
- Solan, M.; Wigham, B.; Hudson, I.; Kennedy, R.; Coulon, C.; Norling, K.; Nilsson, H.; Rosenberg, R. In situ quantification of bioturbation using time-lapse fluorescent sediment profile imaging (f-SPI), luminophore tracers and model simulation. Mar. Ecol. Prog. Ser. 2004, 271, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, E.; Penha-Lopes, G.; Delefosse, M.; Valdemarsen, T.; Quintana, C.O.; Banta, G.T. What is bioturbation? The need for a precise definition for fauna in aquatic sciences. Mar. Ecol. Prog. Ser. 2012, 446, 285–302. [Google Scholar] [CrossRef] [Green Version]
- Milan, M.; Pauletto, M.; Patarnello, T.; Bargelloni, L.; Marin, M.G.; Matozzo, V. Gene transcription and biomarker responses in the clam Ruditapes philippinarum after exposure to ibuprofen. Aquat. Toxicol. 2013, 126, 17–29. [Google Scholar] [CrossRef]
- Rato, L.D.; Novais, S.C.; Lemos, M.F.L.; Alves, L.M.F.; Leandro, S.M. Homarus gammarus (Crustacea: Decapoda) larvae under an ocean acidification scenario: Responses across different levels of biological organization. Comp. Biochem. Physiol. Part C 2017, 203, 29–38. [Google Scholar] [CrossRef]
- Rato, L.D.; Crespo, D.; Lemos, M.F.L. Mechanisms of bioinvasions by coastal crabs using integrative approaches—A conceptual review. Ecol. Indic. 2021, 125, 107578. [Google Scholar] [CrossRef]
- Cordero, D.; Delgado, M.; Liu, B.; Ruesink, J.; Saavedra, C. Population genetics of the Manila clam (Ruditapes philippinarum) introduced in North America and Europe. Sci. Rep. 2017, 7, 39745. [Google Scholar] [CrossRef] [PubMed]
- Dias, E.; Chainho, P.; Barrocas-Dias, C.; Adão, H. Food sources of the non-indigenous bivalve Ruditapes philippinarum (Adams and Reeve, 1850) and trophic niche overlap with native species. Aquat. Invasions 2019, 14, 638–655. [Google Scholar] [CrossRef]
- Chainho, P.; Fernandes, A.; Amorim, A.; Ávila, S.P.; Canning-Clode, J.; Castro, J.J.; Costa, A.C.; Costa, J.L.; Cruz, T.; Gollasch, S.; et al. Non-indigenous species in Portuguese coastal areas, coastal lagoons, estuaries and islands. Estuar. Coast. Shelf Sci. 2015, 167, 199–211. [Google Scholar] [CrossRef]
- Chiesa, S.; Lucentini, L.; Freitas, R.; Nonnis Marzano, F.; Breda, S.; Figueira, E.; Caill-Milly, N.; Herbert, R.J.H.; Soares, A.M.V.M.; Argese, E. A history of invasion: COI phylogeny of Manila clam Ruditapes philippinarum in Europe. Fish. Res. 2017, 186, 25–35. [Google Scholar] [CrossRef]
- European Commission EUROSTAT. Available online: https://ec.europa.eu/eurostat/web/products-datasets/-/fish_ld_main (accessed on 22 June 2020).
- De Marchi, L.; Rocha, R.J.M.; Rodrigues, A.C.M.; Soares, A.M.V.M.; Pretti, C.; Chiellini, F.; Freitas, R. Environmental fate of multistressors on carpet shell clam Ruditapes decussatus: Carbon nanoparticles and temperature variation. Sustainability 2020, 12, 4939. [Google Scholar] [CrossRef]
- Sobral, P.; Widdows, J. Effects of elevated temperatures on the scope for growth and resistance to air exposure of the clam Ruditapes decussatus (L.), from southern Portugal. Sci. Mar. 1997, 61, 163–171. [Google Scholar] [CrossRef]
- Paul-Pont, I.; de Montaudouin, X.; Gonzalez, P.; Soudant, P.; Baudrimont, M. How life history contributes to stress response in the Manila clam Ruditapes philippinarum. Environ. Sci. Pollut. Res. 2010, 17, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, H.R.; Pestana, J.L.T.; Novais, S.C.; Leston, S.; Ramos, F.; Soares, A.M.V.M.; Devreese, B.; Lemos, M.F.L. Assessment of fipronil toxicity to the freshwater midge Chironomus riparius: Molecular, biochemical, and organismal responses. Aquat. Toxicol. 2019, 216, 105292. [Google Scholar] [CrossRef]
- Paul, N.; Novais, S.C.; Silva, C.S.E.; Mendes, S.; Kunzmann, A.; Lemos, M.F.L. Global warming overrides physiological anti-predatory mechanisms in intertidal rock pool fish Gobius paganellus. Sci. Total Environ. 2021, 776, 145736. [Google Scholar] [CrossRef]
- De Coen, W.M.; Janssen, C.R. The use of biomarkers in Daphnia magna toxicity testing. IV. Cellular Energy Allocation: A new methodology to assess the energy budget of toxicant-stressed Daphnia populations. J. Aquat. Ecosyst. Stress Recover. 1997, 6, 43–55. [Google Scholar] [CrossRef]
- Calow, P. Physiological costs of combating chemical toxicants: Ecological implications. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1991, 100, 3–6. [Google Scholar] [CrossRef]
- Brey, T. A collection of empirical relations for use in ecological modelling. Naga ICLARM Q. 1999, 22, 24–28. [Google Scholar]
- Blott, S.J.; Pye, K. GRADISTAT: A grain size distribution and statistic package for the analysis of unconsolidated sediments. Earth Surf. Process. Landf. 2001, 26, 1237–1248. [Google Scholar] [CrossRef]
- Vaz, N.; Dias, J.M.; Leitão, P.; Martins, I. Horizontal patterns of water temperature and salinity in an estuarine tidal channel: Ria de Aveiro. Ocean Dyn. 2005, 55, 416–429. [Google Scholar] [CrossRef] [Green Version]
- Matias, D.; Joaquim, S.; Matias, A.M.; Moura, P.; de Sousa, J.T.; Sobral, P.; Leitão, A. The reproductive cycle of the European clam Ruditapes decussatus (L., 1758) in two Portuguese populations: Implications for management and aquaculture programs. Aquaculture 2013, 406–407, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Bueno-Pardo, J.; García-Seoane, E.; Sousa, A.I.; Coelho, J.P.; Morgado, M.; Frankenbach, S.; Ezequiel, J.; Vaz, N.; Quintino, V.; Rodrigues, A.M.; et al. Trophic web structure and ecosystem attributes of a temperate coastal lagoon (Ria de Aveiro, Portugal). Ecol. Model. 2018, 378, 13–25. [Google Scholar] [CrossRef]
- Revelle, W.R. Psych: Procedures for Personality and Psychological Research. 2020. Available online: https://personality-project.org/r/psych/psych-manual.pdf (accessed on 7 December 2020).
- R Development Core Team. R: A Language and Environment for Statistical Computing. 2020. Available online: https://www.R-project.org (accessed on 10 November 2020).
- Københavns Universitet. Limnologisk Metodik; Universite; Ferskvandsbiologisk Laboratorium, Akademisk Forlag: København, Denmark, 1992. [Google Scholar]
- Hale, R.; Mavrogordato, M.N.; Tolhurst, T.J.; Solan, M. Characterizations of how species mediate ecosystem properties require more comprehensive functional effect descriptors. Sci. Rep. 2014, 4, 6463. [Google Scholar] [CrossRef] [Green Version]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase activity. In CRC Handbook of Methods for Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ellis, G.; Goldberg, D.M. An improved manual and semi-automatic assay for NADP-dependent isocitrate dehydrogenase activity, with a description of some kinetic properties of human liver and serum enzyme. Clin. Biochem. 1971, 4, 175–185. [Google Scholar] [CrossRef]
- Lima, I.; Moreira, S.M.; Osten, J.R.-V.; Soares, A.M.V.M.; Guilhermino, L. Biochemical responses of the marine mussel Mytilus galloprovincialis to petrochemical environmental contamination along the North-western coast of Portugal. Chemosphere 2007, 66, 1230–1242. [Google Scholar] [CrossRef]
- Vassault, A. Lactate Dehydrogenase, UV-method with Pyruvate and NADH. Methods Enzym. Anal. 1983, 3, 118. [Google Scholar]
- Diamantino, T.C.; Almeida, E.; Soares, A.M.V.M.; Guilhermino, L. Lactate dehydrogenase activity as an effect criterion in toxicity tests with Daphnia magna straus. Chemosphere 2001, 45, 553–560. [Google Scholar] [CrossRef] [Green Version]
- King, F.D.; Packard, T.T. Respiration and the activity of the respiratory electron transport system in marine zooplankton. Limnol. Oceanogr. 1975, 20, 849–854. [Google Scholar] [CrossRef]
- Olive, P.L. DNA precipitation assay: A rapid and simple method for detecting DNA damage in mammalian cells. Environ. Mol. Mutagen. 1988, 11, 487–495. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- De Coen, W.M.; Janssen, C.R. The missing biomarker link: Relationships between effects on the cellular energy allocation biomarker of toxicant-stressed Daphnia magna and corresponding population characteristics. Environ. Toxicol. Chem. 2003, 22, 1632–1641. [Google Scholar] [CrossRef]
- Verslycke, T.; Ghekiere, A.; Janssen, C.R. Seasonal and spatial patterns in cellular energy allocation in the estuarine mysid Neomysis integer (Crustacea: Mysidacea) of the Scheldt estuary (The Netherlands). J. Exp. Mar. Biol. Ecol. 2004, 306, 245–267. [Google Scholar] [CrossRef]
- Catherine, T.; Vanessa, M.; Evangelia, S.; Valentina, C.; Andreja, R.; Rana, A.A.; Susana, C.; Serena, F.; Alisa, K.; Yiota, L.; et al. Biochemical biomarker responses to pollution in selected sentinel organisms across the Eastern Mediterranean and the Black Sea. Environ. Sci. Pollut. Res. 2016, 23, 1789–1804. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, W.; Burgeot, T.; Porcher, J. A novel “Integrated Biomarker Response” calculation based on reference deviation concept. Environ. Sci. Pollut. Res. 2013, 20, 2721–2725. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, D.; Solan, M.; Godbold, J.A. Species contributions to ecosystem process and function can be population dependent and modified by biotic and abiotic setting. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162805. [Google Scholar] [CrossRef]
- Murray, F.; Douglas, A.; Solan, M. Species that share traits do not necessarily form distinct and universally applicable functional effect groups. Mar. Ecol. Prog. Ser. 2014, 516, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, J.; Bates, D. Mixed-Effects Models in S and S-PLUS; Statistics and Computing; Springer: New York, NY, USA, 2000; ISBN 9780387989570. [Google Scholar]
- Zuur, A.; Ieno, E.; Walker, N.; Saveliev, A.; Smith, G. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; ISBN 9780387874579. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models; R Package Version 3.1-107. 2014. Available online: https://cran.r-project.org/web/packages/nlme/nlme.pdf (accessed on 15 November 2020).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Nerlović, V.; Korlević, M.; Mravinac, B. Morphological and molecular differences between the invasive bivalve Ruditapes philippinarum (Adams & Reeve, 1850) and the native species Ruditapes decussatus (Linnaeus, 1758) from the Northeastern Adriatic Sea. J. Shellfish. Res. 2016, 35, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Glazier, D.S. The 3/4-Power Law Is Not Universal: Evolution of Isometric, Ontogenetic Metabolic Scaling in Pelagic Animals. Bioscience 2006, 56, 325. [Google Scholar] [CrossRef] [Green Version]
- Gabbott, P. Storage cycles in marine bivalve molluscs: An hypothesis concerning the relation between glycogen and gametogenesis. In Proceedings of the Ninth European Marine Biology Symposium; Barnes, H., Ed.; Aberdeen University Press: Aberdeen, UK, 1975; pp. 191–211. [Google Scholar]
- Dridi, S.; Romdhane, M.S.; Elcafsi, M. Seasonal variation in weight and biochemical composition of the Pacific oyster, Crassostrea gigas in relation to the gametogenic cycle and environmental conditions of the Bizert lagoon, Tunisia. Aquaculture 2007, 263, 238–248. [Google Scholar] [CrossRef]
- Gabbott, P.A. Energy metabolism. In Marine Mussels: Their Ecology and Physiology; Bayne, B.L., Ed.; Cambridge University Press: Cambridge, UK, 1976; pp. 293–357. [Google Scholar]
- Matias, D.; Joaquim, S.; Leitão, A.; Massapina, C. Effect of geographic origin, temperature and timing of broodstock collection on conditioning, spawning success and larval viability of Ruditapes decussatus (Linné, 1758). Aquac. Int. 2009, 17, 257–271. [Google Scholar] [CrossRef]
- Matias, D.; Joaquim, S.; Ramos, M.; Sobral, P.; Leitão, A. Biochemical compounds’ dynamics during larval development of the carpet-shell clam Ruditapes decussatus (Linnaeus, 1758): Effects of mono-specific diets and starvation. Helgol. Mar. Res. 2011, 65, 369–379. [Google Scholar] [CrossRef] [Green Version]
- McMahon, R.F. Evolutionary and physiological adaptations of aquatic invasive animals: R selection versus resistance. Can. J. Fish. Aquat. Sci. 2002, 59, 1235–1244. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, G.E.; Somero, G.N. Interspecific variation in thermal denaturation of proteins in the congeneric mussels Mytilus trossulus and M. galloprovincialis: Evidence from the heat-shock response and protein ubiquitination. Mar. Biol. 1996, 126, 65–75. [Google Scholar] [CrossRef]
- Verbrugge, L.N.H.; Schipper, A.M.; Huijbregts, M.A.J.; Van der Velde, G.; Leuven, R.S.E.W. Sensitivity of native and non-native mollusc species to changing river water temperature and salinity. Biol. Invasions 2011, 14, 1187–1199. [Google Scholar] [CrossRef] [Green Version]
- Dukes, J.; Mooney, H. Does global change increase the success of biological invaders? Trends Ecol. Evol. 1999, 14, 135–139. [Google Scholar] [CrossRef]
- Verberk, W.C.E.P.; Leuven, R.S.E.W.; van der Velde, G.; Gabel, F. Thermal limits in native and alien freshwater peracarid Crustacea: The role of habitat use and oxygen limitation. Funct. Ecol. 2018, 32, 926–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahel, F.J. Biogeographic barriers, connectivity and homogenization of freshwater faunas: It’s a small world after all. Freshw. Biol. 2007, 52, 696–710. [Google Scholar] [CrossRef]
- Crespo, D.; Leston, S.; Martinho, F.; Pardal, M.A.; Dolbeth, M. Survival of Corbicula fluminea (Müller, 1774) in a natural salinity and temperature gradient: A field experiment in a temperate estuary. Hydrobiologia 2017, 784, 337–347. [Google Scholar] [CrossRef]
 constant temperature;
constant temperature;  heat wave; n = 20.
heat wave; n = 20.
 constant temperature;
constant temperature;  heat wave; n = 20.
heat wave; n = 20.
 constant temperature;
constant temperature;  heat wave; n = 20.
heat wave; n = 20.
 constant temperature;
constant temperature;  heat wave; n = 20.
heat wave; n = 20.
 constant temperature;
constant temperature;  heat wave.
heat wave.
 constant temperature;
constant temperature;  heat wave.
heat wave.
 constant temperature;
constant temperature;  heat wave.
heat wave.
 constant temperature;
constant temperature;  heat wave.
heat wave.
 constant temperature;
constant temperature;  heat wave.
heat wave.
 constant temperature;
constant temperature;  heat wave.
heat wave.
 constant temperature;
constant temperature;  heat wave.
heat wave.
 constant temperature;
constant temperature;  heat wave.
heat wave.

| Salinity | Temperature (°C) | pH | Dissolved O2 (mg L−1) | ||
|---|---|---|---|---|---|
| Before D6 | Control | 28.14 ± 0.02 | 17.31 ± 0.07 | 8.08 ± 0.04 | 9.43 ± 0.08 |
| R. decussatus: CT | 28.19 ± 0.02 | 17.41 ± 0.04 | 8.06 ± 0.03 | 9.27 ± 0.06 | |
| R. decussatus: HW | 28.18 ± 0.03 | 17.42 ± 0.06 | 8.07 ± 0.06 | 9.42 ± 0.05 | |
| R. philippinarum: CT | 28.17 ± 0.01 | 17.23 ± 0.04 | 8.08 ± 0.02 | 9.37 ± 0.05 | |
| R. philippinarum: HW | 28.19 ± 0.03 | 17.29 ± 0.06 | 8.10 ± 0.03 | 9.46 ± 0.05 | |
| After D6 | Control | 28.41 ± 0.06 | 18.18 ± 0.10 | 8.32 ± 0.01 | 9.41 ± 0.03 |
| R. decussatus: CT | 28.53 ± 0.03 | 18.15 ± 0.09 | 8.29 ± 0.02 | 9.39 ± 0.07 | |
| R. decussatus: HW | 28.70 ± 0.09 | 21.90 ± 0.19 | 8.27 ± 0.04 | 8.64 ± 0.14 | |
| R. philippinarum: CT | 28.35 ± 0.04 | 18.03 ± 0.08 | 8.24 ± 0.01 | 9.27 ± 0.09 | |
| R. philippinarum: HW | 28.71 ± 0.13 | 22.35 ± 0.20 | 8.24 ± 0.02 | 8.27 ± 0.13 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crespo, D.; Leston, S.; Rato, L.D.; Martinho, F.; Novais, S.C.; Pardal, M.A.; Lemos, M.F.L. Does an Invasive Bivalve Outperform Its Native Congener in a Heat Wave Scenario? A Laboratory Study Case with Ruditapes decussatus and R. philippinarum. Biology 2021, 10, 1284. https://doi.org/10.3390/biology10121284
Crespo D, Leston S, Rato LD, Martinho F, Novais SC, Pardal MA, Lemos MFL. Does an Invasive Bivalve Outperform Its Native Congener in a Heat Wave Scenario? A Laboratory Study Case with Ruditapes decussatus and R. philippinarum. Biology. 2021; 10(12):1284. https://doi.org/10.3390/biology10121284
Chicago/Turabian StyleCrespo, Daniel, Sara Leston, Lénia D. Rato, Filipe Martinho, Sara C. Novais, Miguel A. Pardal, and Marco F. L. Lemos. 2021. "Does an Invasive Bivalve Outperform Its Native Congener in a Heat Wave Scenario? A Laboratory Study Case with Ruditapes decussatus and R. philippinarum" Biology 10, no. 12: 1284. https://doi.org/10.3390/biology10121284