Nitrogen and Sulfur Additions Improved the Diversity of nirK- and nirS-Type Denitrifying Bacterial Communities of Farmland Soil
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. Soil Sampling and Physicochemical Analysis
2.3. Microbial DNA Extraction, nirK, and nirS Genes Amplification and Sequencing
2.4. Statistical Analysis
3. Results
3.1. Physico-Chemical Analysis of Soil Samples
3.2. Alpha-Diversity Indices of nirS- and nirK-Type Denitrifying Communities
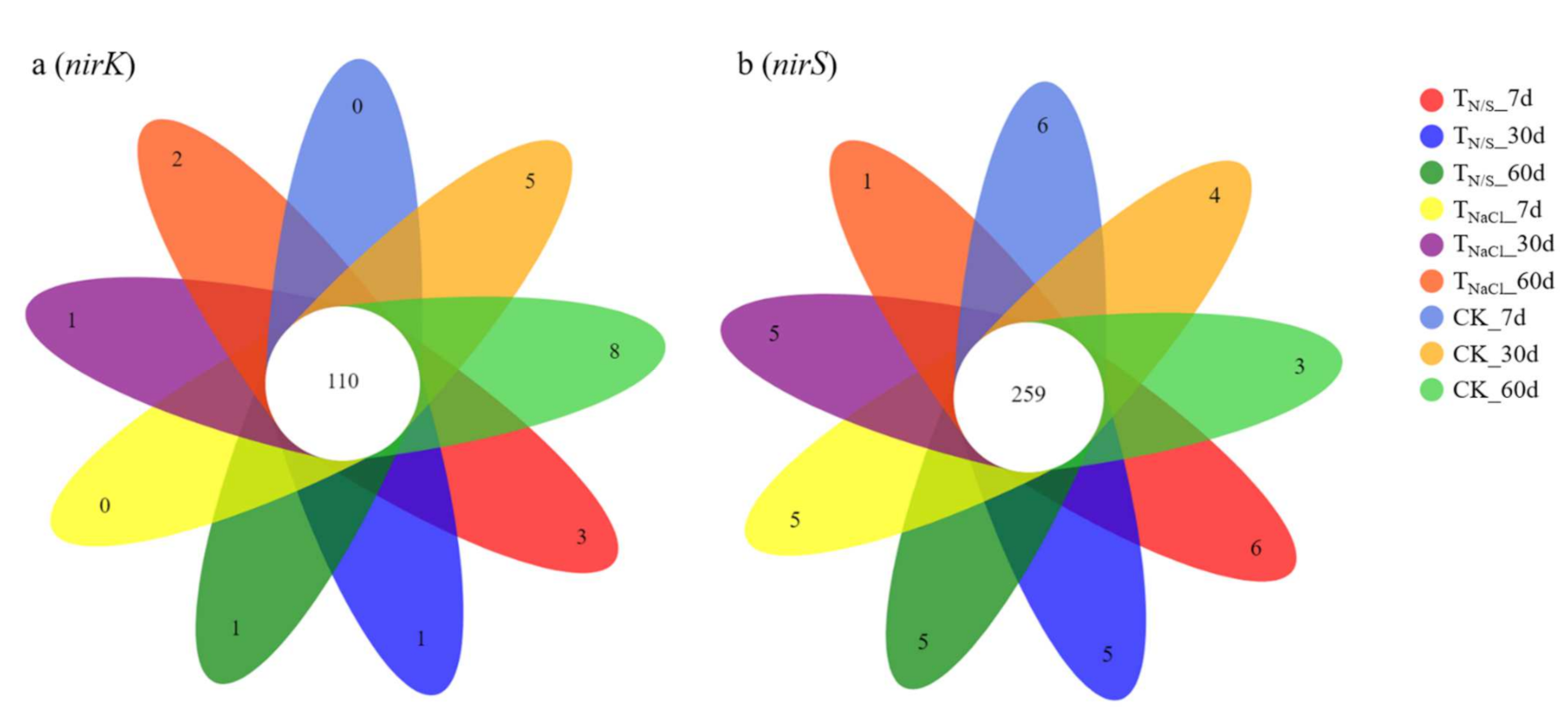
3.3. Beta Diversity of nirS- and nirK-Type Denitrifying Microorganisms
3.4. Enzyme Activities of Soil Samples
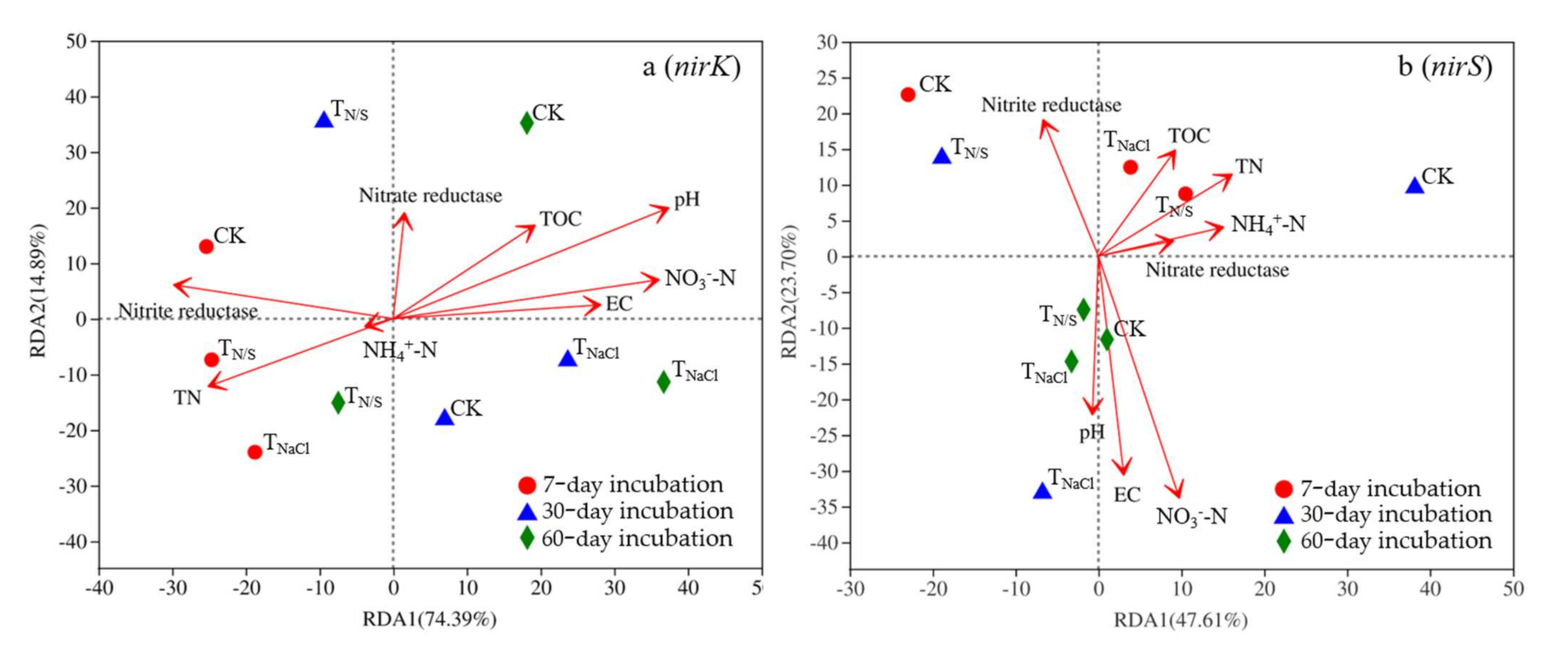
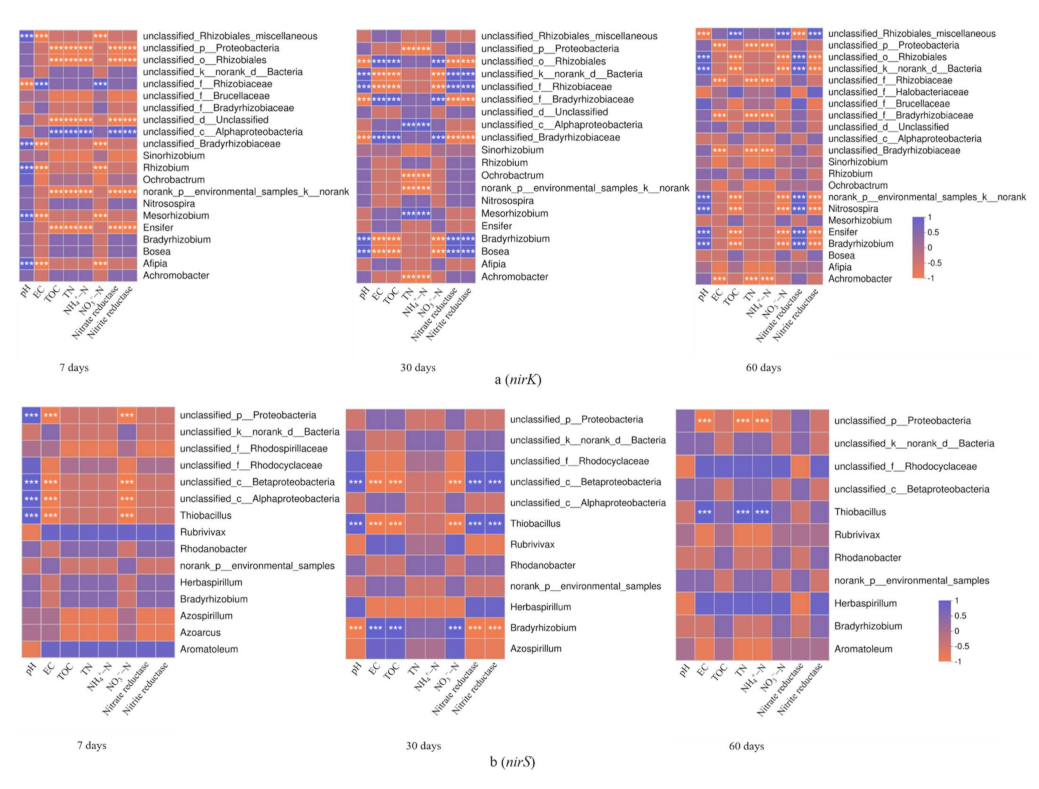
3.5. Correlations between Functional Genes and Soil Properties
4. Discussion
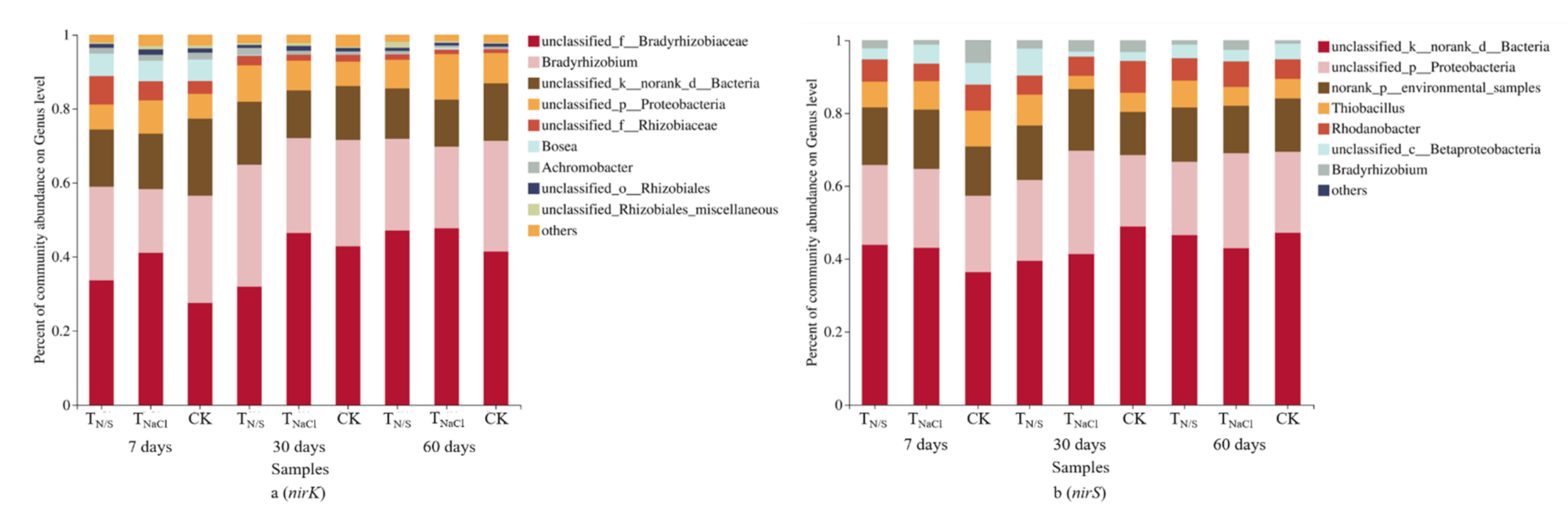
4.1. Impact of N and S Additions on the Community Structure and Diversity of Soil nirK- and nirS-Type Denitrifying Microorganisms
4.2. Impact of Treatment Duration on the Diversity of Soil nirK- and nirS-Type Denitrifying Microorganisms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability statement
Acknowledgments
Conflicts of Interest
References
- Kaiser, J. Environmental Policy: The Other Global Pollutant: Nitrogen Proves Tough to Curb. Science 2001, 294, 1268–1269. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.; Howarth, R.; Seitzinger, S.P.; Asner, G.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen Cycles: Past, Present, and Future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Galloway, J.N.; Cowling, E.B. Reactive Nitrogen and the World: 200 Years of Change. Ambio 2002, 31, 64–71. [Google Scholar] [CrossRef]
- Liu, X.; Duan, L.; Mo, J.; Du, E.; Shen, J.; Lu, X.; Zhang, Y.; Zhou, X.; He, C.; Zhang, F. Nitrogen Deposition and its Ecological Impact in China: An overview. Environ. Pollut. 2011, 159, 2251–2264. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced Nitrogen Deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Ju, X.; Chang, J.; Ge, Y.; Vitousek, P.M. Integrated Reactive Nitrogen Budgets and Future Trends in China. Proc. Natl. Acad. Sci. USA 2015, 112, 8792–8797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.; Kuang, F.; Skiba, U.; Zhu, B.; Liu, X.; Levy, P.; Dore, A.; Fowler, D. Bulk Deposition of Organic and Inorganic Nitrogen in Southwest China from 2008 to 2013. Environ. Pollut. 2017, 227, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.A.; Bleeker, A. Environmental Science: The Shape of Nitrogen to come. Nature 2013, 494, 435–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, C.J.; Dise, N.B.; Mountford, J.O.; Gowing, D.J. Impact of Nitrogen Deposition on the Species Richness of Grasslands. Science 2004, 303, 1876–1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, C.M.; Tilman, D. Loss of Plant Species after Chronic Low-Level Nitrogen Deposition to Prairie Grasslands. Nature 2008, 451, 712–715. [Google Scholar] [CrossRef]
- Asner, G.P.; Townsend, A.R.; Riley, W.J.; Matson, P.A.; Neff, J.C.; Cleveland, C.C. Physical and Biogeochemical Controls over Terrestrial Ecosystem Responses to Nitrogen Deposition. Biogeochemistry 2001, 54, 1–39. [Google Scholar] [CrossRef]
- Matson, P.; Lohse, K.A.; Hall, S.J. The Globalization of Nitrogen Deposition: Consequences for Terrestrial Ecosystems. Ambio 2002, 31, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, Q.; Ma, W.; Chen, L. Atmospheric Deposition of Inorganic Nitrogen to the Eastern China Seas and its Implications to Marine Biogeochemistry. J. Geophys. Res. Space Phys. 2010, 115. [Google Scholar] [CrossRef]
- Hobbs, W.O.; Lafrancois, B.M.; Stottlemyer, R.; Toczydlowski, D.; Engstrom, D.R.; Edlund, M.B.; Almendinger, J.E.; Strock, K.E.; VanderMeulen, D.; Elias, J.E.; et al. Nitrogen Deposition to Lakes in National Parks of the Western Great Lakes Region: Isotopic Signatures, Watershed Retention, and Algal Shifts. Glob. Biogeochem. Cycles 2016, 30, 514–533. [Google Scholar] [CrossRef] [Green Version]
- Pakeman, R.J.; Alexander, J.; Brooker, R.; Cummins, R.; Fielding, D.; Gore, S.; Hewison, R.; Mitchell, R.; Moore, E.; Orford, K.; et al. Long-term Impacts of Nitrogen Deposition on Coastal Plant Communities. Environ. Pollut. 2016, 212, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The Global Nitrogen Cycle in the Twenty-First Century. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130164. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, A.F.; Van Vuuren, D.P.; Derwent, R.G.; Posch, M. A Global Analysis of Acidification and Eutrophication of Terrestrial Ecosystems. Water Air Soil Pollut. 2002, 141, 349–382. [Google Scholar] [CrossRef]
- Bowman, W.D.; Cleveland, C.C.; Halada, L.; Hreško, J.; Baron, J.S. Negative Impact of Nitrogen Deposition on Soil Buffering Capacity. Nat. Geosci. 2008, 1, 767–770. [Google Scholar] [CrossRef]
- Ding, S.Y.; She, J.Y.; Yang, Q.P.; Wang, S.L. Effects of Thinning and Pruning on Soil Microbial Biomass Carbon and Soil Enzyme Activities in Chinese Fir Plantation. J. Cent. South Univ. For. Technol. 2015, 35, 75–79. [Google Scholar]
- Kazda, M. Indications of Unbalanced Nitrogen Nutrition of Norway Spruce Stands. Plant Soil 1990, 128, 97–101. [Google Scholar] [CrossRef]
- Lovett, G.M.; Reiners, W.A.; Olson, R.K. Cloud Droplet Deposition in Subalpine Balsam Fir Forests: Hydrological and Chemical Inputs. Science 1982, 218, 1303–1304. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, R.T.; Liu, Y.N.; Xu, N.; Ni, H.W. Influence of Simulation Nitrogen Deposition on Soil Microbial Functional Diversity of Calamagrostis angustifolia Wetland in Sanjiang Plain. Acta Agrestia Sin. 2016, 24, 1226–1233. [Google Scholar]
- Fierer, N.; Lauber, C.L.; Ramirez, K.; Zaneveld, J.; Bradford, M.; Knight, R. Comparative Metagenomic, Phylogenetic and Physiological Analyses of Soil Microbial Communities across Nitrogen Gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, K.S.; Lauber, C.L.; Knight, R.; Bradford, M.; Fierer, N. Consistent Effects of Nitrogen Fertilization on Soil Bacterial Communities in Contrasting Systems. Ecology 2010, 91, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Cederlund, H.; Wessén, E.; Enwall, K.; Jones, C.; Juhanson, J.; Pell, M.; Philippot, L.; Hallin, S. Soil Carbon Quality and Nitrogen Fertilization Structure Bacterial Communities with Predictable Responses of Major Bacterial Phyla. Appl. Soil Ecol. 2014, 84, 62–68. [Google Scholar] [CrossRef]
- Yu, P.Y.; Zhu, F.; Wang, Z.Y.; Yan, W.D.; Su, S.F.; Li, T.P. Effects of Nitrogen Addition on Metabolic Function of Microbial Community in Red Soil of Cinnamomum camphora Forest. J. Cent. South Univ. For. Technol. 2013, 33, 70–74. [Google Scholar]
- Wu, S.Q.; Wang, C.Z.; Li, M.S. On Soil Functional Diversity of Native Coastal Wetland under Simulated Nitrogen Deposition. Soils 2017, 49, 1153–1158. [Google Scholar]
- Zhang, A.; Olatunji, O.A.; Tariq, A.; Li, T.; Wang, R.; Jiang, Y. Sulfur Deposition Changed the Community Structure of Soil Nematodes by Affecting Omnivores-Predators. Sci. Total Environ. 2021, 771, 144912. [Google Scholar] [CrossRef]
- Piertri, J.C.A.; Brookes, P.C. Relationships between Soil pH and Microbial Properties in a UK Arable Soil. Soil Biol. Biochem. 2008, 40, 1856–1861. [Google Scholar]
- Guo, L.L.; Yuan, Z.G.; Zhu, W.W.; Yi, Z.X.; Tu, N.M. Research Progress in Soil Acidification Effect on Soil Biological Characteristics. Hunan Agric. Sci. 2014, 24, 30–32. [Google Scholar]
- Wang, F.G.; Song, L.; Feng, Y.; Hong, Y.C.; Cui, D.J.; Yuan, Y.B. Characteristics of Soil Microbiology in Different Planting-Life Orchard Acid Soils. Chin. J. Soil Sci. 2011, 42, 46–50. [Google Scholar]
- Sierra, J.; Noel, C.; Dufour, L.; Ozier-Lafontaine, H.; Welcker, C.; Desfontaines, L. Mineral Nutrition and Growth of Tropical Maize as Affected by Soil Acidity. Plant Soil 2003, 252, 215–226. [Google Scholar] [CrossRef]
- Wang, X.T.; Lan, X.F.; An, W.L.; Xu, X.P.; Wang, W.Q. Effect of Simulated Acid Rain on Paddy Soil Bacterial Abundance and Diversity in Fuzhou Plain. China Environ. Sci. 2019, 39, 1237–1244. [Google Scholar]
- Wang, H.T.; Zheng, T.L.; Yang, X.R. Molecular Ecology Research Progress for Soil Denitrification and Research Status for its Incluencing Factors. J. Agro-Environ. Sci. 2013, 32, 1915–1924. [Google Scholar]
- Senbayram, M.; Budai, A.; Bol, R.; Chadwick, D.; Marton, L.; Gündogan, R.; Wu, D. Soil NO3− Level and O2 Availability are Key Factors in Controlling N2O Reduction to N2 following Long-Term Liming of an Acidic Sandy Soil. Soil Biol. Biochem. 2019, 132, 165–173. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.J.; Li, J.H.; Cao, R.; Chen, M.K.; Li, S.H. Response of Soil Readily Oxidizable Carbon to Community Succession of Xishuangbanna Tropical Forests. Acta Ecol. Sin. 2019, 39, 6257–6263. [Google Scholar]
- Zumft, W.G. Cell biology and Molecular Basis of Denitrification. Microbiol. Mol. Biol. Rev. 1997, 61, 533–616. [Google Scholar] [PubMed]
- Ding, B.J.; Zhang, H.; Luo, W.Q.; Sun, S.Y.; Cheng, F.; Li, Z.K. Nitrogen Loss Through Denitrification, Anammox and Feammox in a Paddy Soil. Sci. Total Environ. 2021, 773, 145601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lin, X.-G.; Yin, R. Advances in Functional Gene Diversity of Microorganism in Relation to Soil Nitrogen Cycling. Chin. J. Eco-Agric. 2009, 17, 1029–1034. [Google Scholar] [CrossRef]
- Marcel, M.M.K.; Hannah, K.M.; Boran, K. The Microbial Nitrogen-Cycling Network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar]
- Ding, H.; Wang, Y.S. Influence of Herbicides on Denitrification Loss of Nitrogen Fertilizer and Emission of N2O. Chin. Environ. Sci. 2004, 24, 596–599. [Google Scholar]
- Zhang, Y.S.; Ding, H.; Qin, S.J. Progress in the Studies of Nitrogen Denitrification and N2O Emission in Agroecosystem. Chin. Agric. Sci. Bull. 2010, 26, 253–259. [Google Scholar]
- Jones, C.M.; Hallin, S. Ecological and Evolutionary Factors Underlying Global and Local Assembly of Denitrifier Communities. ISME J. 2010, 4, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Penton, C.R.; Johnson, T.A.; Quensen, J.F.; Iwai, S.; Cole, J.R.; Tiedje, J.M. Functional Fenes to Assess Nitrogen Cycling and Aromatic Hydrocarbon Degradation: Primers and Processing Matter. Front. Microbiol. 2013, 4, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braker, G.; Zhou, J.; Wu, L.; Devol, A.H.; Tiedje, J.M. Nitrite Reductase Genes (nirK and nirS) as Functional Markers to Investigate Diversity of Denitrifying Bacteria in Pacific Northwest Marine Sediment Communities. Appl. Environ. Microbiol. 2000, 66, 2096–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Zhang, X.; Li, D.; Wang, H.; Chen, F.; Fu, X.; Fang, X.; Sun, X.; Yu, G. Impacts of Nitrogen and Phosphorus Additions on the Abundance and Community Structure of Ammonia Oxidizers and Denitrifying Bacteria in Chinese Fir Plantations. Soil Biol. Biochem. 2016, 103, 284–293. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, X.; Hu, R.; Wu, M.; Wu, J.; Wei, W. Impact of Long-Term Fertilization on the Composition of Denitrifier Communities Based on Nitrite Reductase Analyses in a Paddy Soil. Microb. Ecol. 2010, 60, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Bárta, J.; Melichová, T.; Vaněk, D.; Picek, T.; Šantrůčková, H. Effect of pH and Dissolved Organic Matter on the Abundance of nirK and nirS Denitrifiers in Spruce Forest Soil. Biogeochemistry 2010, 101, 123–132. [Google Scholar] [CrossRef]
- Szukics, U.; Hackl, E.; Zechmeister-Boltenstern, S.; Sessitsch, A. Contrasting Response of Two Forest Soils to Nitrogen Input: Rapidly Altered NO and N2O Emissions and nirK Abundance. Biol. Fertil. Soils 2009, 45, 855–863. [Google Scholar] [CrossRef]
- Zhongjun, J.; Angel, R.; Veraart, A.; Daebeler, A.; Jia, Z.; Kim, S.Y.; Kerckhof, F.-M.; Boon, N.; Bodelier, P. Biotic Interactions in Microbial Communities as Modulators of Biogeochemical Processes: Methanotrophy as a Model System. Front. Microbiol. 2016, 7, 1285. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Zeng, J.; Zhao, D.; Yong, B.; Yu, Z. Co-Association of Two nir Denitrifiers under the Influence of Emergent Macrophytes. Microb. Ecol. 2020, 80, 809–821. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.-H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular Ecological Network Analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef] [Green Version]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using Network Analysis to Explore Co-Occurrence Patterns in Soil Microbial Communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.F.; Liu, L.L. Study on Increasing Resistance of Rice with Salicylic Acid. Guangdong Sci. Technol. News 2016, 2, 4–15. [Google Scholar]
- Luo, G.B. FAO World Soil Legend Classification System Revision. Soil Prog. 1988, 6, 22–27. [Google Scholar]
- Bao, S. Soil Agro-Chemistrical Analysis, 1st ed.; China Agriculture Press: Beijing, China, 2000; pp. 1–495. [Google Scholar]
- Di, H.J.; Cameron, K.; Podolyan, A.; Robinson, A. Effect of Soil Moisture Status and a Nitrification Inhibitor, Dicyandiamide, on Ammonia Oxidizer and Denitrifier Growth and Nitrous Oxide Emissions in a Grassland Soil. Soil Biol. Biochem. 2014, 73, 59–68. [Google Scholar] [CrossRef]
- Li, Z.G.; Luo, Y.M.; Teng, E. Soil and Environmental Microbiological Research, 1st ed.; Science Press: Beijing, China, 2008; pp. 50–100. [Google Scholar]
- Caporaso, J.C.; Christian, L.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-High-Throughput Microbial Community Analysis on the lllumina HiSeq and MiSeq Platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Ai, G.; Zhou, W.; Liang, G.; He, P. Structure and assembly cues for rhizospheric nirK- and nirS-type denitrifier communities in long-term fertilized soils. Soil Biol. Biochem. 2018, 119, 32–40. [Google Scholar] [CrossRef]
- Liu, C.H.; Wu, D.M.; Liu, Y.H.; Chen, H.; Shen, B.G.; Jang, Z.K.; Liu, X.F. Effects of Nitrogen Deposition on Soil Organic Carbon and Soil Microbial Communities in a Natural Castanopsis Carlesii Forest. For. Res. 2021, 34, 42–49. [Google Scholar]
- Campbell, B.J.; Polson, S.; Hanson, T.; Mack, M.C.; Schuur, E.A.G. The Effect of Nutrient Deposition on Bacterial Communities in Arctic Tundra Soil. Environ. Microbiol. 2010, 12, 1842–1854. [Google Scholar] [CrossRef]
- Herold, M.B.; Giles, M.E.; Alexander, C.J.; Baggs, E.M.; Daniell, T.J. Variable Response of nirK and nirS Containing Denitrifier Communities to Long-Term pH Manipulation and Cultivation. FEMS Microbiol. Lett. 2018, 365, fny035. [Google Scholar] [CrossRef]
- Ouyang, Y.; Evans, S.E.; Friesen, M.; Tiemann, L.K. Effect of Nitrogen Fertilization on the Abundance of Nitrogen Cycling Genes in Agricultural Soils: A Meta-Analysis of Field Studies. Soil Biol. Biochem. 2018, 127, 71–78. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, J.; Jiang, Y.; Hu, Y.; Zhang, M.; Zeng, Z. Response of Bacteria Harboring nirS and nirK Genes to Different N Fertilization Rates in an Alkaline Northern Chinese Soil. Eur. J. Soil Biol. 2017, 82, 1–9. [Google Scholar] [CrossRef]
- Xie, Z.; LE Roux, X.; Wang, C.; Gu, Z.; An, M.; Nan, H.; Chen, B.; Li, F.; Liu, Y.; Du, G.; et al. Identifying Response Groups of Soil Nitrifiers and Denitrifiers to Grazing and Associated Soil Environmental Drivers in Tibetan Alpine Meadows. Soil Biol. Biochem. 2014, 77, 89–99. [Google Scholar] [CrossRef]
- Fan, K.; Delgado-Baquerizo, M.; Guo, X.; Wang, D.; Wu, Y.; Zhu, M.; Yu, W.; Yao, H.; Zhu, Y.-G.; Chu, H. Suppressed N Fixation and Diazotrophs after Four Decades of Fertilization. Microbiome 2019, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, H.; Wu, B.K.; Yu, F.; Liu, Z.Y.; Jin, T. Effects of Integrated Rice-Crayfish Farming System on Community Structure and Diversity of nirK Denitrification Microbe in Paddy Soils. Chin. J. Eco-Agric. 2018, 26, 1324–1332. [Google Scholar]
- Bremer, C.; Braker, G.; Matthies, D.; Reuter, A.; Engels, C.; Conrad, R. Impact of Plant Functional Group, Plant Species, and Sampling Time on the Composition of nirK-type Denitrifier Communities in Soil. Appl. Environ. Microbiol. 2007, 73, 6876–6884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Wu, J.; Zhou, T.; Liang, Y.; Zheng, L.X.; Sun, Y.X. Effects of Copper and Florfenicol on nirS- and nirK-type Denitrifier Communities and Related Antibiotic Resistance in Vegetable Soils. Ecotoxicol. Environ. Saf. 2021, 213, 112011. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wei, Q.; Zhang, Y.; Li, H.; Jiang, Y.; Zheng, J. Nitrogen Removal from Ammonium- and Sulfate-Rich Wastewater in an Upflow Anaerobic Sludge Bed Reactor: Performance and Microbial Community Structure. Ecotoxicology 2021, 30, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Enwall, K.; Philippot, L.; Hallin, S. Activity and Composition of the Denitrifying Bacterial Community Respond Differently to Long-Term Fertilization. Appl. Environ. Microbiol. 2005, 71, 8335–8343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, S.L.; Dandie, C.E.; Patten, C.L.; Zebarth, B.J.; Burton, D.L.; Trevors, J.T.; Goyer, C. Changes in Denitrifier Abundance, Denitrification Gene mRNA Levels, Nitrous Oxide Emissions, and Denitrification in Anoxic Soil Microcosms Amended with Glucose and Plant Residues. Appl. Environ. Microbiol. 2010, 76, 2155–2164. [Google Scholar] [CrossRef] [Green Version]
- Yin, C.; Fan, F.; Song, A.; Cui, P.; Li, T.; Liang, Y. Denitrification Potential under Different Fertilization Regimes is Closely Coupled with Changes in the Denitrifying Community in a Black Soil. Appl. Microbiol. Biotechnol. 2015, 99, 5719–5729. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Zhang, B.; Hu, F.; Qiao, J.; Zhang, W.J. Carbon and Nitrogen Mineralization of Paddy Soils as Affected by Wetting and Drying. Soils 2008, 40, 554–560. [Google Scholar]
- Tang, H.; Shen, J.L.; Zhang, Y.Z.; Liu, J.Y.; Wang, C. Effect of Rice Straw Incorporation and Water Management on Soil Microbial Biomass Carbon, Nitrogen and Dissolved Organic Carbon, Nitrogen in a Rice Paddy Field. J. Soil Water Conserv. 2013, 27, 240–246. [Google Scholar]
- Karasu, A.; Dogan, M.Z. The Effect of Bacterial Inoculation and Different Nitrogen Dose on Yield and Yield Components of Some Chickpea Genotypes (Cicer arietinum L.). Afr. J. Biotechnol. 2009, 8, 59–64. [Google Scholar]
- Rinklebe, J.; Langer, U. Microbial Diversity in Three Flood Plain Soils at the Elbe River (Germany). Soil Biol. Biochem. 2006, 38, 2144–2151. [Google Scholar] [CrossRef]
- Chen, Z.; Hou, H.; Zheng, Y.; Qin, H.; Zhu, Y.; Wu, J.; Wei, W. Influence of Fertilization Regimes on a nosZ-Containing Denitrifying Community in a Rice Paddy Soil. J. Sci. Food Agr. 2012, 92, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Wakelin, S.; Liang, Y.; Hu, B.; Chu, G. Nitrous Oxide Emission and Denitrifier Communities in Drip-Irrigated Calcareous Soil as Affected by Chemical and Organic Fertilizers. Sci. Total Environ. 2018, 612, 739–749. [Google Scholar] [CrossRef]
- Delmont, T.O.; Francioli, D.; Jacquesson, S.; Laoudi, S.; Mathieu, A.; Nesme, J.; Ceccherini, M.T.; Nannipieri, P.; Simonet, P.; Vogel, T.M. Microbial Community Development and Unseen Diversity Recovery in Inoculated Sterile Soil. Biol. Fertil. Soils 2014, 50, 1069–1076. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.; Lu, M.; Qin, C.; Chen, Y.; Yang, L.; Huang, Q.; Wang, J.; Shen, Z.; Shen, Q. Microbial Communities of an Arable Soil Treated For 8 Years with Organic and Inorganic Fertilizers. Biol. Fertil. Soils 2016, 52, 455–467. [Google Scholar] [CrossRef]
- Fang, L.-N.; Yang, X.-D.; Du, J. Effects of Land Use Pattern on Soil Microbial Biomass Carbon in Xishuangbanna. J. Appl. Ecol. 2011, 22, 837–844. [Google Scholar]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-Analysis of the Impacts of Global Change Factors on Soil Microbial Diversity and Functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef] [PubMed]
- Enwall, K.; Throbäck, I.N.; Stenberg, M.; Söderström, M.; Hallin, S. Soil Resources Influence Spatial Patterns of Denitrifying Communities at Scales Compatible with Land Management. Appl. Environ. Microb. 2010, 76, 2243–2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Days | Treatments | pH | EC (μS·cm−1) | TN (g·kg−1) | TOC (g·kg−1) | NO3−-N (mg·kg−1) | NH4+-N (mg·kg−1) |
|---|---|---|---|---|---|---|---|
| 7 | TN/S | 5.9 + 0.0 b | 115 + 8.9 a | 1.14 + 0.28 a | 28 + 0.6 a | 28 + 0.6 a | 11 + 0.2 a |
| TNaCl | 6.2 + 0.0 a | 82 + 4.6 b | 1.03 + 0.02 b | 20 + 0.5 b | 17 + 0.1 b | 7 + 0.3 b | |
| CK | 6.2 + 0.1 a | 70 + 3.5 b | 1.05 + 0.04 b | 20 + 0.8 b | 16 + 0.4 c | 7 + 0.2 b | |
| 30 | TN/S | 6.8 + 0.1 a | 128 + 7.5 a | 1.09 + 0.07 a | 22 + 0.3 a | 44 + 0.7 a | 14 + 0.6 a |
| TNaCl | 6.7 + 0.1 a | 137 + 3.6 a | 1.11 + 0.08 a | 22 + 0.7 a | 45 + 0.9 a | 14 + 0.5 a | |
| CK | 6.8 + 0.1 a | 130 + 7.5 a | 1.15 + 0.09 a | 22 + 0.4 a | 45 + 0.6 a | 15 + 0.7 a | |
| 60 | TN/S | 6.3 + 0.0 b | 266 + 2.1 a | 1.14 + 0.02 a | 23 + 0.6 a | 51 + 0.8 a | 6 + 0.1 a |
| TNaCl | 6.8 + 0.0 a | 220 + 8.5 b | 0.90 + 0.02 b | 17 + 0.4 b | 48 + 0.6 b | 5 + 0.1 b | |
| CK | 6.9 + 0.1 a | 224 + 8.1 b | 0.96 + 0.03 c | 17 + 0.1 b | 48 + 0.5 b | 5 + 0.0 b |
| Gene Types | Days | Sample | Shannon | Chao 1 |
|---|---|---|---|---|
| nirK | 7 | TN/S | 2.77 ± 0.01 a | 246 ± 1.8 a |
| TNaCl | 2.56 ± 0.00 c | 219 ± 4.4 c | ||
| CK | 2.73 ± 0.00 b | 237 ± 5.4 b | ||
| 30 | TN/S | 3.38 ± 0.01 a | 274 ± 8.5 a | |
| TNaCl | 3.07 ± 0.01 b | 242 ± 10.8 b | ||
| CK | 3.03 ± 0.01 c | 238 ± 8.4 b | ||
| 60 | TN/S | 3.07 ± 0.01 a | 262 ± 3.0 a | |
| TNaCl | 2.79 ± 0.01 c | 254 ± 5.3 b | ||
| CK | 3.03 ± 0.01 b | 228 ± 2.7 c | ||
| nirS | 7 | TN/S | 4.49 ± 0.01 b | 619 ± 4.8 a |
| TNaCl | 4.34 ± 0.00 c | 620 ± 9.6 a | ||
| CK | 4.51 ± 0.01 a | 620 ± 12.4 a | ||
| 30 | TN/S | 4.60 ± 0.01 a | 619 ± 9.2 a | |
| TNaCl | 4.61 ± 0.01 a | 623 ± 13.1 a | ||
| CK | 4.55 ± 0.00 b | 619 ± 7.2 a | ||
| 60 | TN/S | 4.53 ± 0.01 a | 602 ± 11.2 b | |
| TNaCl | 4.54 ± 0.01 a | 596 ± 7.3 b | ||
| CK | 4.51 ± 0.00 b | 615 ± 7.7 a |
| Enzymes | Treatments | 7 days | 30 days | 60 days |
|---|---|---|---|---|
| Nitrate reductase | TN/S | 0.54 + 0.02 a | 0.54 + 0.00 a | 0.54 + 0.01 a |
| TNaCl | 0.49 + 0.01 b | 0.51 + 0.00 b | 0.51 + 0.01 b | |
| CK | 0.50 + 0.02 b | 0.51 + 0.01 b | 0.51 + 0.01 b | |
| Nitrite reductase | TN/S | 1.53 + 0.00 a | 1.53 + 0.01 a | 1.39 + 0.01 a |
| TNaCl | 1.14 + 0.01 b | 1.12 + 0.00 b | 1.03 + 0.00 b | |
| CK | 1.11 + 0.01 c | 1.12 + 0.01 b | 1.01 + 0.01 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wei, H.; Zhang, J. Nitrogen and Sulfur Additions Improved the Diversity of nirK- and nirS-Type Denitrifying Bacterial Communities of Farmland Soil. Biology 2021, 10, 1191. https://doi.org/10.3390/biology10111191
Chen X, Wei H, Zhang J. Nitrogen and Sulfur Additions Improved the Diversity of nirK- and nirS-Type Denitrifying Bacterial Communities of Farmland Soil. Biology. 2021; 10(11):1191. https://doi.org/10.3390/biology10111191
Chicago/Turabian StyleChen, Xuan, Hui Wei, and Jiaen Zhang. 2021. "Nitrogen and Sulfur Additions Improved the Diversity of nirK- and nirS-Type Denitrifying Bacterial Communities of Farmland Soil" Biology 10, no. 11: 1191. https://doi.org/10.3390/biology10111191





