Sex Associated Effects of Noise Pollution in Stone Sculpin (Paracottus knerii) as a Model Object in the Context of Human-Induced Rapid Environmental Change
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Noise Exposure
2.3. Ethical Standards and Tissue Collection
2.4. Blood Parameters
2.5. Inner Ear Analysis
2.6. Telomere Length and Telomerase Activity Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Noise-Induced and Sex-Dependent Changes in Stone Sculpin Blood
3.2. Noise Causes Saccular Hair Cell Loss in Both Male and Female Stone Sculpins
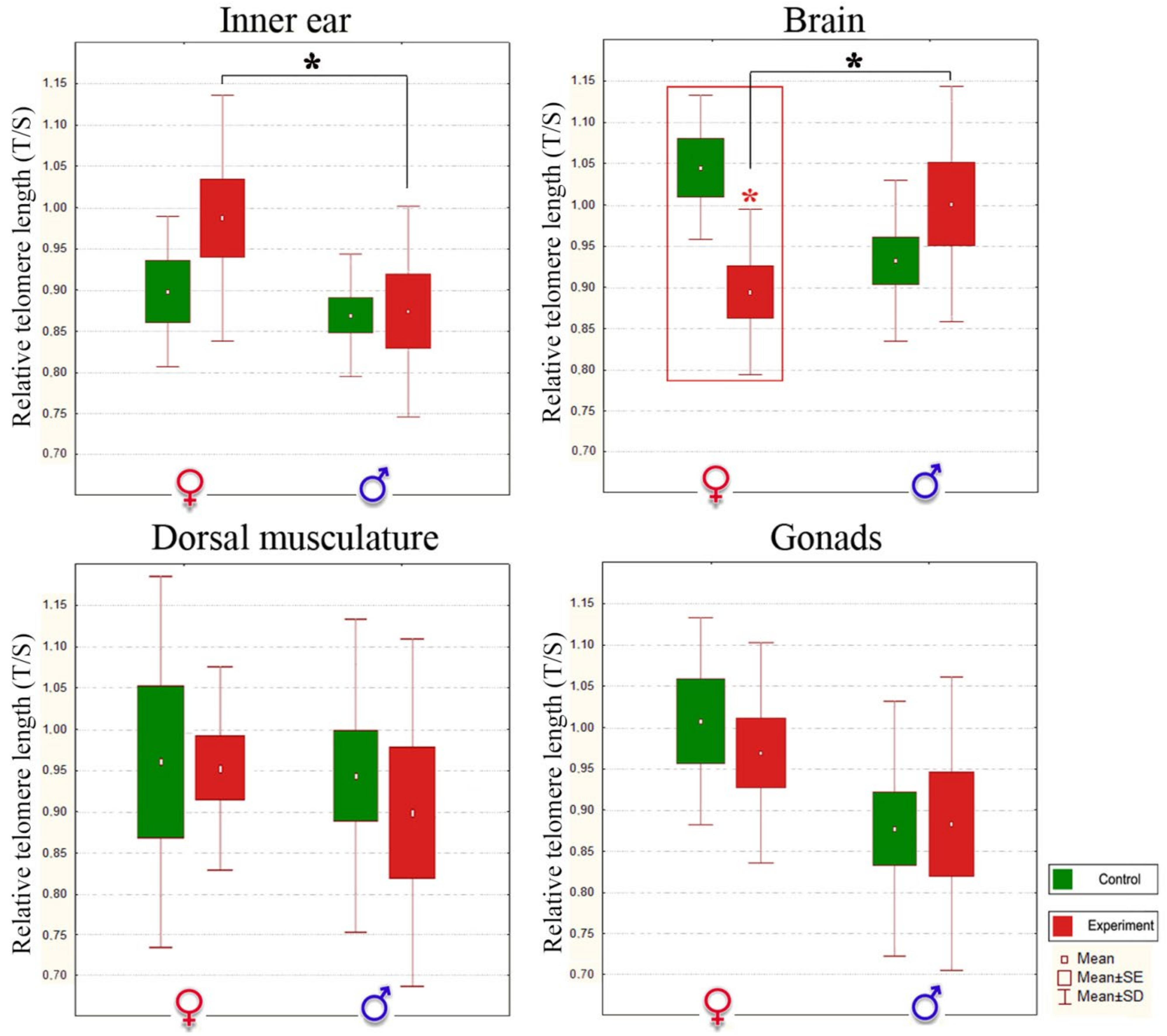
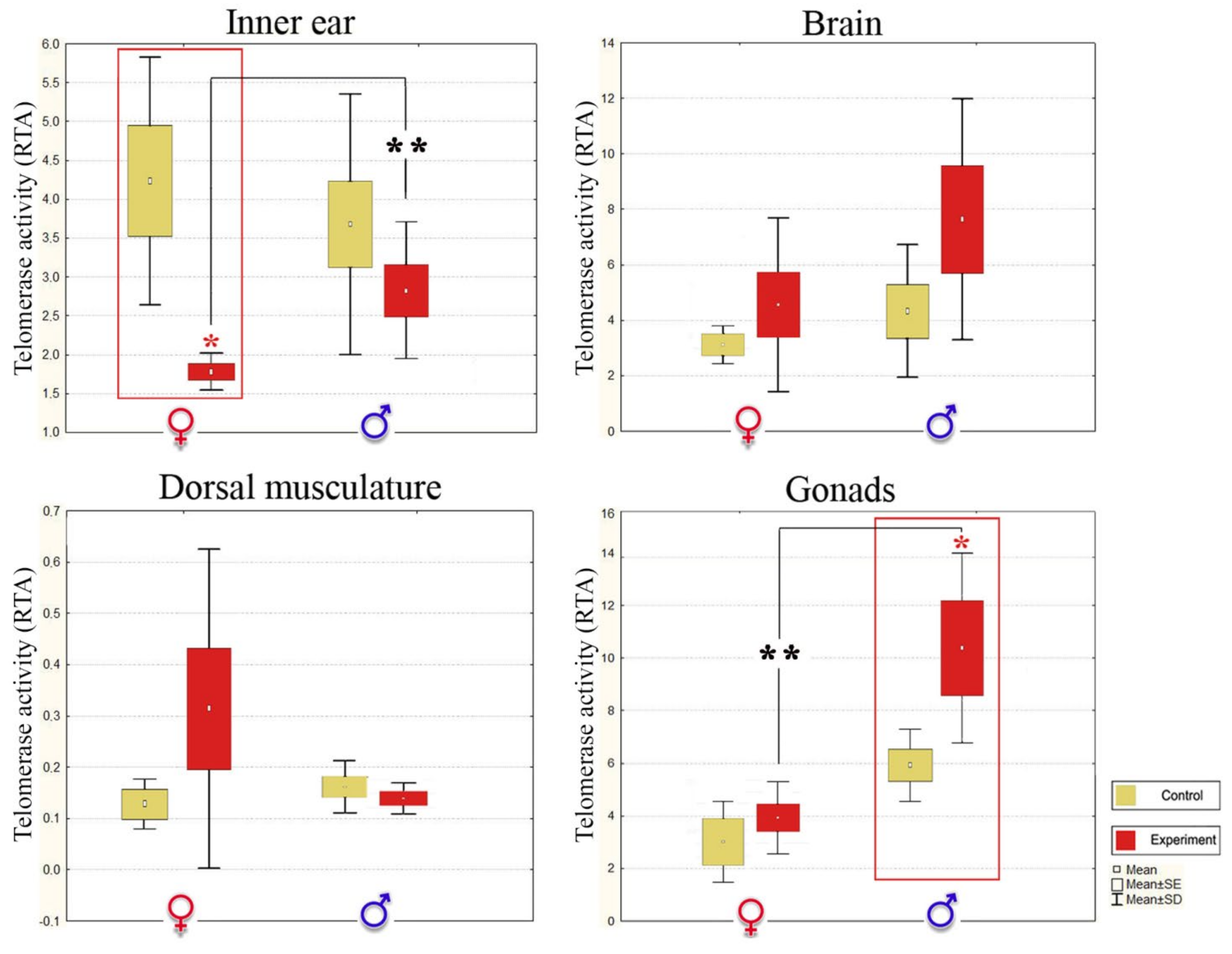
3.3. Noise Forces Accelerated Senescence Differently in Stone Sculpin Males and Females
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duarte, C.M.; Chapuis, L.; Collin, S.P.; Costa, D.P.; Devassy, R.P.; Eguiluz, V.M.; Erbe, C.; Gordon, T.A.C.; Halpern, B.S.; Harding, H.R.; et al. The soundscape of the Anthropocene ocean. Science 2021, 371, eaba4658. [Google Scholar] [CrossRef]
- Erbe, C.; MacGillivray, A.; Williams, R. Mapping cumulative noise from shipping to inform marine spatial planning. J. Acoust. Soc. Am. 2012, 132, EL423–EL438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sertlek, H.O.; Slabbekoorn, H.; Ten Cate, C.; Ainslie, M.A. Source specific sound mapping: Spatial, temporal and spectral distribution of sound in the Dutch North Sea. Environ. Pollut. 2019, 247, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.F.; Ross, D.; Wiggins, S.M.; Hildebrand, J.A. Underwater radiated noise from modern commercial ships. J. Acoust. Soc. Am. 2012, 131, 92–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jong, K.; Forland, T.N.; Amorim, M.C.P.; Rieucau, G.; Slabbekoorn, H.; Sivle, L.D. Predicting the effects of anthropogenic noise on fish reproduction. Rev. Fish Biol. Fish. 2020, 30, 245–268. [Google Scholar] [CrossRef] [Green Version]
- Veirs, S.; Veirs, V.; Williams, R.; Jasny, M.; Wood, J. A key to quieter seas: Half of ship noise comes from 15% of the fleet. PeerJ Prepr. 2018, 6, e26525v1. [Google Scholar]
- Popper, A.N.; Hawkins, A.D. An overview of fish bioacoustics and the impacts of anthropogenic sounds on fishes. J. Fish Biol. 2019, 94, 692–713. [Google Scholar] [CrossRef] [Green Version]
- Sih, A.; Trimmer, P.C.; Ehlman, S.M. A conceptual framework for understanding behavioral responses to HIREC. Curr. Opin. Behav. Sci. 2016, 12, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Balasch, J.C.; Tort, L. Netting the stress responses in fish. Front. Endocrinol. 2019, 10, 62. [Google Scholar] [CrossRef]
- Popper, A.N.; Hawkins, A.D.; Fay, R.R.; Mann, D.; Bartol, S.; Carlson, T.; Coombs, S.; Ellison, W.T.; Gentry, R.; Halvorsen, M.B.; et al. ASA S3/SC1.4 TR-2014 Sound Exposure Guidelines for Fishes and Sea Turtles: A Technical Report Prepared by ANSI-Accredited Standards Committee S3/SC1 and Registered with ANSI; Springer and ASA Press: New York, NY, USA, 2014. [Google Scholar]
- Jawad, L.A. The Effects of Man-Made Noise on the Fishes in the Marshes of Iraq. In Southern Iraq’s Marshes; Springer: Cham, Switzerland, 2021; pp. 517–525. [Google Scholar]
- Di Franco, E.; Pierson, P.; Di Iorio, L.; Calò, A.; Cottalorda, J.M.; Derijard, B.; Di Franco, A.; Galvé, A.; Guibbolini, M.; Lebrun, J.; et al. Effects of marine noise pollution on Mediterranean fishes and invertebrates: A review. Mar. Pollut. Bull. 2020, 159, 111450. [Google Scholar] [CrossRef]
- Vincenzi, G.; Micarelli, P.; Viola, S.; Buffa, G.; Sciacca, V.; Maccarrone, V.; Corrias, V.; Reinero, F.R.; Giacoma, C.; Filiciotto, F. Biological Sound vs. Anthropogenic Noise: Assessment of Behavioural Changes in Scyliorhinus canicula Exposed to Boats Noise. Animals 2021, 11, 174. [Google Scholar] [CrossRef]
- Radford, A.N.; Kerridge, E.; Simpson, S.D. Acoustic communication in a noisy world: Can fish compete with anthropogenic noise? Behav. Ecol. 2014, 25, 1022–1030. [Google Scholar] [CrossRef] [Green Version]
- Alves, D.; Vieira, M.; Amorim, M.C.P.; Fonseca, P.J. Boat noise interferes with Lusitanian toadfish acoustic communication. J. Exp. Biol. 2021, 224, jeb234849. [Google Scholar]
- Ladich, F. Sound production by the river bullhead, Cottus gobio L. (Cottidae, Teleostei). J. Fish. Biol. 2006, 35, 531–538. [Google Scholar] [CrossRef]
- Popper, A.N.; Fay, R.R. Rethinking sound detection by fishes. Hear. Res. 2011, 273, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Nedelec, S.L.; Radford, A.N.; Pearl, L.; Nedelec, B.; McCormick, M.I.; Meekan, M.G.; Simpson, S.D. Motorboat noise impacts parental behaviour and offspring survival in a reef fish. Proc. R. Soc. B 2017, 284, 20170143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahad, O.; Frenis, K.; Kuntic, M.; Daiber, A.; Münzel, T. Accelerated Aging and Age-Related Diseases (CVD and Neurological) Due to Air Pollution and Traffic Noise Exposure. Int J. Mol. Sci. 2021, 22, 2419. [Google Scholar] [CrossRef] [PubMed]
- Schreck, C.B.; Tort, L.; Farrell, A.; Brauner, C. (Eds.) Biology of Stress in Fish. Fish Physiology; Elsevier: London, UK, 2016; pp. 1–34. [Google Scholar]
- Petitjean, Q.; Jean, S.; Gandar, A.; Côtea, J.; Laffailleb, P.; Jacquin, L. Stress responses in fish: From molecular to evolutionary processes. Sci. Total Environ. 2019, 684, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Selye, H. The Stress of Life, Rev. ed.; McGraw-Hill: Oxford, UK, 1978. [Google Scholar]
- Wingfield, J.C.; Sapolsky, R.M. Reproduction and resistance to stress: When and how. J. Neuroendocrinol. 2003, 15, 711–724. [Google Scholar] [CrossRef]
- Babisch, W. The noise/stress concept, risk assessment and research needs. Noise Health 2002, 4, 1–11. [Google Scholar]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Bose, H.S.; Lingappa, V.R.; Miller, W.L. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature 2002, 417, 87–91. [Google Scholar] [CrossRef]
- Midzak, A.; Papadopoulos, V. Adrenal Mitochondria and Steroidogenesis: From Individual Proteins to Functional Protein Assemblies. Front. Endocrinol. 2016, 7, 106. [Google Scholar] [CrossRef]
- Manoli, I.; Alesci, S.; Blackman, M.R.; Su, Y.A.; Rennert, O.M.; Chrousos, G.P. Mitochondria as key components of the stress response. Trends Endocrinol. Metab. 2007, 18, 190–198. [Google Scholar] [CrossRef]
- Morava, E.; Kozicz, T. Mitochondria and the economy of stress (mal)adaptation. Neurosci. Biobehav. Rev. 2013, 37, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, L.E.; Dittami, J.P.; Ladich, F. Ship noise and cortisol secretion in European freshwater fishes. Biol. Conserv. 2006, 128, 501–508. [Google Scholar] [CrossRef]
- Chang, H.Y.; Lin, T.H.; Anraku, K.; Shao, Y.T. The Effects of Continuous Acoustic Stress on ROS Levels and Antioxidant-related Gene Expression in the Black Porgy (Acanthopagrus schlegelii). Zool Stud. 2018, 57, e59. [Google Scholar] [PubMed]
- Wei, C.A.; Lin, T.H.; Chen, R.D.; Tseng, Y.C.; Shao, Y.T. The effects of continuously acoustical stress on cortisol in milkfish (Chanos chanos). Gen. Comp. Endocrinol. 2018, 257, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Salmon, P.; Nilsson, J.; Nord, A.; Bensch, S.; Isaksson, C. Urban environment shortens telomere length in nestling great tits, Parus major. Biol. Lett. 2016, 12, 20160155. [Google Scholar] [CrossRef] [Green Version]
- Injaian, A.S.; Gonzalez-Gomez, P.L.; Conor, C.T.; Bird, A.K.; Ziure, A.D.; Patricellia, G.L.; Haussmanne, M.F.; Wingfield, J.C. Traffic noise exposure alters nestling physiology and telomere attrition through direct, but not maternal, effects in a free-living bird. Gen. Comp. Endocr. 2019, 276, 14–21. [Google Scholar] [CrossRef]
- Wale, M.A.; Briers, R.A.; Hartl, M.G.J.; Bryson, D.; Diele, K. From DNA to ecological performance: Effects of anthropogenic noise on a reef-building mussel. Sci. Total Environ. 2019, 689, 126–132. [Google Scholar] [CrossRef]
- Grunst, M.L.; Grunst, A.S.; Pinxten, R.; Eens, M. Anthropogenic noise is associated with telomere length and carotenoid-based coloration in free-living nestling songbirds. Environ. Pollut. 2020, 260, 114032. [Google Scholar] [CrossRef]
- Schreck, C.B.; Contreras-Sánchez, W.M.; Contreras-Sánchez, W.M.; Fitzpatrick, M.S. Effects of stress on fish reproduction, gamete quality, and progeny. Aquaculture 2001, 197, 3–24. [Google Scholar] [CrossRef]
- O’Reilly, K.M.; Wingfield, J.C. Seasonal, age, and sex differences in weight, fat reserves, and plasma corticosterone in western sandpipers. Condor 2003, 105, 13–26. [Google Scholar] [CrossRef]
- Astheimer, L.; Buttemer, W.A.; Wingfield, J.C. Corticosterone Treatment Has No Effect on Reproductive Hormones or Aggressive Behavior in Free-living Male Tree Sparrows, Spizella arborea. Horm. Behav. 2000, 37, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Astheimer, L.B.; Buttemer, W.A.; Wingfield, J.C. Seasonal and acute changes in adrenocortical responsiveness in an arctic-breeding bird. Horm. Behav. 1995, 29, 442–457. [Google Scholar] [CrossRef] [PubMed]
- Clutton-Brock, T.H. The Evolution of Parental Care; Princeton University Press: Princeton, NJ, USA, 1991. [Google Scholar]
- Slabbekoorn, H. Aiming for progress in understanding underwater noise impact on fish: Complementary need for indoor and outdoor studies. In The Effects of Noise on Aquatic Life II; Advances in experimental medicine and biology 875; Popper, A.N., Hawkins, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1057–1065. [Google Scholar]
- Melnik, N.G.; Tolstoganova, L.K.; Khanaev, I.V. The Experience of Identifying a Biological Species by Acoustic Signals Emitted by Aquatic Organisms. In Proceedings of the 12th Baikal International Conference Optimization Methods and Their Applications, Irkutsk, Russia, 24 June–1 July 2001; Bulatov, V., Baturin, V., Eds.; Institute of System Dynamics and Control Theory of SB RAS Press: Irkutsk, Russia, 2001; pp. 240–243. [Google Scholar]
- Aĭnutdinov, V.M.; Balkanov, V.A.; Belolaptikov, I.A. High-frequency acoustic noise of Lake Baikal. Acoust. Phys. 2006, 52, 495–504. [Google Scholar] [CrossRef]
- Glotin, H.; Poupard, M.; Marxer, R.; Ferrari, M.; Ricard, J.; Roger, V.; Patris, J.; Malige, F.; Giraudet, P.; Prevot, J.-M.; et al. Big Data Passive Acoustic for Baikal Lake Soundscape & Ecosystem Observatory [B2O]; DYNI CNRS LSIS team: Toulon, France, 2017. [Google Scholar]
- Codarin, A.; Wysocki, L.E.; Ladich, F.; Picciulin, M. Effects of ambient and boat noise on hearing and communication in three fish species living in a marine protected area (Miramare, Italy). Mar. Pollut Bull. 2009, 58, 1880–1887. [Google Scholar] [CrossRef]
- Putland, R.L.; Montgomery, J.C.; Radford, C.A. Ecology of fish hearing. J. Fish Biol. 2018, 95, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Whang, A.; Janssen, J. Sound production through the substrate during reproduction in the mottled sculpin, Cottus bairdi (Cottidae). Environ. Biol. Fishes 1994, 40, 141–148. [Google Scholar] [CrossRef]
- Kierl, N.C.; Johnston, C.E. Sound production in the pygmy sculpin Cottus paulus (Cottidae) during courtship and agonistic behaviours. J. Fish. Biol. 2010, 77, 1268–1281. [Google Scholar] [CrossRef] [PubMed]
- Holt, D.E.; Friebertshauser, R.J.; Johnston, C.E. Sound production of the banded Sculpin, Cottus carolinae. Environ. Biol. Fishes 2020, 103, 299–311. [Google Scholar] [CrossRef]
- Wahlberg, M.; Westerberg, H. Hearing in fish and their reactions to sounds from offshore wind farms. Mar. Ecol. Prog. Ser. 2005, 288, 295–309. [Google Scholar] [CrossRef]
- Colleye, O.; Ovidio, M.; Salmon, A.; Parmentier, E. Contribution to the study of acoustic communication in two Belgian river bullheads (Cottus rhenanus and C. perifretum) with further insight into the sound-producing mechanism. Front. Zool 2013, 10, 71. [Google Scholar] [CrossRef] [Green Version]
- Reshetnikov, Y.S. Atlas of Freshwater Fish of Russia; Science: Moscow, Russia, 2003; p. 379. [Google Scholar]
- Taliev, D.N. The Sculpins of Lake Baikal (Cottoidei); Nalivkin, D.V., Strelkov, A.A., Eds.; Akad Nauk SSSR: Moscow, Russia, 1955; p. 601. [Google Scholar]
- Smith, M.E.; Kane, A.S.; Popper, A.N. Noise-induced stress response and hearing loss in goldfish (Carassius auratus). J. Exp. Biol. 2004, 207, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Arjona, F.J.; Vargas-Chacoff, L.; Ruiz-Jarabo, I.; Martín del Río, M.P.; Mancera, J.M. Osmoregulatory response of Senegalese sole (Solea senegalensis) to changes in environmental salinity. Comp. Biochem. Physiol. A 2007, 148, 413–421. [Google Scholar] [CrossRef]
- Nedelec, S.L.; Radford, A.N.; Simpson, S.D.; Nedelec, B.; Lecchini, D.; Mills, S.C. Anthropogenic noise playback impairs embryonic development and increases mortality in a marine invertebrate. Sci. Rep. 2014, 4, 5891. [Google Scholar] [CrossRef] [Green Version]
- Aguilar de Soto, N.; Kight, C. Physiological effects of noise on aquatic animals. In Stressors in the Marine Environment; Solan, M., Whiteley, N.M., Eds.; Oxford University Press: Oxford, UK, 2016; pp. 135–158. [Google Scholar]
- Sapozhnikova, Y.P.; Koroleva, A.G.; Yakhnenko, V.M.; Tyagun, M.L.; Glyzina, O.Y.; Coffin, A.B.; Makarov, M.M.; Shagun, A.N.; Kulikov, V.A.; Gasarov, P.V.; et al. Molecular and cellular responses to long-term sound exposure in peled (Coregonus peled). J. Acoust. Soc. Am. 2020, 148, 895. [Google Scholar] [CrossRef]
- Picard, M.; McEwen, B.S. Psychological Stress and Mitochondria: A Systematic Review. Psychosom. Med. 2018, 80, 141–153. [Google Scholar] [CrossRef] [Green Version]
- Picard, M.; McEwen, B.S. Psychological Stress and Mitochondria: A Conceptual Framework. Psychosom. Med. 2018, 80, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H. Telomere states and cell fates. Nature 2000, 408, 53–56. [Google Scholar] [CrossRef]
- Puterman, E.; Epel, E. An intricate dance: Life experience, multisystem resiliency, and rate of telomere decline throughout the lifespan. Soc. Personal. Psychol. Compass 2012, 6, 807–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoser, S.; Ladich, F. Are hearing sensitivities of freshwater fish adapted to the ambient noise in their habitats? J. Exp. Biol. 2005, 208, 3533–3542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wysocki, L.E.; Ladich, F. Hearing in fishes under noise conditions. J. Assoc. Res. Otolaryngol. 2005, 6, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapozhnikova, Y.P.; Belous, A.A.; Makarov, M.M.; Glyzina, O.Y.; Klimenkov, I.V.; Yakhnenko, V.M.; Sukhanova, L.V. Ultrastructural correlates of acoustic sensitivity in Baikal coregonid fishes. Fundam. Appl. Limnol. 2017, 189, 267–278. [Google Scholar] [CrossRef]
- Wysocki, L.E.; Codarin, A.; Ladich, F.; Picciulin, M. Sound pressure and particle acceleration audiograms in three marine fish species from the Adriatic Sea. J. Acoust. Soc. Am. 2009, 126, 2100–2107. [Google Scholar] [CrossRef] [Green Version]
- Mikodina, E.V.; Sedova, M.A.; Pyanova, S.V.; Kourzhil, Y.; Gamachko, J. Guidance on the use of an anesthetic “clove oil” in aquaculture. Aquaculture 2011, 6, 58. [Google Scholar]
- Green, N.G.; Thomas, B.J. Numerical determination of the effective moments of non spherical particles. J. Phys. D Appl. Phys. 2007, 40, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Klimenkov, I.V.; Sudakov, N.P.; Pastukhov, M.V.; Kositsyn, N.S. The phenomenon of compensatory cell proliferation in olfactory epithelium in fish caused by prolonged exposure to natural odorants. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Sun, H.; Lin, C.-H.; Smith, M.E. Growth hormone promotes hair cell regeneration in the zebrafish (Danio rerio) inner ear following acoustic trauma. PLoS ONE 2011, 6, e28372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambrook, J.; Fritsch, E.R.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Voropaeva, E.; Maksimov, V.; Malyutina, S.; Bobak, M.; Voevoda, M. Effects of DNA quality on the measurement of telomere length. Mol. Biol. 2015, 49, 508–512. [Google Scholar] [CrossRef]
- Cawthon, R. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef] [PubMed]
- Yip, B.W.; Mok, H.O.; Peterson, D.R.; Wan, M.T.; Taniguchi, Y.; Ge, W.; Au, D.W. Sex-dependent telomere shortening, telomerase activity and oxidative damage in marine medaka Oryzias melastigma during aging. Mar. Pollut. Bull. 2017, 124, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef]
- Kim, N.; Wu, F. Advances in quantification and characterization of telomerase activity by the telomeric repeat amplification protocol (TRAP). Nucleic Acids Res. 1997, 25, 2595–2597. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
- Ellsaesser, C.F.; Clem, L.W. Hematological and immunological changes in channel catfish stressed by handling and transport. J. Fish. Biol 1986, 28, 511–521. [Google Scholar] [CrossRef]
- Harris, J.; Bird, D.J. Modulation of the fish immune system by hormones. Vet. Immunol. Immunopathol. 2000, 77, 163–176. [Google Scholar] [CrossRef]
- Dhabhar, F.S. A hassle a day may keep the doctor away: Stress and the augmentation of immune function. Integr. Comp. Biol. 2002, 42, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.R.; Athens, J.W.; Boggs, D.R.; Warner, H.R.; Cartwrig, G.; Wintrobe, M.M. Leukokinetic Studies XIII. A non-steady-state kinetic evaluation of the mechanism of cortisone-induced granulocytosis. J. Clin. Investig. 1968, 47, 249. [Google Scholar] [CrossRef] [PubMed]
- Houston, A.H.; Dobric, N.; Kahurananga, R. The nature of hematological response in fish. Fish Physiol. Biochem. 1996, 15, 339–347. [Google Scholar] [CrossRef]
- Kind, P.K.; Grigg, G.C.; Booth, D.T. Physiological responses to prolonged aquatic hypoxia in the Queensland lungfish Neoceratodus forsteri. Resp. Physiol. Neurobiol. 2002, 132, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Dhabhar, F.S.; Miller, A.H.; McEwen, B.S.; Spencer, R.L. Stressinduced changes in blood leukocyte distribution—Role of adrenal steroid hormones. J. Immunol. 1996, 157, 1638–1644. [Google Scholar]
- Schmidt, E.E.; Schibler, U. Cell size regulation, a mechanism that controls cellular RNA accumulation: Consequences on regulation of the ubiquitous transcription factors Oct1 and NF-Y and the liver-enriched transcription factor DBP. J. Cell. Biol. 1995, 128, 467–483. [Google Scholar] [CrossRef]
- Webster, M.; Witkin, K.L.; Cohen-Fix, O. Sizing Up the Nucleus: Nuclear Shape, Size and Nuclear-Envelope Assembly. J. Cell Sci. 2009, 122, 1477–1486. [Google Scholar] [CrossRef] [Green Version]
- Martin-Aragon, S.; Villar, A.; Benedi, J. Age-dependent effects of esculetin on mood-related behavior and cognition from stressed mice are associated with restoring brain antioxidant status. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 65, 1–16. [Google Scholar] [CrossRef]
- Ortmann, C.F.; Reus, G.Z.; Ignacio, Z.M.; Abelaira, H.M.; Titus, S.E.; de Carvalho, P.; Arent, C.O.; Dos Santos, M.A.; Matias, B.I.; Martins, M.M.; et al. Enriched flavonoid fraction from Cecropia pachystachya Trécul leaves exerts antidepressant-like behavior and protects brain against oxidative stress in rats subjected to chronic mild stress. Neurotox. Res. 2016, 29, 469–483. [Google Scholar] [CrossRef]
- Filipovic, D.; Zlatkovic, J.; Inta, D.; Bjelobaba, I.; Stojiljkovic, M.; Gass, P. Chronic isolation stress predisposes the frontal cortex but not the hippocampus to the potentially detrimental release of cytochrome c from mitochondria and the activation of caspase-3. J. Neurosci. Res. 2011, 89, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Boeck, C.; Koenig, A.M.; Schury, K.; Geiger, M.L.; Karabatsiakis, A.; Wilker, S.; Waller, C.; Gundel, H.; Fegert, J.M.; Calzia, E.; et al. Inflammation in adult women with a history of child maltreatment: The involvement of mitochondrial alterations and oxidative stress. Mitochondrion 2016, 30, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Tamburrini, M.; Verde, C.; Olianas, A.; Giardina, B.; Corda, M.; Sanna, M.T.; Fais, A.; Deiana, A.M.; di Prisco, G.; Pellegrini, M. The Hemoglobin System of the Brown Moray Gymnothorax Unicolor: Structure/Function Relationships. Eur. J. Biochem. 2001, 268, 4104–4111. [Google Scholar] [CrossRef]
- Lambert, A.J.; Brand, M.D. Reactive oxygen species production by mitochondria. Methods Mol. Biol. 2009, 554, 165–181. [Google Scholar]
- McManus, M.J.; Murphy, M.P.; Franklin, J.L. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2011, 31, 15703–15715. [Google Scholar] [CrossRef] [Green Version]
- Shalev, I.; Entringer, S.; Wadhwa, P.D.; Wolkowitz, O.M.; Puterman, E.; Lin, J.; Epel, E.S. Stress and telomere biology: A lifespan perspective. Psychoneuroendocrinology 2013, 38, 1835–1842. [Google Scholar] [CrossRef] [Green Version]
- Trifunovic, A.; Wredenberg, A.; Falkenberg, M.; Spelbrink, J.N.; Rovio, A.T.; Bruder, C.E.; Bohlooly, Y.M.; Gidlof, S.; Oldfors, A.; Wibom, R.; et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 2004, 429, 417–423. [Google Scholar] [CrossRef]
- Kujoth, G.C.; Hiona, A.; Pugh, T.D.; Someya, S.; Panzer, K.; Wohlgemuth, S.E.; Hofer, T.; Seo, A.Y.; Sullivan, R.; Jobling, W.A.; et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 2005, 309, 481–484. [Google Scholar] [CrossRef]
- Latorre-Pellicer, A.; Moreno-Loshuertos, R.; Lechuga-Vieco, A.V.; Sanchez-Cabo, F.; Torroja, C.; AcinPerez, R.; Calvo, E.; Aix, E.; Gonzalez-Guerra, A.; Logan, A.; et al. Mitochondrial and nuclear DNA matching shapes metabolism and healthy ageing. Nature 2016, 535, 561–565. [Google Scholar] [CrossRef]
- Sapozhnikova, Y.P.; Klimenkov, I.V.; Khanaev, I.V.; Makarov, M.M.; Belous, A.A. Ultrastructure of saccular epithelium sensory cells of four sculpin fish species (Cottoidei) of Lake Baikal in relation to their way of life. J. Ichthyol. 2016, 56, 289–297. [Google Scholar] [CrossRef]
- Monroe, J.D.; Rajadinakaran, G.; Smith, M.E. Sensory hair cell death and regeneration in fishes. Front. Cell. Neurosci. 2015, 9, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurabi, A.; Keithley, E.M.; Housley, G.D.; Ryan, A.F.; Wong, A.C. Cellular mechanisms of noise-induced hearing loss. Hear. Res. 2017, 349, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Batandier, C.; Poulet, L.; Hininger, I.; Couturier, K.; Fontaine, E.; Roussel, A.M.; Canini, F. Acute stress delays brain mitochondrial permeability transition pore opening. J. Neurochem. 2014, 131, 314–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, D.; Bielefeld, E.; Harris, K.C.; Hu, B.H. The role of oxidative stress in noise induced hearing loss. Ear Hear. 2006, 27, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.H.; Henderson, D.; Nicotera, T.M. Extremely rapid induction of outer hair cell apoptosis in the chinchilla cochlea following exposure to impulse noise. Hear. Res. 2006, 211, 16–25. [Google Scholar] [CrossRef]
- Hirose, K.; Hockenbery, D.M.; Rubel, E.W. Reactive oxygen species in chick hair cells after gentamicin exposure in vitro. Hear. Res. 1997, 104, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Breitzler, L.; Lau, I.H.; Fonseca, P.J.; Vasconcelos, R.O. Noise-induced hearing loss in zebrafish: Investigating structural and functional inner ear damage and recovery. Hear. Res. 2020, 391, 107952. [Google Scholar] [CrossRef]
- Kositsyn, I.S.; Klimenkov, I.V.; Dmitrieva, T.M. Ultrastructural rearrangements of the receptor cells of the olfactory analyzer in fish in different phases of reproductive behavior. Dokl. RAS 1990, 311, 739–742. [Google Scholar]
- Choung, Y.H.; Taura, A.; Pak, K.; Choi, S.J.; Masuda, M.; Ryan, A.F. Generation of highly-reactive oxygen species is closely related to hair cell damage in rat organ of Corti treated with gentamicin. Neuroscience 2009, 161, 214–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamane, H.; Nakai, Y.; Takayama, M.; Iguchi, H.; Nakagawa, T.; Kojima, A. Appearance of free radicals in the Guinea pig inner ear after noise-induced acoustic trauma. Eur. Arch. Otorhinolaryngol. 1995, 252, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.; Brennan, L.P.; Gerwing, T.G. Sound the alarm: A meta-analysis on the effect of aquatic noise on fish behavior and physiology. Glob. Chang. Biol. 2018, 24, 3105–3116. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Epel, E.; Cheon, J.; Kroenke, C.; Sinclair, E.; Bigos, M.; Wolkowitz, O.; Mellon, S.; Blackburn, E. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: Insights for epidemiology of telomere maintenance. J. Immunol. Methods 2010, 352, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Conklin, Q.; Crosswell, A.; Saron, C.; Epel, E. Meditation, stress processes, and telomere biology. Curr. Opin. Psychol. 2019, 28, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Stress-induced suppression of testicular function in the wild baboon: Role of glucocorticoids. Endocrinology 1985, 116, 2273–2278. [Google Scholar] [CrossRef]
- Dong, Q.; Salva, A.; Sottas, C.M.; Niu, E.; Holmes, M.; Hardy, M.P. Rapid glucocorticoid mediation of suppressed testosterone biosynthesis in male mice subjected to immobilization stress. J. Androl. 2004, 25, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Nakajima, M.; Ibañez-Tallon, I.; Heintz, N.A. Cortical Circuit for Sexually Dimorphic Oxytocin-Dependent Anxiety Behaviors. Cell 2016, 167, 60–72.e11. [Google Scholar] [CrossRef] [Green Version]
- Chiodi, I.; Mondello, C. Telomere-independent functions of telomerase in nuclei, cytoplasm, and mitochondria. Front. Oncol. 2012, 2, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafiei-Sabet, S.; van Dooren, D.; Slabbekoorn, H. Son et lumière: Sound and light effects on spatial distribution and swimming behavior in captive zebrafish. Environ. Epidemiol. 2016, 212, 480–488. [Google Scholar] [CrossRef] [PubMed]
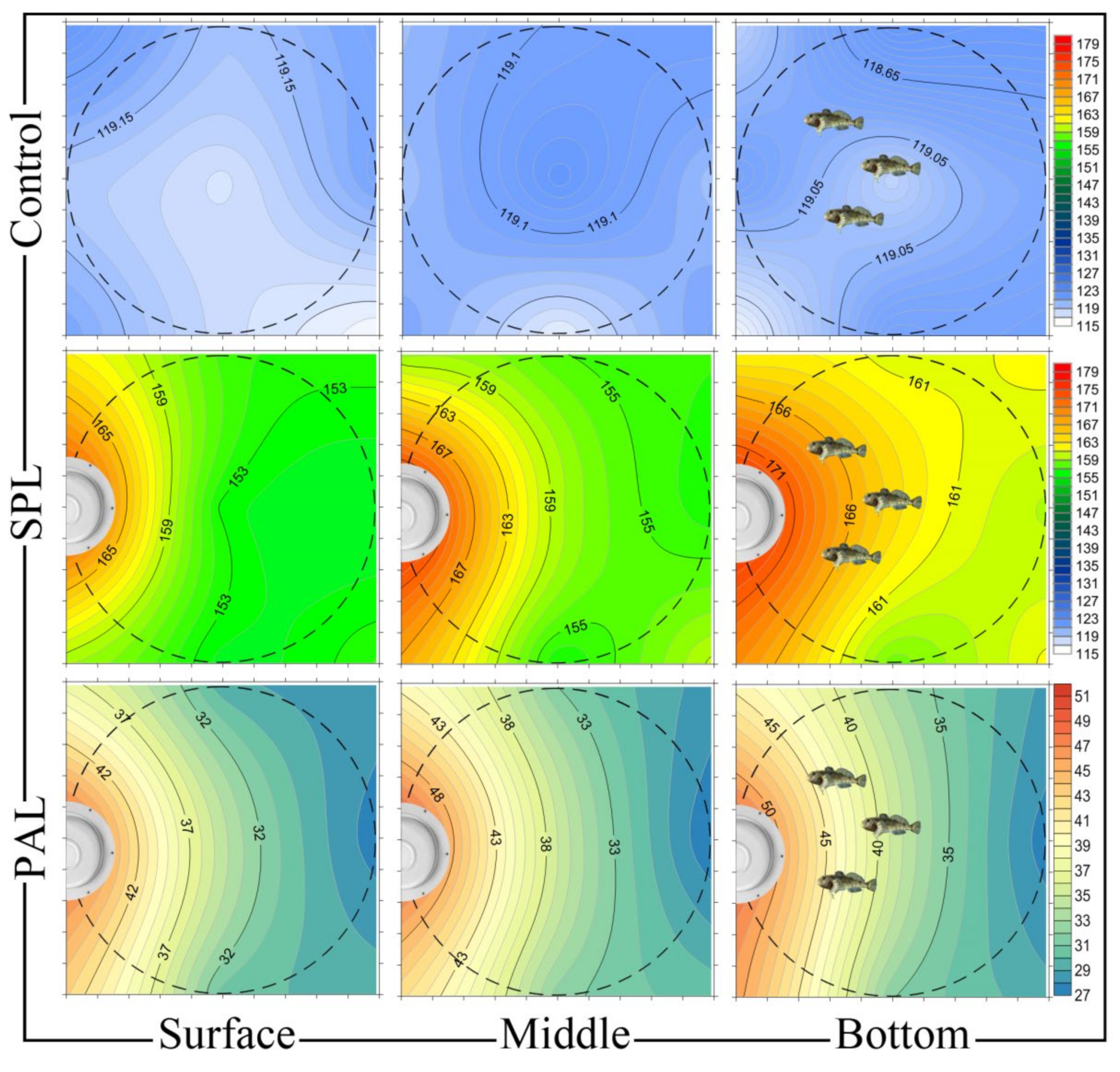


| Blood Cells 1, % | Control | Noise-Exposed | |||
|---|---|---|---|---|---|
| ♂ | ♀ | ♂ | ♀ | ||
| Peripheral blood | |||||
| Erythroblasts 2,* | 26.69 ± 3.86 | 33.38 ± 0.56 | 26.21 ± 2.9 | 23.32 ± 4.3 3,**↓ | |
| Mature red blood cells | 73.31 ± 3.9 | 66.62 ± 0.60 | 73.79 ± 2.89 | 76.68 ± 4.82 3,**↑ | |
| White blood cells | Lymphocytes 2,** | 92.11 ± 6.04 | 91.90 ± 5.3 | 94.10 ± 1.6 | 87.68 ± 7.7 3,**↓ |
| Monocytes 2,*** | 0 | 0 | 0.41 ± 0.04 3,***↑ | 0 | |
| Immature neutrophils 2,*** | 6.17 ± 0.35 | 2.72 ± 0.14 | 2.29 ± 0.13 3,***↓ | 5.73 ± 0.3 3,***↑ | |
| Polymorphonuclear neutrophils 2,*** | 1.72 ± 0.08 | 5.38 ± 0.4 | 3.20 ± 0.15 3,***↑ | 6.59 ± 0.5 3,**↑ | |
| Neutrophils to lymphocytes ratio, N:L 2,* | 0.08 | 0.08 | 0.06 | 0.14 3,*↑ | |
| Hematopoietic organs: Kidneys | |||||
| Blasts 2,*** | 3.46 ± 0.14 | 12.7 ± 0.22 | 9.68 ± 0.36 3,***↑ | 5.05 ± 0.25 3,***↓ | |
| Phagocytes | 58.00 ± 0.8 | 27.1 ± 0.24 | 30.1 ± 0.34 3,***↓ | 29.1 ± 0.39 | |
| Lymphocytes 2,** | 47.99 ± 8.94 | 56.25 ± 4.92 | 49.45 ± 5.19 | 58.48 ± 6.51 | |
| Plasmocytes 2,* | 4.23 ± 0.24 | 3.95 ± 1.73 | 10.75 ± 0.32 3,***↑ | 7.35 ± 3.59 3,***↑ | |
| Hematopoietic organs: Spleen | |||||
| Blasts | 6.63 ± 0.29 | 2.77 ± 0.15 | 5.53 ± 0.13 | 5.23 ± 0.33 3,***↑ | |
| Phagocytes 2,*** | 43.8 ± 1.74 | 52.4 ± 1.44 | 47.6 ± 0.54 3,*↑ | 36.4 ± 1.01 3,***↓ | |
| Lymphocytes 2,** | 47.07 ± 17.77 | 43.7 ± 13.10 | 41.85 ± 8.11 | 54.35 ± 8.36 3,***↑ | |
| Plasmocytes 2,* | 2.50 ± 0.12 | 1.13 ± 0.11 | 4.98 ± 0.15 3,***↑ | 4.05 ± 0.23 3,***↑ | |
| Eccentricity 2,*** | Nuclear Area 2,*** | Cell Area 2,*** | Cell Volume 2,*** | Nuclear Volume 2,** | NCR 2,*** | |
|---|---|---|---|---|---|---|
| Control | ||||||
| ♂ | 0.714 ± 0.003 | 23.74 ± 0.14 | 117.8 ± 0.68 | 1232 ± 11.6 | 112.1 ± 1.1 | 0.107 ± 0.001 |
| ♀ | 0.739 ± 0.004 | 23.51 ± 0.17 | 108.1 ± 1.24 | 1111 ± 18.8 | 110 ± 1.32 | 0.126 ± 0.003 |
| Noise-exposed | ||||||
| ♂ | 0.673 ± 0.003 3,***↓ | 25.5 ± 0.13 3,*↑ | 118.1 ± 0.75 | 1200 ± 11.3 3,*↓ | 122.8 ± 1.04 3,*↑ | 0.125 ± 0.002 3,*↑ |
| ♀ | 0.704 ± 0.003 3,***↓ | 24.7 ± 0.15 3,*↑ | 122.4 ± 0.81 3,***↑ | 1294 ± 13.4 3,***↑ | 118.4 ± 1.19 3,*↑ | 0.109 ± 0.002 3,*↓ |
| EP 2,*** | Hb, g/% 2,*** | RBC 2,* | BOC | MCHC2,* | K/A | |
| Control | ||||||
| ♂ | 361.05 ± 65 | 7,92 ± 0.74 | 1.73 ± 0.02 | 12,52 ± 0,76 | 56,39 ± 8,7 | 1 |
| ♀ | 456.88 ± 44 | 6.41 ± 0.61 | 1.63 ± 0.03 | 10.36 ± 0.39 | 55.44 ± 0.39 | 1 |
| Noise-exposed | ||||||
| ♂ | 631.30 ± 72 3,*↑ | 5.2 ± 0.38 3,***↓ | 2.11 ± 0.06 | 12.31 ± 0.32 | 44.82 ± 5.88 3,*↓ | 2 3,*↑ |
| ♀ | 170.46 ± 47 3,*↓ | 7.89 ± 0.30 3,***↑ | 1.52 ± 0.04 | 12.49 ± 0.31 3,***↑ | 53.01 ± 5.35 | 2 3,*↑ |
| RTL ± SD | ||||||||
| Inner Ear Tissue 2,* | Brain 2,* | Dorsal Musculature | Gonads | |||||
| Control | Noise-Exposed | Control | Noise-Exposed | Control | Noise-Exposed | Control | Noise-Exposed | |
| ♂ | 0.866 ± 0.07 | 0.871 ± 0.13 | 0.931 ± 0.09 | 1.001 ± 0.14 | 0.954 ± 0.21 | 0.792 ± 0.38 | 0.877 ± 0.16 | 0.881 ± 0.18 |
| ♀ | 0.898 ± 0.09 | 0.987 ± 0.15 | 1.045 ± 0.09 | 0.895 ± 0.10 3,**↓ | 0.963 ± 0.23 | 0.956 ± 0.13 | 1.008 ± 0.13 | 0.968 ± 0.13 |
| RTA ± SD | ||||||||
| Inner ear Tissue 2,** | Brain | Dorsal Musculature | Gonads 2,** | |||||
| Control | Noise-Exposed | Control | Noise-Exposed | Control | Noise-Exposed | Control | Noise-Exposed | |
| ♂ | 3.114 ± 1.34 | 2.782 ± 1.01 | 4.323 ± 2.39 | 7.630 ± 4.34 | 0.158 ± 0.05 | 0.133 ± 0.02 | 5.911 ± 1.37 | 10.408 ± 3.72 3,*↑ |
| ♀ | 4.630 ± 1.60 | 2.113 ± 0.85 3,**↓ | 3.130 ± 2.01 | 4.567 ± 3.82 | 0.127 ± 0.05 | 0.314 ± 0.25 | 3.074 ± 1.97 | 4.000 ± 3.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapozhnikova, Y.P.; Koroleva, A.G.; Yakhnenko, V.M.; Khanaev, I.V.; Glyzina, O.Y.; Avezova, T.N.; Volkova, A.A.; Mushinskaya, A.V.; Tyagun, M.L.; Shagun, A.N.; et al. Sex Associated Effects of Noise Pollution in Stone Sculpin (Paracottus knerii) as a Model Object in the Context of Human-Induced Rapid Environmental Change. Biology 2021, 10, 1063. https://doi.org/10.3390/biology10101063
Sapozhnikova YP, Koroleva AG, Yakhnenko VM, Khanaev IV, Glyzina OY, Avezova TN, Volkova AA, Mushinskaya AV, Tyagun ML, Shagun AN, et al. Sex Associated Effects of Noise Pollution in Stone Sculpin (Paracottus knerii) as a Model Object in the Context of Human-Induced Rapid Environmental Change. Biology. 2021; 10(10):1063. https://doi.org/10.3390/biology10101063
Chicago/Turabian StyleSapozhnikova, Yulia P., Anastasia G. Koroleva, Vera M. Yakhnenko, Igor V. Khanaev, Olga Yu. Glyzina, Tatyana N. Avezova, Aleksandra A. Volkova, Angela V. Mushinskaya, Marina L. Tyagun, Artem N. Shagun, and et al. 2021. "Sex Associated Effects of Noise Pollution in Stone Sculpin (Paracottus knerii) as a Model Object in the Context of Human-Induced Rapid Environmental Change" Biology 10, no. 10: 1063. https://doi.org/10.3390/biology10101063









