Atmospheric Pressure Plasma Coating of Bismuth Oxide Circular Droplets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coating Process
2.2. Characterization
3. Results and Discussion
3.1. Morphology Overview
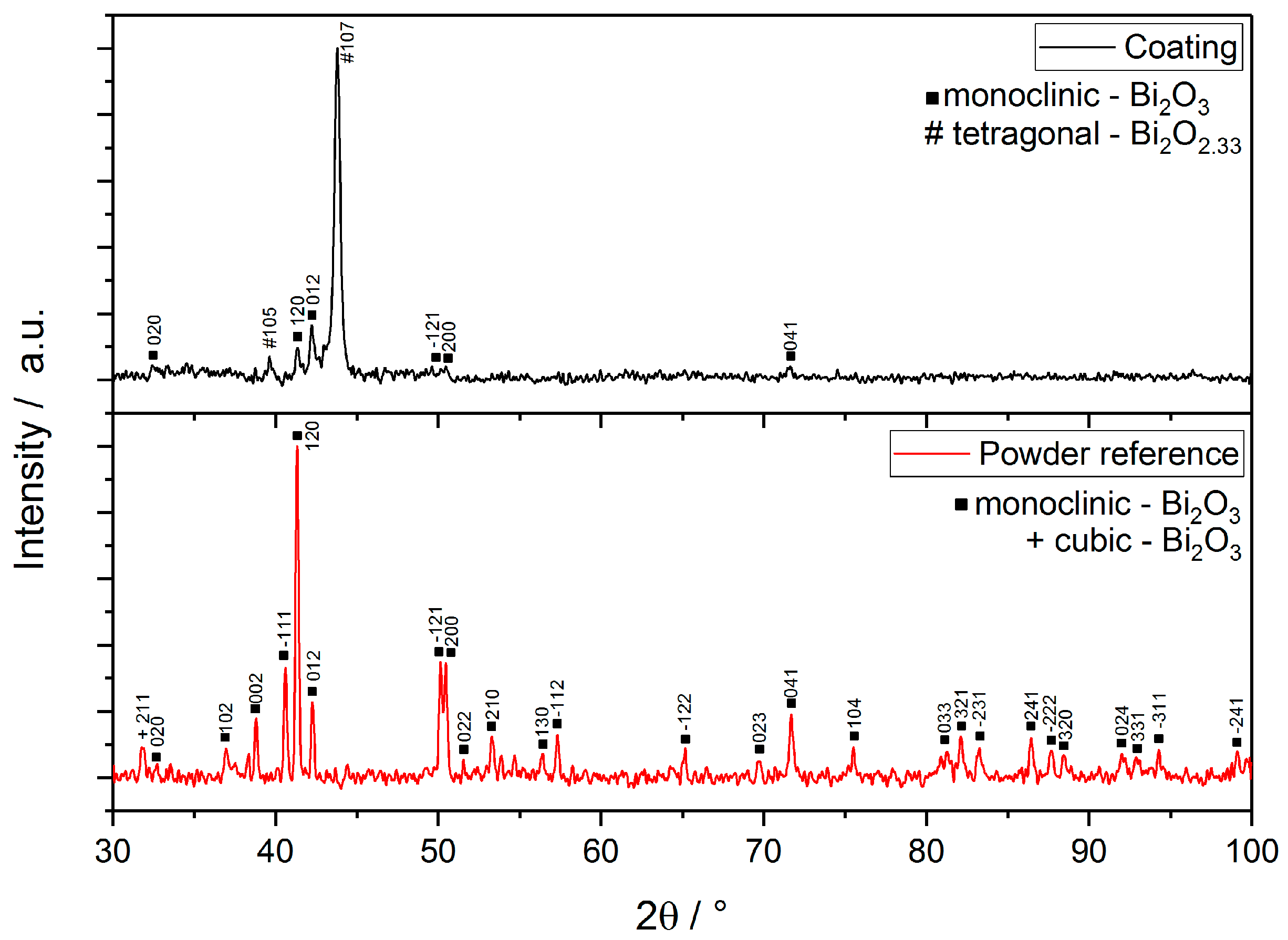
3.2. X-ray Diffraction (XRD)
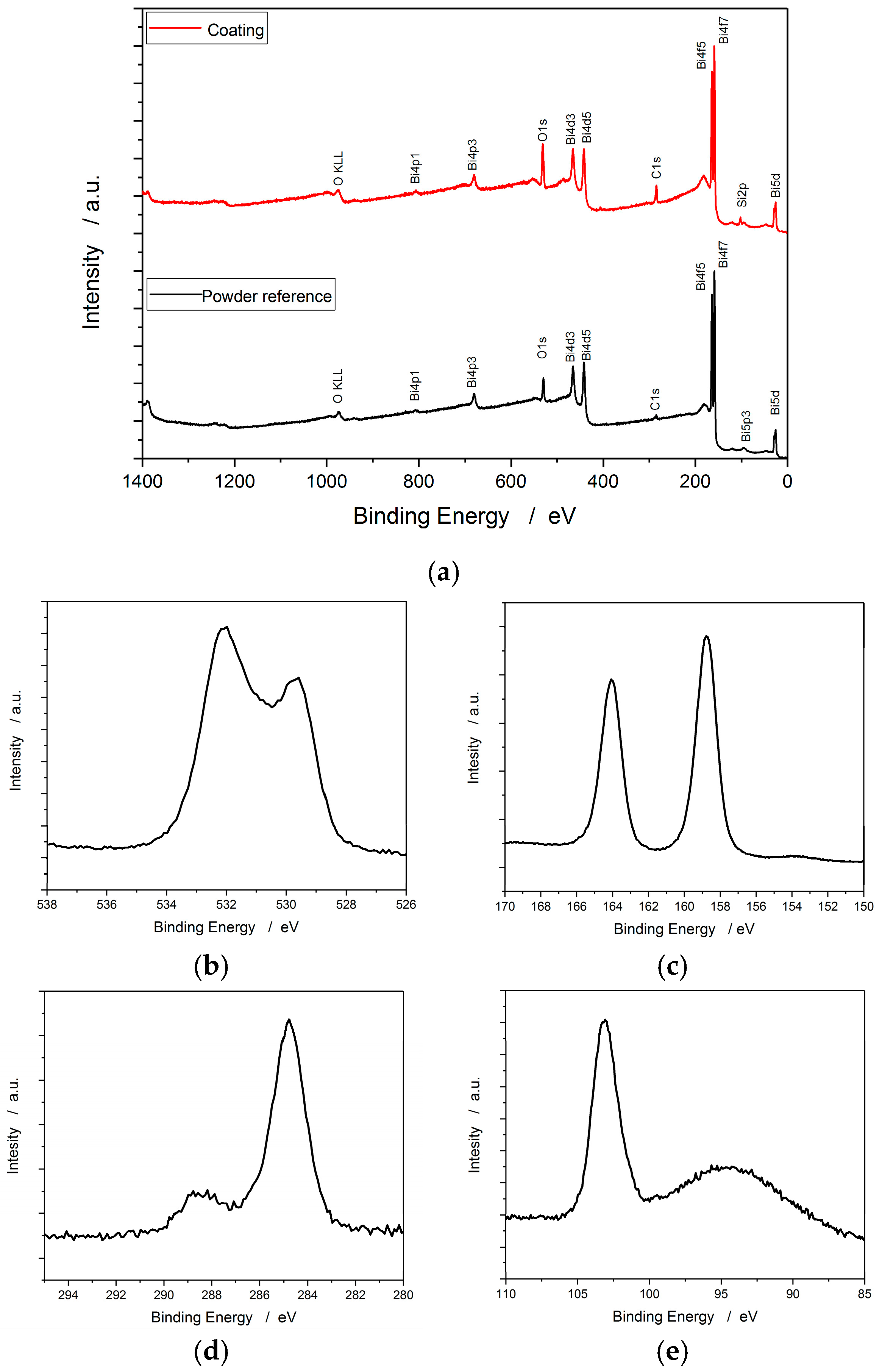
3.3. X-ray Photoelectron Spectroscopy (XPS)
3.4. Optical Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iljinas, A.; Burinskas, S.; Dudonis, J. Synthesis of bismuth oxide thin films deposited by reactive magnetron sputtering. Acta Phys. Pol. A 2011, 120, 60–62. [Google Scholar] [CrossRef]
- Xia, J.-Y.; Tang, M.-T.; Cui, C.; Jin, S.-M.; Chen, Y.-M. Preparation of α-Bi2O3 from bismuth powders through low-temperature oxidation. Trans. Nonferrous Metals Soc. China 2012, 22, 2289–2294. [Google Scholar] [CrossRef]
- Tezel, F.M.; Kariper, İ.A. Synthesis, surface tension, optical and dielectric properties of bismuth oxide thin film. Mater. Sci. Pol. 2017, 35, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Yang, C.; Wang, Z.; Zhou, W.; Jiao, S.; Zhu, H. In situ synthesis of α-β phase heterojunction on Bi2O3 nanowires with exceptional visible-light photocatalytic performance. Appl. Catal. B Environ. 2013, 142–143, 504–511. [Google Scholar] [CrossRef]
- Shen, Y.D.; Li, Y.W.; Li, W.M.; Zhang, J.Z.; Hu, Z.G.; Chu, J.H. Growth of Bi2O3 ultrathin films by atomic layer deposition. J. Phys. Chem. C 2012, 116, 3449–3456. [Google Scholar] [CrossRef]
- Shimanoe, K. Bismuth oxide thin film as new electrochromic material. Solid State Ion. 1998, 113–115, 415–419. [Google Scholar] [CrossRef]
- Gujar, T.P.; Shinde, V.R.; Lokhande, C.D.; Mane, R.S.; Han, S.-H. Bismuth oxide thin films prepared by chemical bath deposition (CBD) method: Annealing effect. Appl. Surf. Sci. 2005, 250, 161–167. [Google Scholar] [CrossRef]
- Cornei, N.; Tancret, N.; Abraham, F.; Mentré, O. New ε-Bi2O3 metastable polymorph. Inorg. Chem. 2006, 45, 4886–4888. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.Y.; Xu, H.Z.; Hao, W.C. A Transition phase in the transformation from α-, β- and ε- to δ-bismuth oxide. Chin. Phys. Lett. 2011, 28, 056101. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Immovilli, S.; Prudenziati, M. Powder X-ray diffraction data for the new polymorphic compound ω-Bi2O3. Powder Diffr. 1997, 12, 90–92. [Google Scholar] [CrossRef]
- Harwig, H.A.; Gerards, A.G. The polymorphism of bismuth sesquioxide. Thermochim. Acta 1979, 28, 121–131. [Google Scholar] [CrossRef]
- Mehring, M. From molecules to bismuth oxide-based materials: Potential homo- and heterometallic precursors and model compounds. Coord. Chem. Rev. 2007, 251, 974–1006. [Google Scholar] [CrossRef]
- Klinkova, L.A.; Nikolaichik, V.I.; Barkovskii, N.V.; Fedotov, V.K. Thermal stability of Bi2O3. Russ. J. Inorg. Chem. 2007, 52, 1822–1829. [Google Scholar] [CrossRef]
- Patil, R.B.; Puri, R.K.; Puri, V. Intrinsic stress of bismuth oxide thin films: Effect of vapour chopping and air ageing. J. Phys. Conf. Ser. 2008, 114, 012036. [Google Scholar] [CrossRef]
- Kumari, L.; Lin, J.-H.; Ma, Y.-R. Synthesis of bismuth oxide nanostructures by an oxidative metal vapour phase deposition technique. Nanotechnology 2007, 18, 295605. [Google Scholar] [CrossRef]
- Takeyama, T.; Takahashi, N.; Nakamura, T.; Itoh, S. Microstructure characterization of δ-Bi2O3 thin film under atmospheric pressure by means of halide CVD on c-sapphire. J. Cryst. Grow. 2005, 275, 460–466. [Google Scholar] [CrossRef]
- Yang, X.; Lian, X.; Liu, S.; Wang, G.; Jiang, C.; Tian, J.; Chen, J.; Wang, R. Enhanced photocatalytic performance: A β-Bi2O3 thin film by nanoporous surface. J. Phys. D Appl. Phys. 2013, 46, 035103. [Google Scholar] [CrossRef]
- Ratova, M.; Marcelino, R.B.P.; de Souza, P.P.; Amorim, C.C.; Kelly, P.J. Reactive Magnetron Sputter Deposition of Bismuth Tungstate Coatings for Water Treatment Applications under Natural Sunlight. Catalysts 2017, 7, 283. [Google Scholar] [CrossRef]
- Sabaghiana, M.; Behzada, M.; Jahromib, H.S. Alfa-bismuth(III) oxide catalyzed biginelli reactions using experimentally designed optimized condition. J. Adv. Mater. Process. 2015, 3, 61–69. [Google Scholar]
- Condurache-Bota, S.; Constantinescu, C.; Praisler, M.; Tiron, V.; Tigau, N.; Gheorghies, C. The influence of laser wavelength and pulses number on the structure and the optical properties of pulsed laser-deposited bismuth oxide thin films. In Proceedings of the 2014 International Semiconductor Conference (CAS), Sinaia, Romania, 13–15 October 2014; pp. 87–90. [Google Scholar]
- Condurache-Bota, S.; Rusu, G.I.; Tigau, N.; Drasovean, R.; Gheorghies, C. Structural and optical characterization of thermally oxidized bismuth films. Rom. J. Chem. 2009, 54, 205–211. [Google Scholar]
- Killedar, V.V.; Bhosale, C.H.; Lokhande, C.D. Characterization of spray deposited bismuth oxide thin films from non-aqueous medium. Turk. J. Phys. 1998, 22, 825–830. [Google Scholar]
- Wang, X.; Zhu, A.; Li, Z.; Liu, Z. Ag doped Bi2O2.33 microrods: Photocatalytic activity investigation. RSC Adv. 2016, 6, 25409–25415. [Google Scholar] [CrossRef]
- Salim, E.T.; Al-Douri, Y.; Al Wazny, M.S.; Fakhri, M.A. Optical properties of Cauliflower-like Bi2O3 nanostructures by reactive pulsed laser deposition (PLD) technique. Sol. Energy 2014, 107, 523–529. [Google Scholar] [CrossRef]
- Fang, G.; Chen, G.; Liu, J.; Wang, X. Ultraviolet-Emitting Bi2O2.33 Nanosheets Prepared by Electrolytic Corrosion of Metal Bi. J. Phys. Chem. C 2009, 114, 864–867. [Google Scholar] [CrossRef]
- Schuisky, M.; Hårsta, A. Epitaxial growth of Bi2O2.33 by halide CVD. Chem. Vap. Depos. 1996, 2, 235–238. [Google Scholar] [CrossRef]
- Huang, X.; Yan, J.; Zeng, F.; Yuan, X.; Zou, W.; Yuan, D. Facile preparation of orange-like Bi2O2.33 microspheres for high performance supercapacitor application. Mater. Lett. 2013, 90, 90–92. [Google Scholar] [CrossRef]
- Köhler, R.; Sauerbier, P.; Militz, H.; Viöl, W. Atmospheric pressure plasma coating of wood and MDF with polyester powder. Coatings 2017, 7, 171. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; St. Smart, R.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef] [Green Version]
- Barr, T.L.; Seal, S. Nature of the use of adventitious carbon as a binding energy standard. J. Vac. Sci. Technol. A Vac. Surf. Films 1995, 13, 1239–1246. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; Wiley: New York, NY, USA; Chichester, UK, 1992. [Google Scholar]
- Guan, H.; Zhang, X.; Xie, Y. Soft-chemical synthetic nonstoichiometric Bi2O2.33 nanoflower: A new room-temperature ferromagnetic semiconductor. J. Phys. Chem. C 2014, 118, 27170–27174. [Google Scholar] [CrossRef]
- Gondal, M.A.; Saleh, T.A.; Drmosh, Q. Optical properties of bismuth oxide nanoparticles synthesized by pulsed laser ablation in liquids. Sci. Adv. Mater. 2012, 4, 507–510. [Google Scholar] [CrossRef]
- Sirota, B.; Reyes-Cuellar, J.; Kohli, P.; Wang, L.; McCarroll, M.E.; Aouadi, S.M. Bismuth oxide photocatalytic nanostructures produced by magnetron sputtering deposition. Thin Solid Films 2012, 520, 6118–6123. [Google Scholar] [CrossRef]
- Leontie, L.; Caraman, M.; Visinoiu, A.; Rusu, G.I. On the optical properties of bismuth oxide thin films prepared by pulsed laser deposition. Thin Solid Films 2005, 473, 230–235. [Google Scholar] [CrossRef]
- Enhessari, M.; Shaterian, M.; Esfahani, M.J.; Motaharian, M.N. Synthesis, characterization and optical band gap of La2CuO4 nanoparticles. Mater. Sci. Semicond. Process. 2013, 16, 1517–1520. [Google Scholar] [CrossRef]

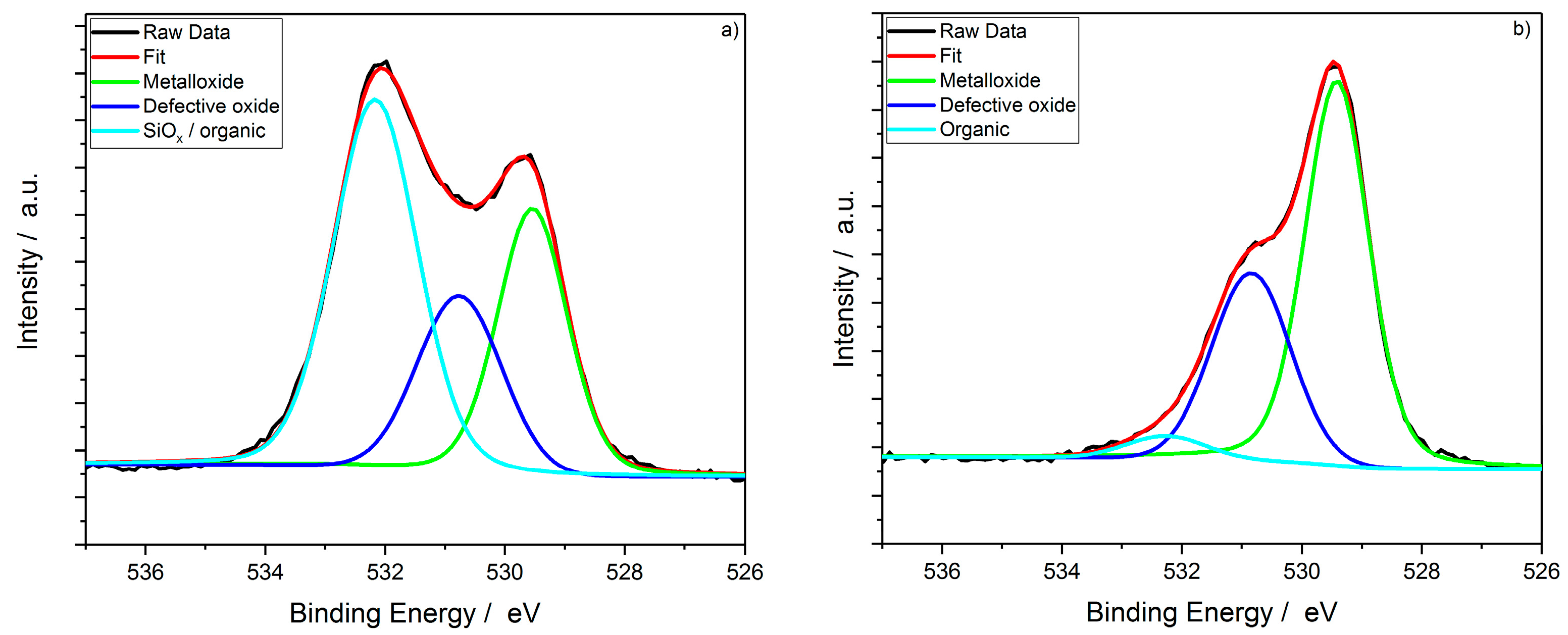


| Sample | C 1s | MO | DO | org. C | SiOx and org. C |
| Powder reference | 18.3 | 30.86 | 16.55 | 2.16 | – |
| Coating | 24.95 | 13.97 | 10.32 | – | 23.12 |
| Sample | Si 2p | Bi 4f | Ratio of MO/Bi 4f | Ratio of MO + DO/Bi 4f | Ratio of DO/Bi 4f |
| Powder reference | – | 32.13 | 0.96 | 1.48 | 0.52 |
| Coating | 11.05 | 16.59 | 0.84 | 1.46 | 0.62 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köhler, R.; Ohms, G.; Militz, H.; Viöl, W. Atmospheric Pressure Plasma Coating of Bismuth Oxide Circular Droplets. Coatings 2018, 8, 312. https://doi.org/10.3390/coatings8090312
Köhler R, Ohms G, Militz H, Viöl W. Atmospheric Pressure Plasma Coating of Bismuth Oxide Circular Droplets. Coatings. 2018; 8(9):312. https://doi.org/10.3390/coatings8090312
Chicago/Turabian StyleKöhler, Robert, Gisela Ohms, Holger Militz, and Wolfgang Viöl. 2018. "Atmospheric Pressure Plasma Coating of Bismuth Oxide Circular Droplets" Coatings 8, no. 9: 312. https://doi.org/10.3390/coatings8090312





