Anti-Corrosion Characteristics of Electrodeposited Self-Doped Polyaniline Films on Mild Steel in Low Acidity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Test Samples
2.3. Electrochemical Polymerization of SPAN on Mild Steel
2.4. Materials Analysis
2.5. Electrochemical Measurements
3. Results and Discussion
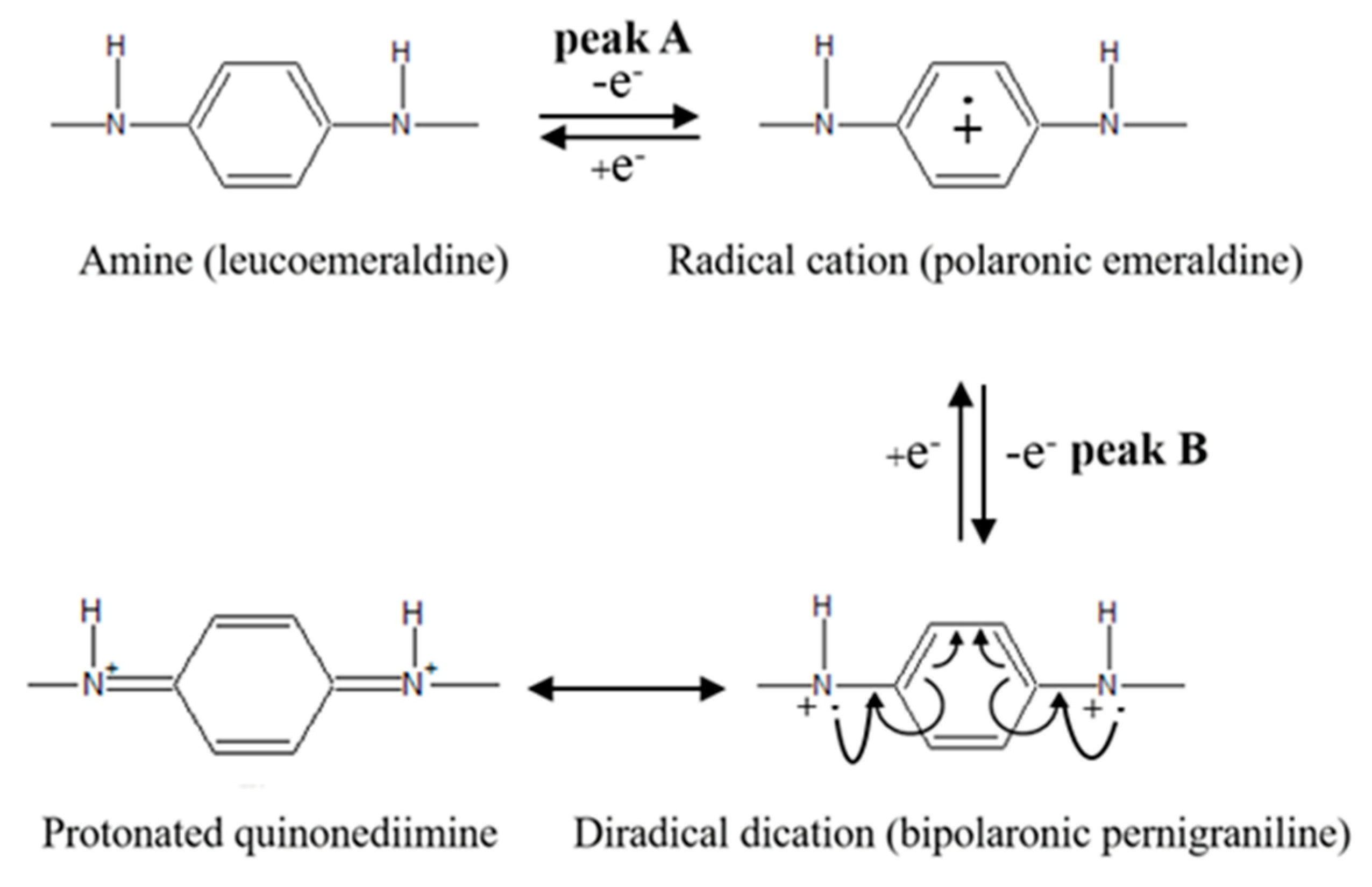
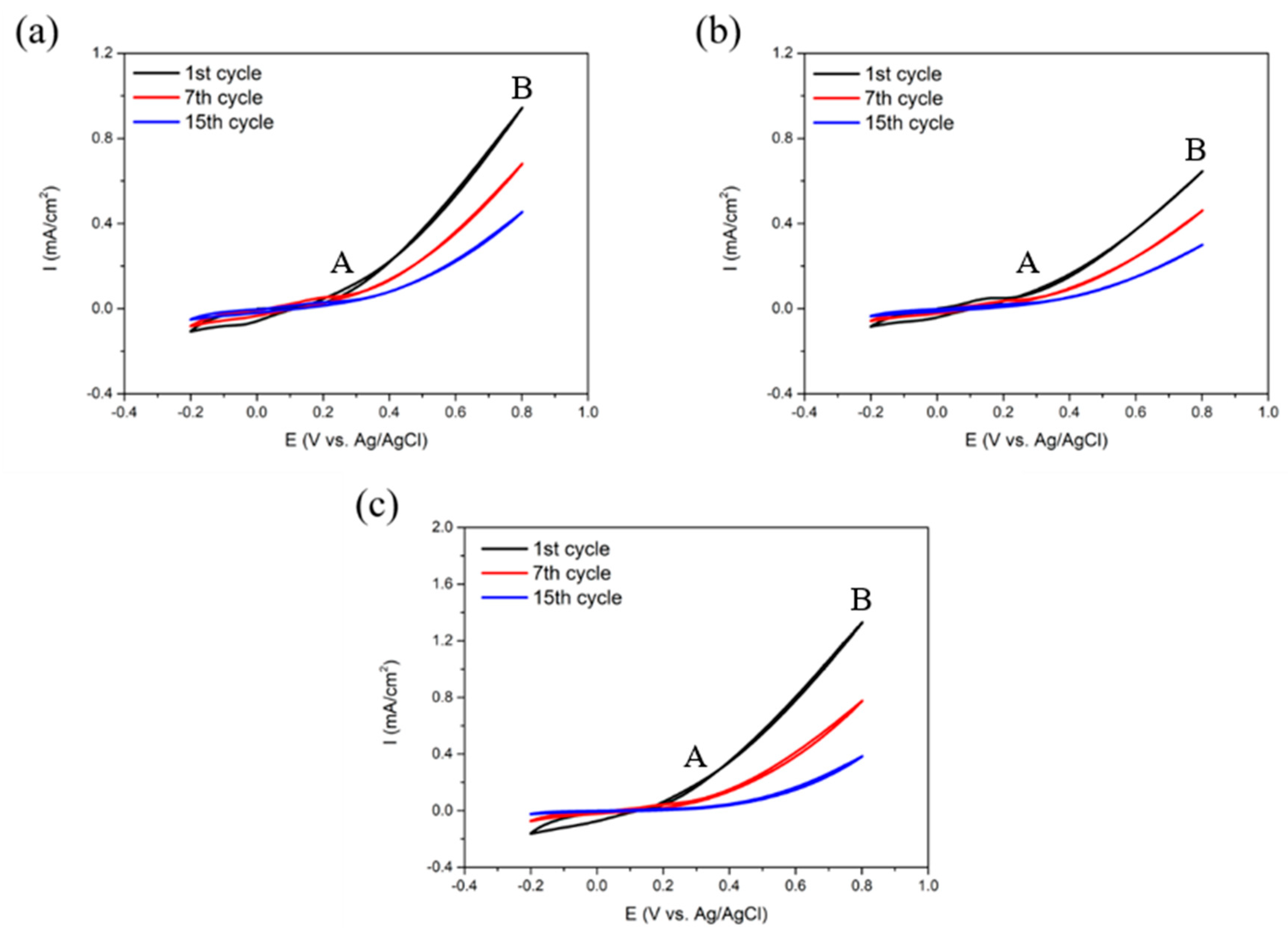
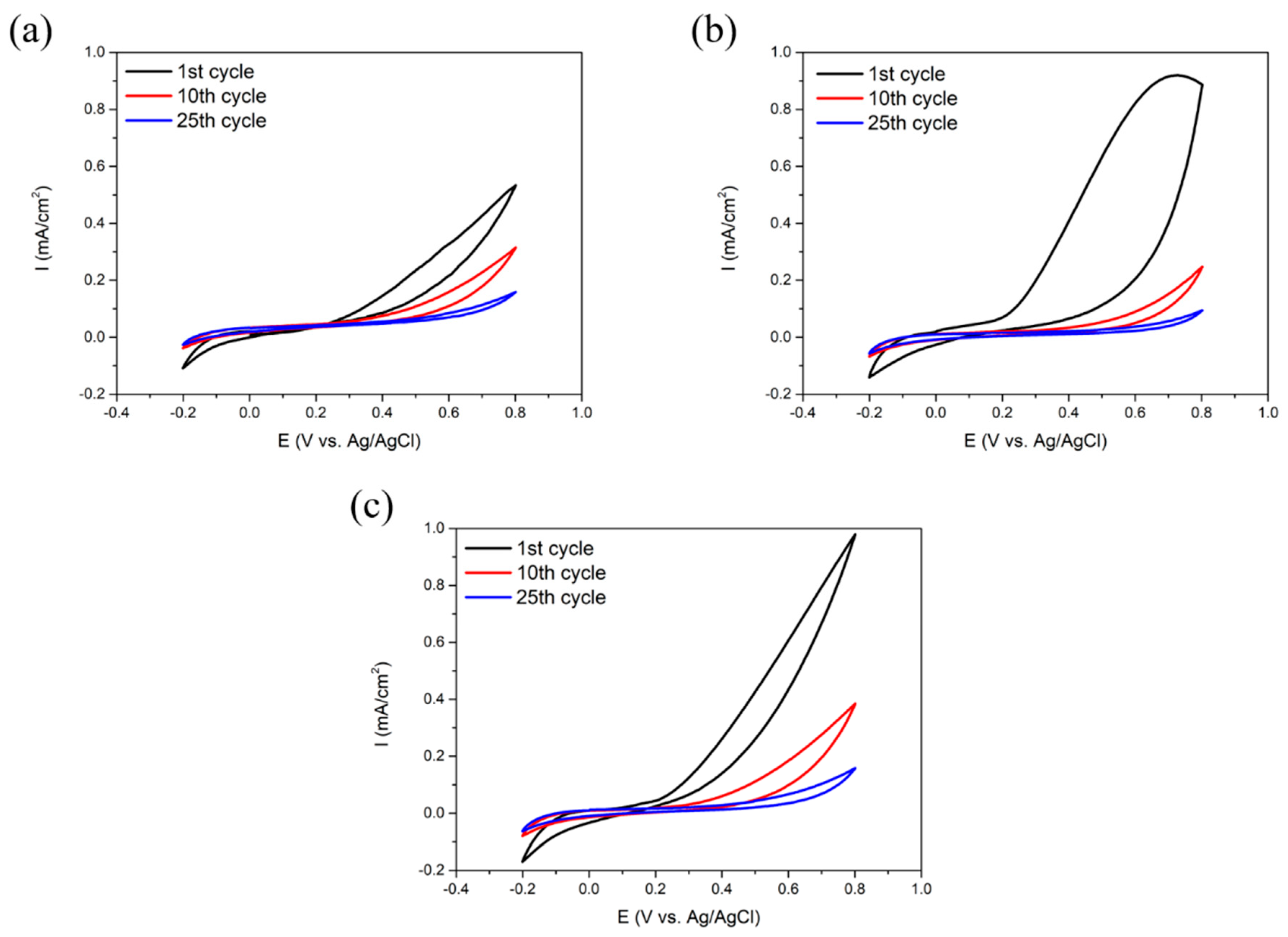
3.1. Electrodeposition of SPAN
3.2. Material Analysis
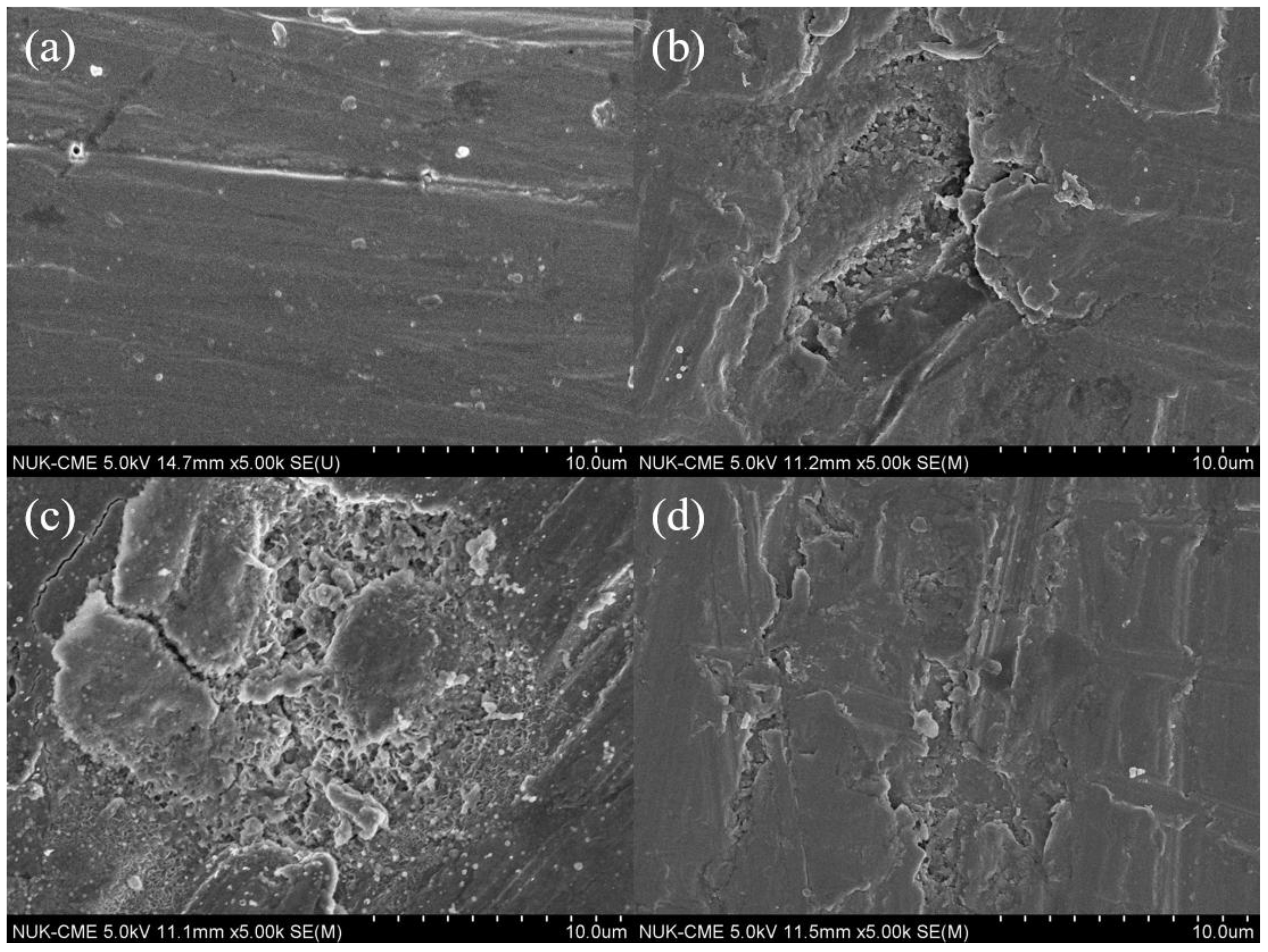
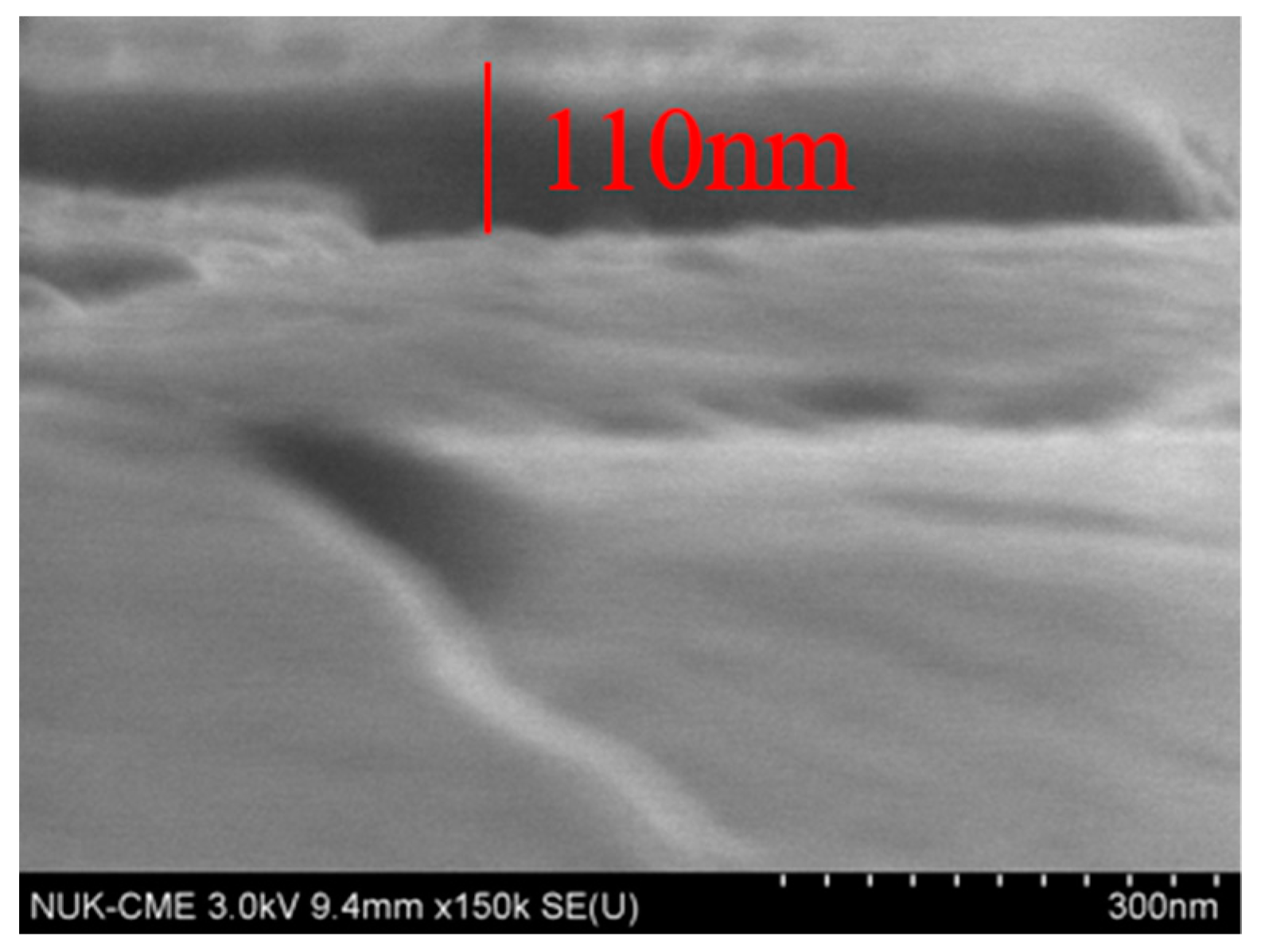
3.2.1. Morphology of SPAN Deposits
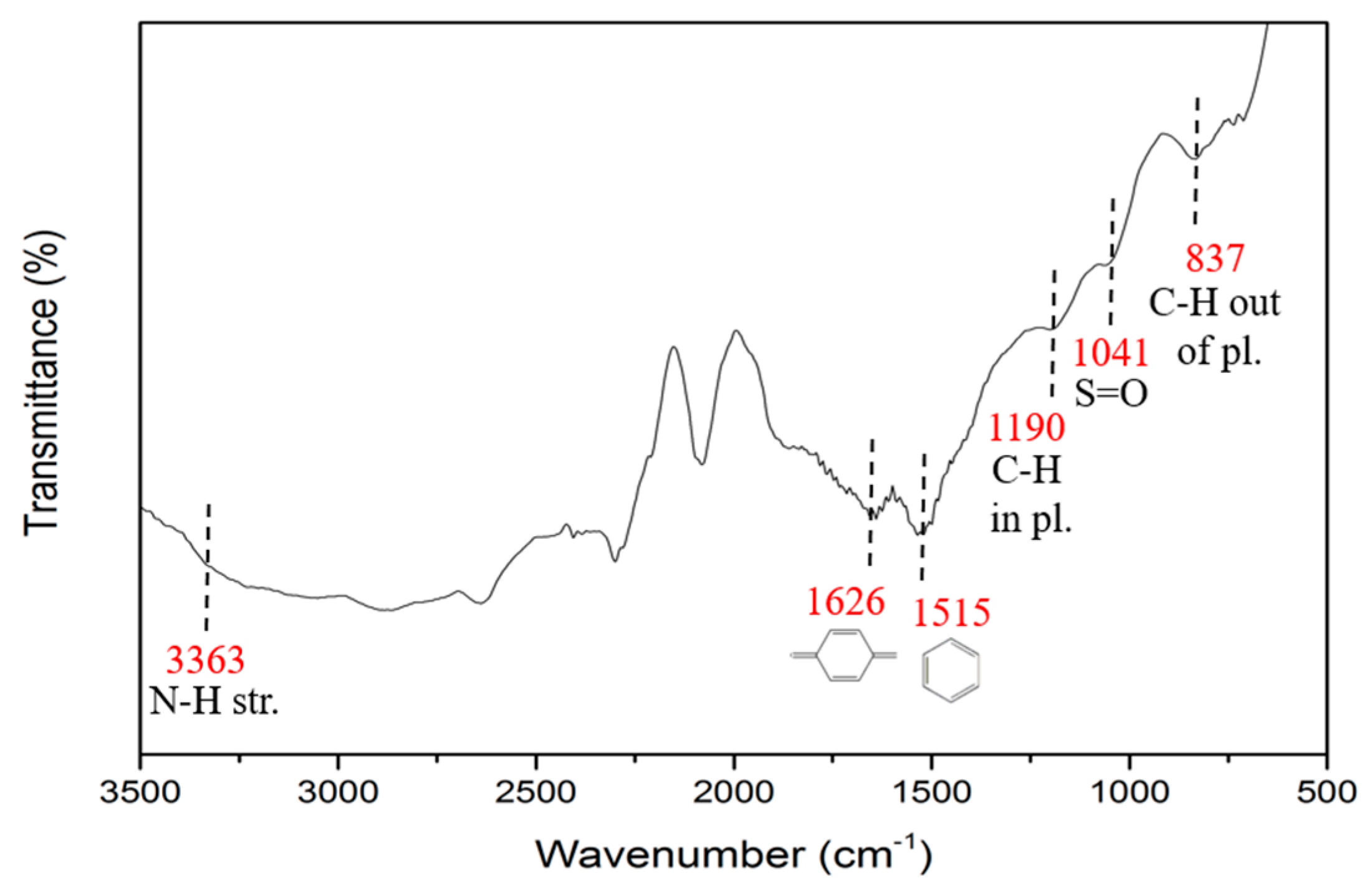
3.2.2. FTIR Spectra
3.3. Characterization of the Anticorrosion Properties of the Deposited Polymer Layers
3.3.1. Electrochemical Measurements
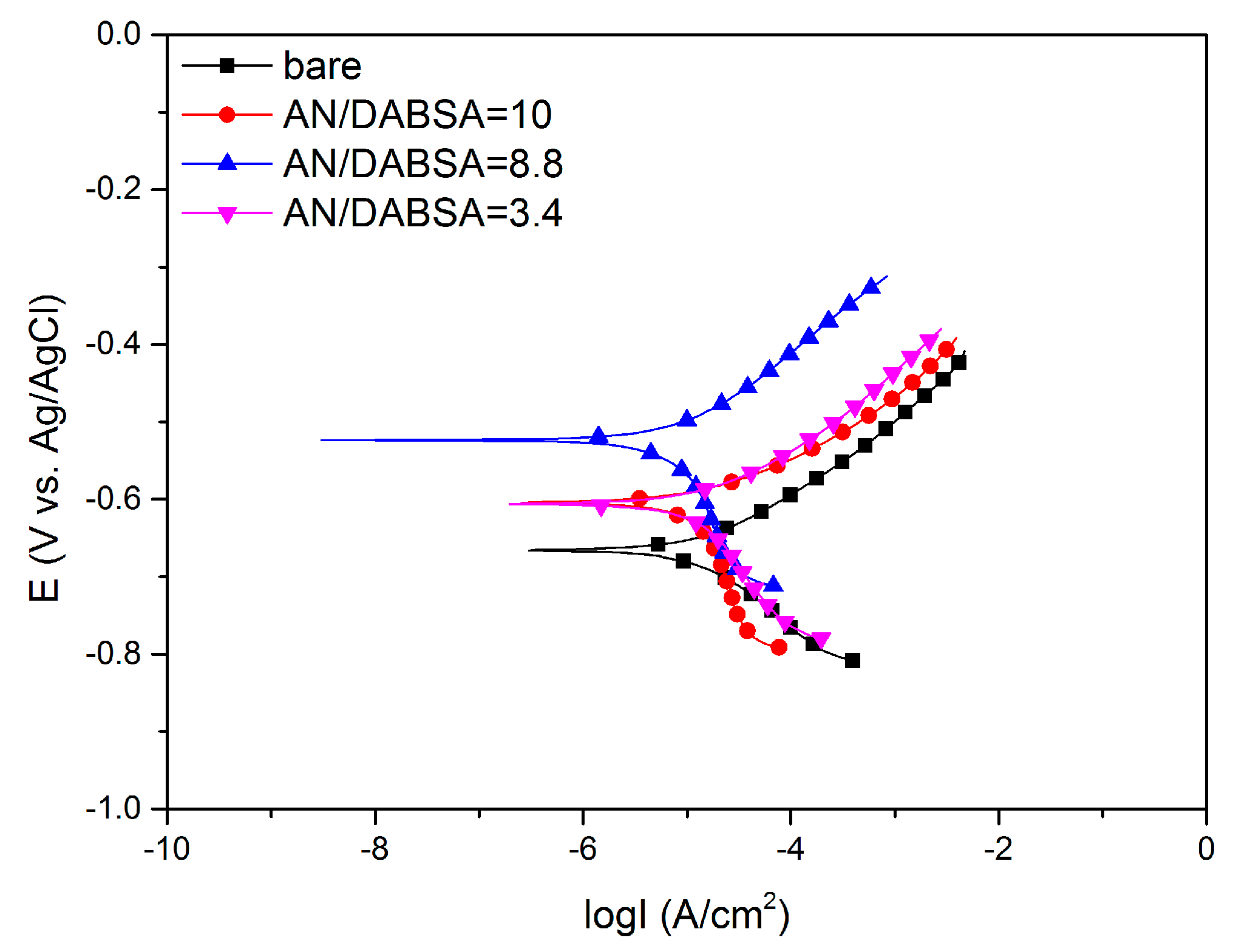
Corrosion Test in HCl
Corrosion Test in NaCl
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Santos, J.; Mattoso, L.; Motheo, A. Investigation of corrosion protection of steel by polyaniline films. Electrochim. Acta 1998, 43, 309–313. [Google Scholar] [CrossRef]
- Cai, K.; Zuo, S.; Luo, S.; Yao, C.; Liu, W.; Ma, J.; Mao, H.; Li, Z. Preparation of polyaniline/graphene composites with excellent anti-corrosion properties and their application in waterborne polyurethane anticorrosive coatings. RSC Adv. 2016, 6, 95965–95972. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Zhang, L.; Hou, B. Preparation and photocathodic protection performance of CdSe/reduced graphene oxide/TiO2 composite. Corros. Sci. 2015, 94, 342–349. [Google Scholar] [CrossRef]
- Inagaki, M. Carbon coating for enhancing the functionalities of materials. Carbon 2012, 50, 3247–3266. [Google Scholar] [CrossRef]
- Khan, M.I.; Chaudhry, A.U.; Hashim, S.; Zahoor, M.K.; Iqbal, M.Z. Recent developments in intrinsically conductive polymer coatings for corrosion protection. Chem. Eng. Res. Bull. 2010, 14, 73–86. [Google Scholar] [CrossRef]
- Mooss, V.A.; Bhopale, A.A.; Deshpande, P.P.; Athawale, A.A. Graphene oxide-modified polyaniline pigment for epoxy based anti-corrosion coatings. Chem. Pap. 2017, 71, 1515–1528. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Jadhav, N.G.; Gelling, V.J.; Sazou, D. Conducting polymers for corrosion protection: A review. J. Coat. Technol. Res. 2014, 11, 473–494. [Google Scholar] [CrossRef]
- Liao, Y.; Strong, V.; Chian, W.; Wang, X.; Li, X.-G.; Kaner, R.B. Sulfonated polyaniline nanostructures synthesized via rapid initiated copolymerization with controllable morphology, size, and electrical properties. Macromolecules 2012, 45, 1570–1579. [Google Scholar] [CrossRef]
- Wessling, B. Passivation of metals by coating with polyaniline: Corrosion potential shift and morphological changes. Adv. Mater. 1994, 6, 226–228. [Google Scholar] [CrossRef]
- DeBerry, D.W. Modification of the electrochemical and corrosion behavior of stainless steels with an electroactive coating. J. Electrochem. Soc. 1985, 132, 1022–1026. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, J.; Jia, X.; Yeh, J.M.; Spellane, P. Polyaniline as corrosion protection coatings on cold rolled steel. Polymer 1995, 36, 4535–4537. [Google Scholar] [CrossRef]
- Williams, G.; Mamurray, H.N. Polyaniline inhibition of filiform corrosion on organic coated AA2024-T3. Electrochim. Acta 2009, 54, 4245–4252. [Google Scholar] [CrossRef]
- Qiu, S.; Chen, C.; Zheng, W.; Li, W.; Zhao, H.; Wang, L. Long-term corrosion protection of mild steel by epoxy coating containing self-doped polyaniline nanofiber. Synth. Met. 2017, 229, 39–46. [Google Scholar] [CrossRef]
- Syed, J.A.; Tang, S.; Lu, H.; Meng, X. Water-soluble polyaniline–polyacrylic acid composites as efficient corrosion inhibitors for 316SS. Ind. Eng. Chem. Res. 2015, 54, 2950–2959. [Google Scholar] [CrossRef]
- Motheo, A.J.; Pantoja, M.F.; Venancio, E.C. Effect of monomer ratio in the electrochemical synthesis of poly(aniline-co-o-methoxyaniline). Solid State Ion. 2004, 171, 91–98. [Google Scholar] [CrossRef]
- Li, X.G.; Huang, M.R.; Lu, Y.Q.; Zhu, M.F. Synthesis and properties of processible copolymer microparticles from chloroanilines and aniline. J. Mater. Chem. 2005, 15, 1343–1352. [Google Scholar]
- Ates, M. A review on conducting polymer coatings for corrosion protection. J. Adhes. Sci. Technol. 2016, 30, 1510–1536. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Yang, C.H.; Wen, T.C. Polyaniline derivative with external and internal doping via electrochemical copolymerization of aniline and 2,5-diaminobenzenesulfonic acid on IrO2-coated titanium electrode. J. Electrochem. Soc. 1994, 141, 2624–2632. [Google Scholar] [CrossRef]
- Ping, Z. In situ FTIR-attenuated total reflection spectroscopic investigations on the base-acid transitions of polyaniline. Base-acid transition in the emeraldine form of polyaniline. J. Chem. Soc. Faraday Trans. 1996, 92, 3063–3067. [Google Scholar] [CrossRef]
- Miroslava, T.; Šeděnková, I.; Tobolková, E.; Stejskal, J. FTIR spectroscopic and conductivity study of the thermal degradation of polyaniline films. Polym. Degrad. Stab. 2004, 86, 179–185. [Google Scholar]
- Yüce, A.O.; Mert, B.D.; Kardaş, G. Electrochemical and quantum chemical studies of 2-amino-4-methyl-thiazole as corrosion inhibitor for mild steel in HCl solution. Corros. Sci. 2014, 83, 310–316. [Google Scholar] [CrossRef]
- Grgur, B.; Elkais, A.; Gvozdenović, M.; Drmanić, S.; Trišović, T.L.; Jugović, B. Corrosion of mild steel with composite polyaniline coatings using different formulations. Prog. Org. Coat. 2015, 79, 17–24. [Google Scholar] [CrossRef]
- Dkhireche, N.; Dahami, A.; Rochdi, A.; Hmimou, J.; Touir, R.; Touhami, M.E.; El Bakri, M.; El Hallaoui, A.; Anouar, A.; Takenouti, H. Corrosion and scale inhibition of low carbon steel in cooling water system by 2-propargyl-5-o-hydroxyphenyltetrazole. J. Ind. Eng. Chem. 2013, 19, 1996–2003. [Google Scholar] [CrossRef]
- Danaee, I.; Khomami, M.N.; Attar, A. Corrosion behavior of AISI 4130 steel alloy in ethylene glycol–water mixture in presence of molybdate. Mater. Chem. Phys. 2012, 135, 658–667. [Google Scholar] [CrossRef]
- Li, P.; Tan, T.; Lee, J. Corrosion protection of mild steel by electroactive polyaniline coatings. Synth. Met. 1997, 88, 237–242. [Google Scholar] [CrossRef]
- Holness, R.J.; Williams, G.; Worsley, D.A.; McMurray, H.N. Polyaniline inhibition of corrosion-driven organic coating cathodic delamination on iron. J. Electrochem. Soc. 2005, 152, B73–B81. [Google Scholar] [CrossRef]
- Williams, G.; Gabriel, A.; Cook, A.; McMurray, H.N. Dopant anion effects in the inhibition by polyaniline of corrosion-driven organic coating delamination on iron. J. Electrochem. Soc. 2006, 153, B425–B433. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Muthukrishnan, S.; Venkatachari, G.; Trivedi, D.C. Corrosion protection of steel by polyaniline (PANI) pigmented paint coating. Prog. Org. Coat. 2005, 53, 297–301. [Google Scholar] [CrossRef]
- De Souza, S.; da Silva, J.E.P.; de Torresi, S.I.C.; Temperini, M.L.A.; Torresi, R.M. Polyaniline based acrylic blends for iron corrosion protection. Electrochem. Solid-State Lett. 2001, 4, B27–B30. [Google Scholar] [CrossRef]
- Kendig, M.; Hon, M.; Warren, L. Smart corrosion inhibiting coatings. Prog. Org. Coat. 2003, 47, 183–189. [Google Scholar] [CrossRef]
- Sazou, D.; Kourouzidou, M. Electrochemical synthesis and anticorrosive properties of Nafion®-poly(aniline-co-aminophenol) coatings on stainless steel. Electrochim. Acta 2009, 54, 2425–2433. [Google Scholar] [CrossRef]
- Kosseoglou, D.; Kokkinofta, R.; Sazou, D. FTIR spectroscopic characterization of Nafion®-polyaniline composite films employed for the corrosion control of stainless steel. J. Solid State Electrochem. 2011, 15, 2619–2631. [Google Scholar] [CrossRef]



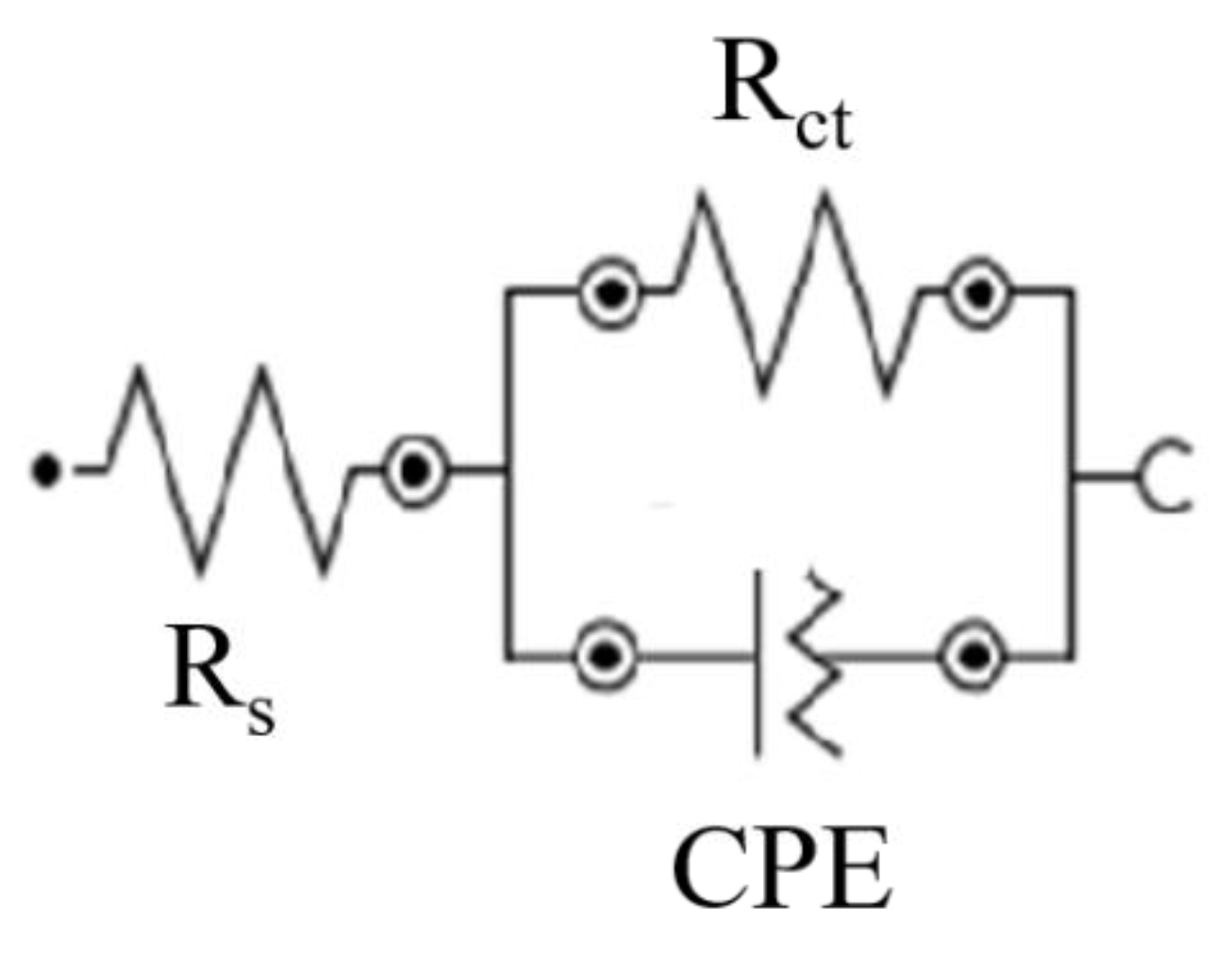
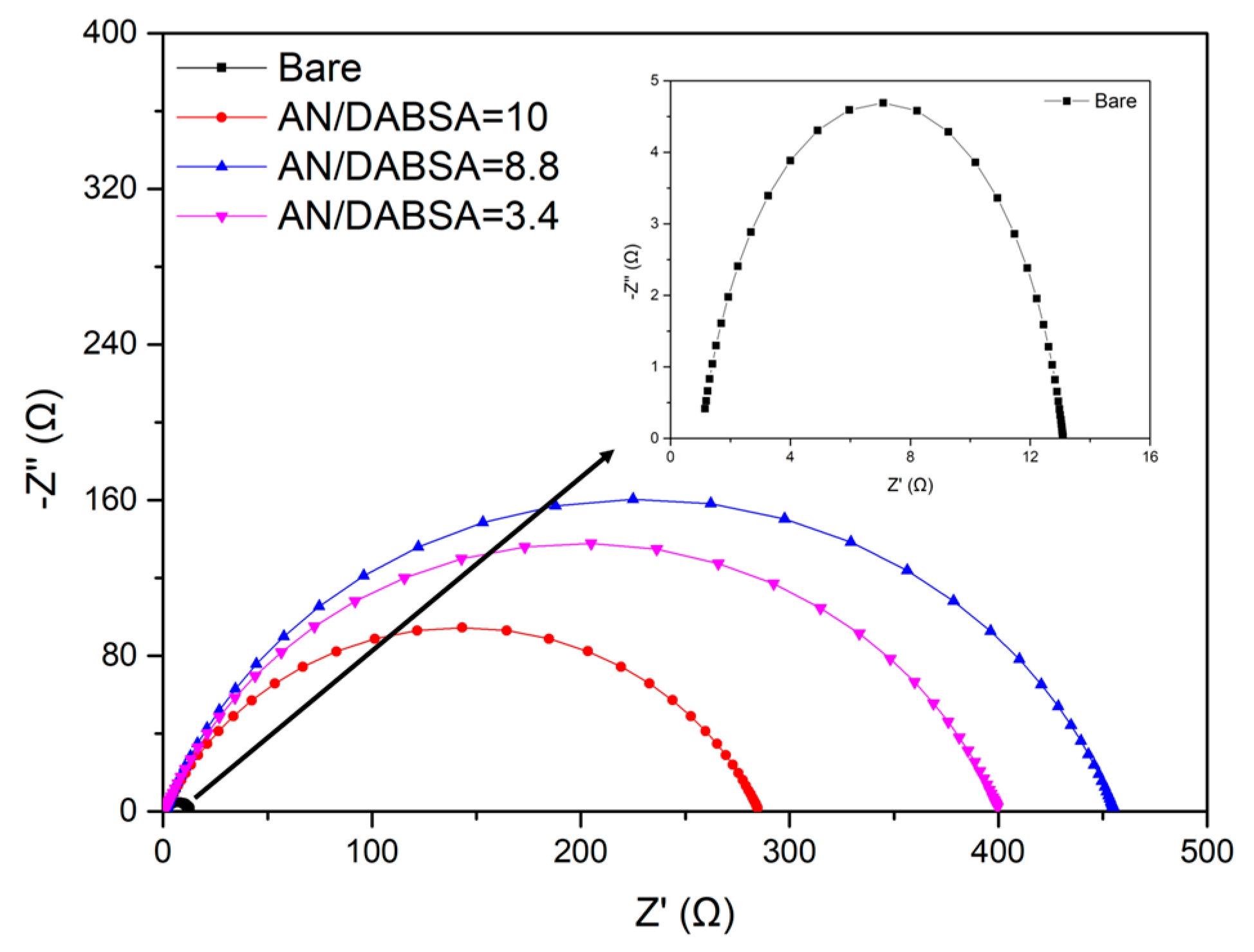


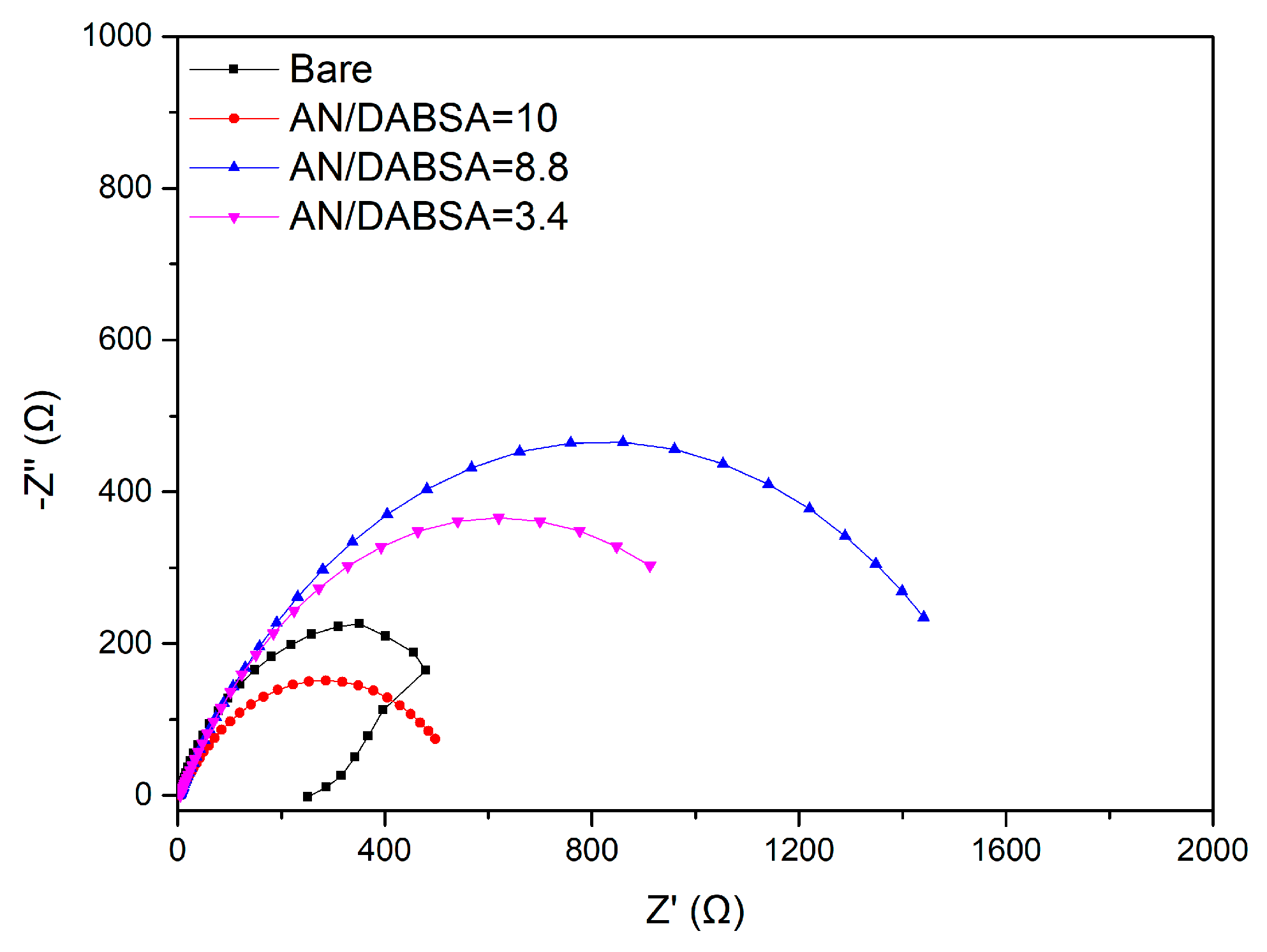
| Sample | Rct (Ω cm2) | PE (%) |
|---|---|---|
| Bare | 12.0 (0.16) | – |
| AN/DABSA = 10 | 283.4 (1.51) | 95.8 (0.51) |
| AN/DABSA = 8.8 | 453.5 (3.24) | 97.4 (0.70) |
| AN/DABSA = 3.4 | 398.0 (2.16) | 97.0 (0.53) |
| Sample | Ecorr (mV vs. Ag/AgCl) | Icorr (μA) | Rp (Ω cm2) | C.R. (mm year−1) |
|---|---|---|---|---|
| Bare | −410 (4.14) | 953.0 (9.62) | 16.6 (0.17) | 11.11 (0.11) |
| AN/DABSA = 10 | −408 (6.16) | 78.8 (1.19) | 288.0 (4.34) | 0.92 (0.01) |
| AN/DABSA = 8.8 | −411 (5.59) | 66.9 (0.91) | 331.2 (4.50) | 0.78 (0.01) |
| AN/DABSA = 3.4 | −411 (2.68) | 69.0 (0.45) | 300.5 (1.96) | 0.80 (0.05) |
| Sample | Rct (Ω cm2) | PE (%) |
|---|---|---|
| Bare | 246.5 (2.01) | – |
| AN/DABSA = 10 | 494.0 (4.08) | 50.1 (0.42) |
| AN/DABSA = 8.8 | 1437.5 (22.46) | 82.9 (1.29) |
| AN/DABSA = 3.4 | 906.9 (17.10) | 72.9 (1.37) |
| Sample | Ecorr (mV vs. Ag/AgCl) | Icorr (μA) | Rp (Ω cm2) | C.R. (mm year−1) |
|---|---|---|---|---|
| Bare | −665 (13.04) | 20.9 (0.41) | 1184.4 (23.23) | 0.24 (0.004) |
| AN/DABSA = 10 | −604 (8.86) | 14.3 (0.21) | 1946.1 (28.57) | 0.16 (0.002) |
| AN/DABSA = 8.8 | −523 (11.88) | 8.8 (0.20) | 4169.0 (94.75) | 0.10 (0.002) |
| AN/DABSA = 3.4 | −606 (19.34) | 14.1 (0.45) | 1916.1 (61.15) | 0.16 (0.005) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.-W.; Wang, T.-L.; Lin, W.-C.; Lin, H.-Y.; Lee, M.-H.; Yang, C.-H. Anti-Corrosion Characteristics of Electrodeposited Self-Doped Polyaniline Films on Mild Steel in Low Acidity. Coatings 2018, 8, 155. https://doi.org/10.3390/coatings8050155
Wu J-W, Wang T-L, Lin W-C, Lin H-Y, Lee M-H, Yang C-H. Anti-Corrosion Characteristics of Electrodeposited Self-Doped Polyaniline Films on Mild Steel in Low Acidity. Coatings. 2018; 8(5):155. https://doi.org/10.3390/coatings8050155
Chicago/Turabian StyleWu, Jhen-Wei, Tzong-Liu Wang, Wen-Churng Lin, Hung-Yin Lin, Mei-Hwa Lee, and Chien-Hsin Yang. 2018. "Anti-Corrosion Characteristics of Electrodeposited Self-Doped Polyaniline Films on Mild Steel in Low Acidity" Coatings 8, no. 5: 155. https://doi.org/10.3390/coatings8050155






