Photocatalytic Behavior of Water-Based Styrene-Acrylic Coatings Containing TiO2 Sensitized with Metal-Phthalocyanine Tetracarboxylic Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Modification of TiO2 with Metal-Phthalocyanine Tetracarboxylic Acids
2.3. Characterization of TiO2 Sensitized with Metal-Phthalocyanine Tetracarboxylic Acids
2.4. Preparation of Styrene-Acrylic Coatings
2.5. Coatings Characterization and Evaluation of Photocatalytic Performances
3. Results and Discussion
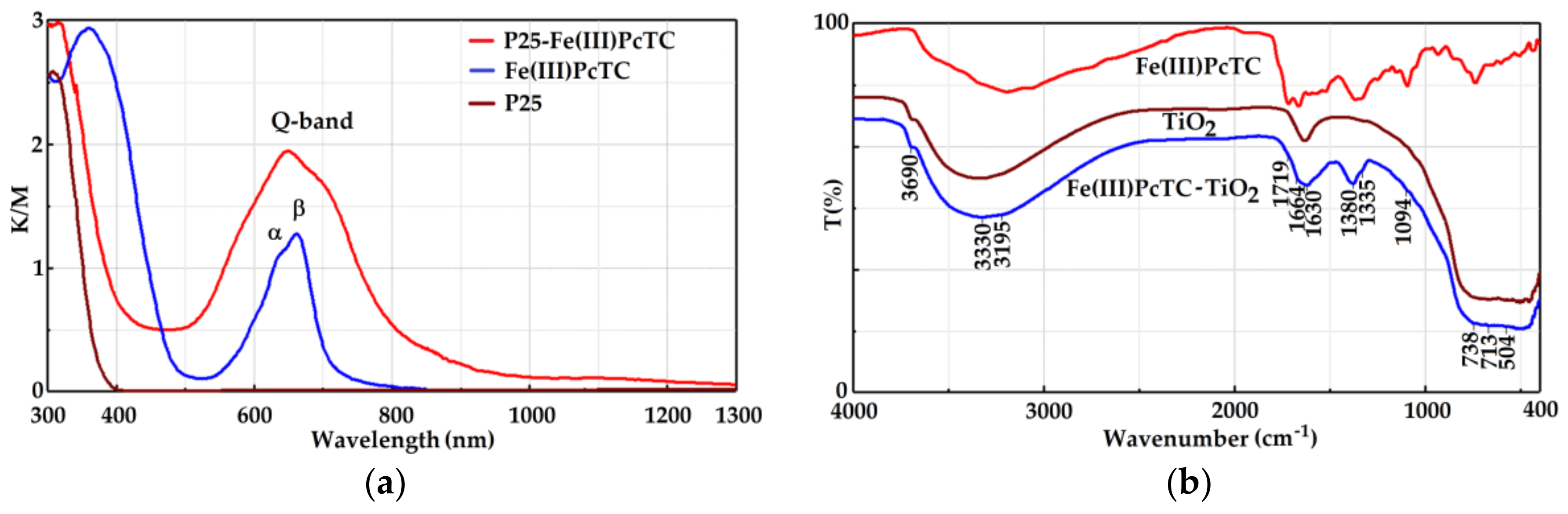
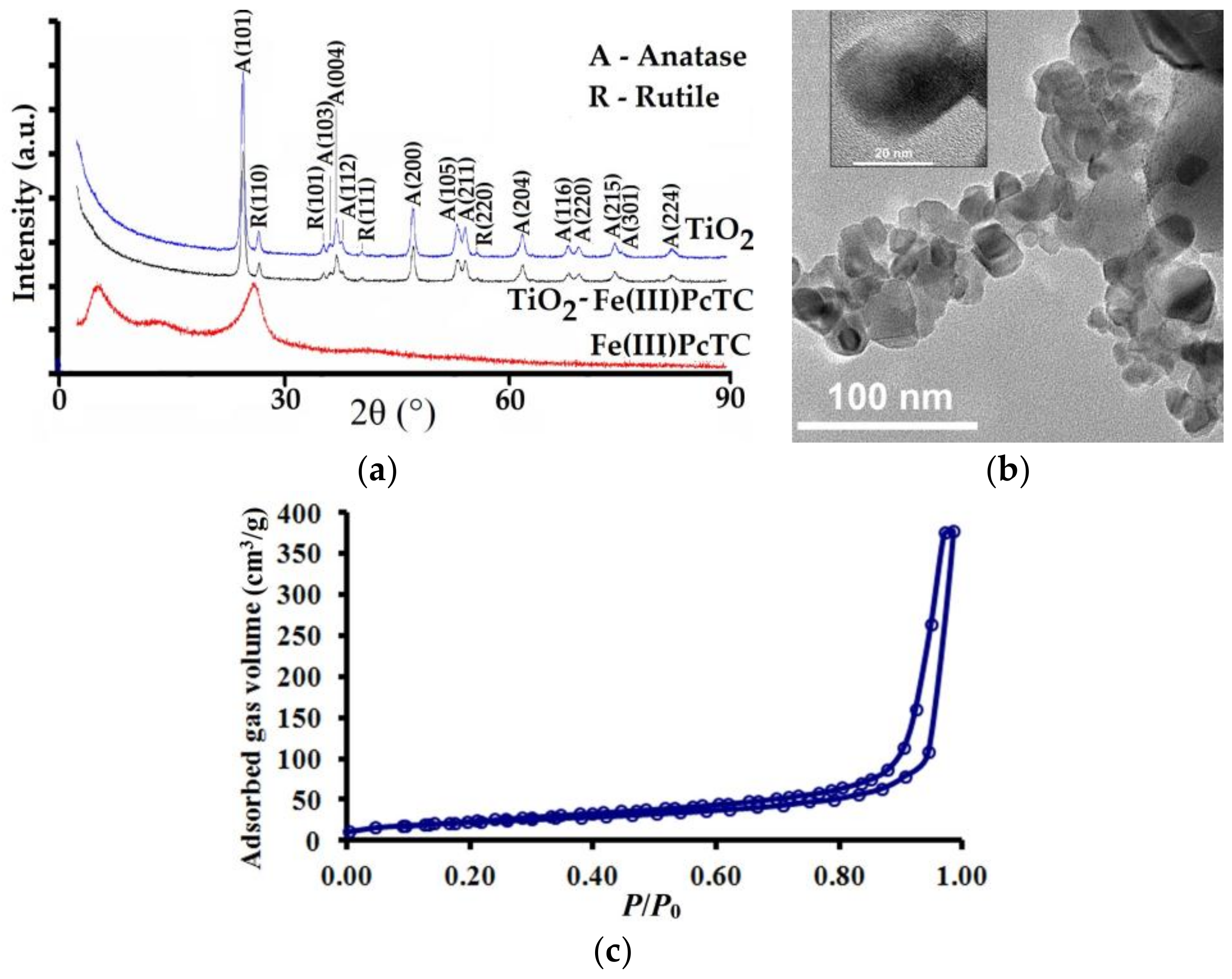
3.1. Characterization of the Photocatalysts
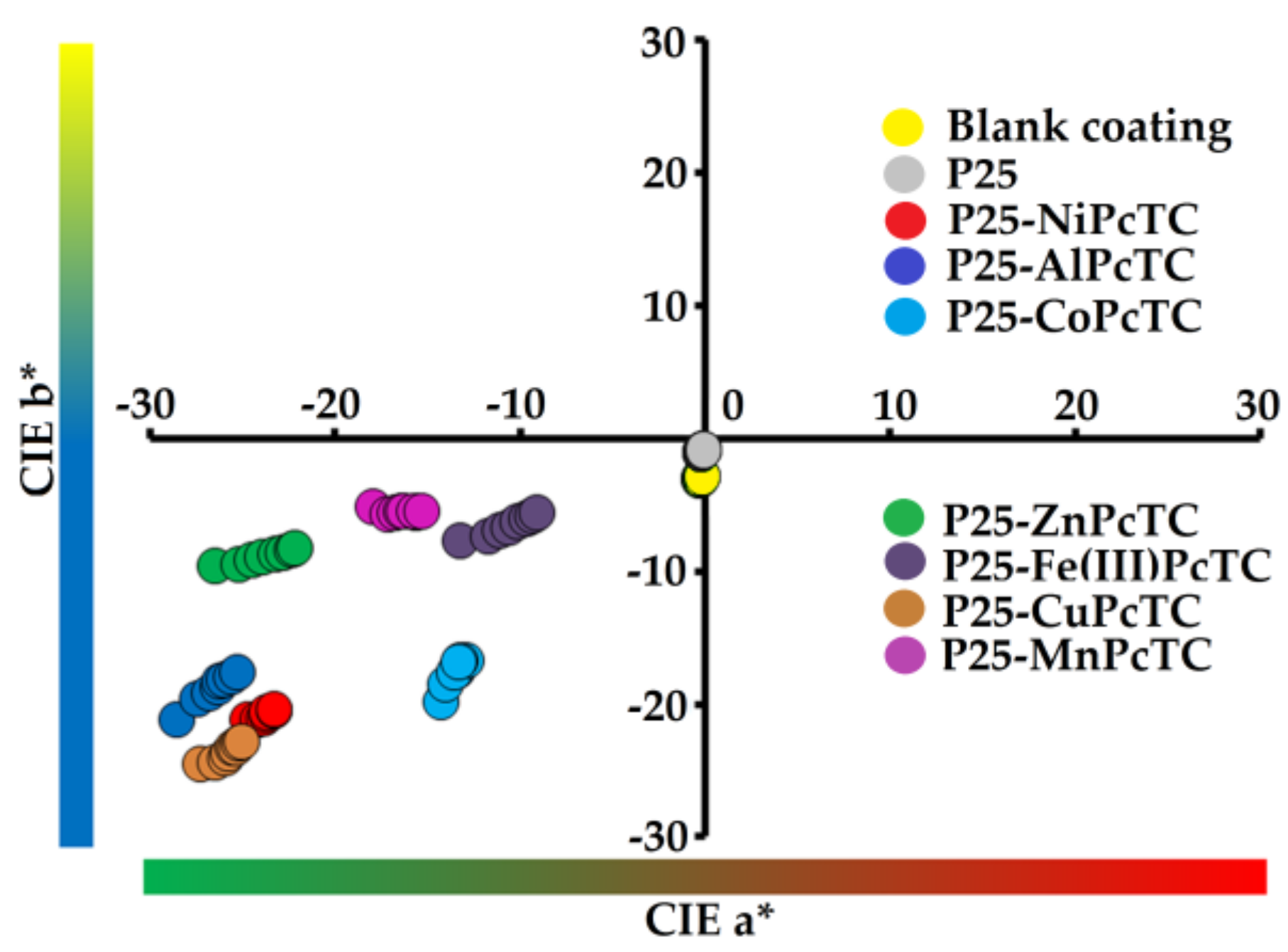
3.2. Coatings Properties
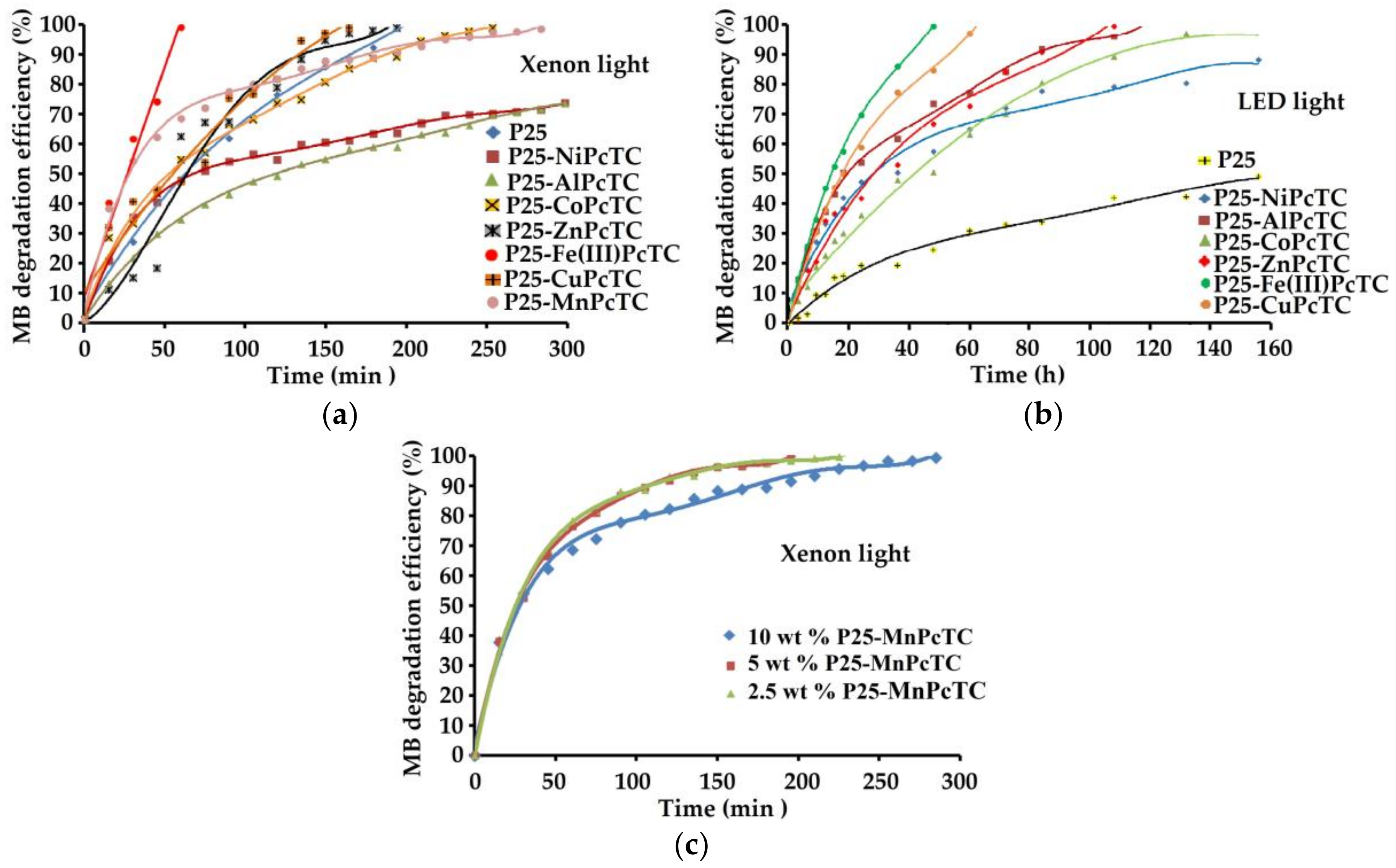
3.3. Coatings Photocatalytic Performances
4. Conclusions
Supplementary Material
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mills, A.; O’Rourke, C.; Moore, K. Powder semiconductor photocatalysis in aqueous solution: An overview of kinetics-based reaction mechanisms. J. Photochem. Photobiol. A 2015, 310, 66–105. [Google Scholar] [CrossRef]
- Bayrak, R.; Albay, C.; Koc, M.; Altin, I.; Degirmencioglu, I.; Sokmen, M. Preparation of phthalocyanine/TiO2 nanocomposites for photocatalytic removal of toxic Cr(VI) ions. Proc. Saf. Environ. Prot. 2016, 102, 294–302. [Google Scholar] [CrossRef]
- Salinas-Guzman, R.R.; Guzman-Mar, J.L.; Hinojosa-Reyes, L.; Peralta-Hernandez, J.M.; Hernandez-Ramirez, A. Enhancement of cyanide photocatalytic degradation using sol-gel ZnO sensitized with cobalt phthalocyanine. J. Sol-Gel Sci. Technol. 2010, 54, 1–7. [Google Scholar] [CrossRef]
- Cozzoli, P.D.; Kornowski, A.; Weller, H. Low-temperature synthesis of soluble and processable organic-capped Anatase TiO2 nanorods. J. Am. Chem. Soc. 2003, 125, 14539–14548. [Google Scholar] [CrossRef] [PubMed]
- Xagas, A.P.; Androulaki, E.; Hiskia, A.; Falaras, P. Preparation, fractal surface morphology and photocatalytic properties of TiO2 films. Thin Solid Films 1999, 357, 173–178. [Google Scholar] [CrossRef]
- Tomovska, R.; Daniloska, V.; Asua, J.M. Surface modification of TiO2 nanoparticles via photocataliticaly induced reaction: Influence of functionality of silane coupling agent. Appl. Surf. Sci. 2013, 264, 670–673. [Google Scholar] [CrossRef]
- Rahimi, R.; Moghaddas, M.M.; Zargari, S. Investigation of the anchoring silane coupling reagent effect in porphyrin sensitized mesoporous V–TiO2 on the photodegradation efficiency of methyl orange under visible light irradiation. J. Sol-Gel Sci. Technol. 2013, 65, 420–429. [Google Scholar] [CrossRef]
- Stefanov, B.I.; Kaneva, N.V.; Li Puma, G.; Dushkin, C.D. Novel integrated reactor for evaluation of activity of supported photocatalytic thin films: Case of methylene blue degradation on TiO2 and nickel modified TiO2 under UV and visible light. Colloids Surf. A 2011, 382, 219–225. [Google Scholar] [CrossRef]
- Madhu Kumar, P.; Badrinarayanan, S.; Sastry, M. Nanocrystalline TiO2 studied by optical, FTIR and X-ray photoelectron spectroscopy: Correlation to presence of surface states. Thin Solid Films 2000, 358, 122–130. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Kruse, O.; Rupprecht, J.; Mussgnug, J.H.; Dismukes, G.C.; Hankamer, B. Photosynthesis: A blueprint for solar energy capture and biohydrogen production technologies. Photochem. Photobiol. Sci. 2005, 4, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Suttponparnit, K.; Jiang, J.; Sahu, M.; Suvachittanont, S.; Charinpanitkul, T.; Biswas, P. Role of surface area, primary particle size and crystal phase on titanium dioxide nanoparticle dispersion properties. Nanoscale Res. Lett. 2010, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, X.; Chen, Z.; Cheng, H.; Lu, G. The role of crystal phase in determining photocatalytic activity of nitrogen doped TiO2. J. Colloid Interface Sci. 2009, 329, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.A.; Vithal, M.; Back, I.C.; Seok, S.I. Morphological and phase evolution of TiO2 nanocrystals prepared from peroxotitanate complex aqueous solution: Influence of acetic acid. J. Solid State Chem. 2009, 182, 749–756. [Google Scholar] [CrossRef]
- Lee, J.W.; Othman, M.R.; Eom, Y.; Lee, T.G.; Kim, W.S.; Kim, J. The effects of sonification and TiO2 deposition on the micro-characteristics of the thermally treated SiO2/TiO2 spherical core-shell particles for photo-catalysis of methyl orange. Microporous Mesoporous Mater. 2008, 116, 561–568. [Google Scholar] [CrossRef]
- Rehman, S.; Ullah, R.; Butt, A.M.; Gohar, N.D. Strategies of making TiO2 and ZnO visible light active. J. Hazard. Mater. 2009, 170, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.; Ntiribinyange, M.; Nyamayaro, K.; Fester, V. Photocatalytic activities of ultra-small β-FeOOH and TiO2 heterojunction structure under simulated solar irradiation. Mater. Res. Bull. 2015, 68, 133–141. [Google Scholar] [CrossRef]
- Rawal, S.B.; Chakraborty, A.K.; Lee, W.I. Heterojunction of FeOOH and TiO2 for the formation of visible light photocatalyst. Bull. Korean Chem. Soc. 2009, 30, 2613–2616. [Google Scholar]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’Shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New insights into the mechanism of visible light photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallejo, W.; Diaz-Uribe, C.; Cantillo, A. Methylene blue photocatalytic degradation under visible irradiation on TiO2 thin films sensitized with Cu and Zn tetracarboxy-phthalocyanines. J. Photochem. Photobiol. A 2015, 299, 80–86. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Z.; Chen, Y.; Shi, L.; Zhu, Y. Enhancement of photocatalytic degradation of polyethylene plastic with CuPc modified TiO2 photocatalyst under solar light irradiation. Appl. Surf. Sci. 2008, 254, 1825–1829. [Google Scholar] [CrossRef]
- Wang, S.; Fang, Y.; Yang, Y.; Liu, J.; Deng, A.; Zhao, X.; Huang, Y. Catalysis of organic pollutant photodegradation by metal phthalocyanines immobilized on TiO2@SiO2. Chin. Sci. Bull. 2011, 56, 969–976. [Google Scholar] [CrossRef]
- Machado, A.E.H.; Franca, M.D.; Velani, V.; Magnino, G.A.; Velani, H.M.M.; Freitas, F.S.; Muller, P.S., Jr.; Sattler, C.; Scmucker, M. Characterization and evaluation of the efficiency of TiO2/Zinc phthalocyanine nanocomposites as photocatalysts for wastewater treatment using solar irradiation. Int. J. Photoenergy 2008, 2008, 482373. [Google Scholar] [CrossRef]
- Ino, D.; Watanabe, K.; Takagi, N.; Matsumoto, Y. Electron transfer dynamics from organic adsorbate to a semiconductor surface: Zinc phthalocyanine on TiO2(110). J. Phys. Chem. B 2005, 109, 18018–18024. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, G.; Gutierrez, M.C.; Ferrer, M.L.; Gil-Luna, M.D.; Augugliaro, V.; Yurdakal, S.; Pagliaro, M. TiO2/ORMOSIL thin films doped with phthalocyanine dyes: New photocatalytic devices activated by solar light. J. Phys. Chem. C 2008, 112, 2667–2670. [Google Scholar] [CrossRef]
- He, J.; Hagfeldt, A.; Lindquist, S.E.; Grennberg, H.; Korodi, F.; Sun, L.; Akermark, B. Phthalocyanine-sensitized nanostructured TiO2 electrodes prepared by a novel anchoring method. Langmuir 2001, 17, 2743–2747. [Google Scholar] [CrossRef]
- Rodriguez, H.B.; Di Iorio, Y.; Meichtry, J.M.; Grela, M.A.; Litter, M.; Roman, E.S. Evidence on dye clustering in the sensitization of TiO2 by aluminum phthalocyanine. Photochem. Photobiol. Sci. 2013, 12, 1984–1990. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jeon, J.D.; Chung, J.W.; Kwak, S.Y. Amplified visible light photocatalytic activity of mesoporous TiO2/ZnPc hybrid by cascade Mie light scattering. Microporous Mesoporous Mater. 2016, 227, 169–175. [Google Scholar] [CrossRef]
- Beyrhouty, M.; Sorokin, A.B.; Daniele, S.; Hubert-Pfalzagraf, L.G. Combination of two catalytic sites in a novel nanocrystalline TiO2–iron tetrasulfophthaocyanine material provides better catalytic properties. New J. Chem. 2005, 29, 1245–1248. [Google Scholar] [CrossRef]
- Pei, D.; Luan, J. Development of visible light-responsive sensitized photocatalysts. Int. J. Photoenergy 2012, 2012, 262831. [Google Scholar] [CrossRef]
- Raza, M.; Bachinger, A.; Zahn, N.; Kickelbick, G. Interaction and UV-stability of various organic capping agents on the surface of anatase nanoparticles. Materials 2014, 7, 2890–2912. [Google Scholar] [CrossRef] [PubMed]
- Pazokifard, S.; Esfandeh, M.; Mirabedini, S.M. Photocatalytic activity of water-based acrylic coatings containing fluorosilane treated TiO2 nanoparticles. Prog. Org. Coat. 2014, 77, 1325–1335. [Google Scholar] [CrossRef]
- Mirabedini, A.; Mirabedini, S.M.; Babalon, A.A.; Pazokifard, S. Synthesis, characterization and enhanced photocatalytic activity of TiO2/SiO2 nanocomposite in an aqueous solution and acrylic-based coatings. Prog. Org. Coat. 2011, 72, 453–460. [Google Scholar] [CrossRef]
- Sun, X.; Wang, L.; Tan, Z. Improved synthesis of soluble metal-free/metal phthalocyanine tetracarboxylic acids and their application in the catalytic epoxidation of cyclohexene. Catal. Lett. 2015, 145, 1094–1102. [Google Scholar] [CrossRef]
- Sorokin, A.B.; Buisson, P.; Pierre, A.C. Encapsulation of iron phthalocyanine in sol-gel materials. Microporous Mesoporous Mater. 2001, 46, 87–98. [Google Scholar] [CrossRef]
- Zhou, X.; Ji, H.; Huang, X. Photocatalytic degradation of Methyl Orange over metalloporphyrins supported on TiO2 Degussa P25. Molecules 2012, 17, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Grinter, D.C.; Nickels, P.; Woolcot, T.; Basahel, S.N.; Obaid, A.Y.; Al-Ghamdi, A.A.; El-Mossalamy, S.H.; Alyoubi, A.O.; Thornton, G. Binding of a benzoate dye-molecule analogue to rutile titanium dioxide surfaces. J. Phys. Chem. C 2012, 116, 1020–1026. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M.; Ortega, A.; Liauw, C.M.; Stratton, J.; McIntyre, R.B. Behaviour of nanoparticle (ultrafine) titanium dioxide pigments and stabilizers on the photooxidative stability of water based acrylic and isocyanate based acrylic coatings. Polym. Degrad. Stab. 2002, 78, 467–478. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M.; Verran, J.; Stratton, J.; Maltby, J.; Bygott, C. Photocatalytic titania based surfaces: Environmental benefits. Polym. Degrad. Stab. 2008, 93, 1632–1646. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M.; Ortega, A.; Sandoval, G.; Liauw, C.M.; Verran, J.; Stratton, J.; McIntyre, R.B. Degradation and stabilization of polymers and coatings: Nano versus pigmentary titania particles. Polym. Degrad. Stab. 2004, 85, 927–946. [Google Scholar] [CrossRef]
- Chatterjee, D. Effect of excited state redox properties of dye sensitizers on hydrogen production through photo-splitting of water over TiO2 photocatalyst. Catal. Commun. 2010, 11, 336–339. [Google Scholar] [CrossRef]
- Barbero, N.; Vione, D. Why dyes should not be used to test the photocatalytic activity of semiconductor oxides. Environ. Sci. Technol. 2016, 50, 2130–2131. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Chern, J.M. Kinetics of photocatalytic decomposition of methylene blue. Ind. Eng. Chem. Res. 2006, 45, 6450–6457. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M.; Sandoval, G.; Verran, J.; Stratton, J.; Maltby, J. Photocatalytic coatings for environmental applications. Photochem. Photobiol. 2005, 81, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Hill, C.; Robertson, P.K.J. Overview of the current ISO tests for photocatalytic materials. J. Photochem. Photobiol. A 2012, 237, 7–23. [Google Scholar] [CrossRef]
- Mills, A. An overview of the methyl blue ISO test for assessing the activities of photocatalytic films. Appl. Catal. B 2012, 128, 144–149. [Google Scholar] [CrossRef]
- Mills, A.; McFarlane, M. Current and possible future methods of assessing the activities of photocatalyst films. Catal. Today 2007, 129, 22–28. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloni, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B 2001, 31, 145–157. [Google Scholar] [CrossRef]



| Component | Role (Active Substance) | Quantity (wt %) |
|---|---|---|
| Water | medium | 20.3 |
| Parmetol A26 | preservative (mixture of 5-Chloro-2-methyl- and 2-Methyl-2H-isothiazol-3-one) | 0.1 |
| Parmetol DF12 | preservative (mixture of isothiazolinones) | 0.1 |
| BYK 034 | defoamer (mixture of paraffin based mineral oils and silicones) | 0.2 |
| ADDAPT DISP 500 | dispersant (ammonium polyacrylate) | 0.4 |
| Ammonia (5 wt %) solution | pH adjustment ( ammonia) | 0.1 |
| Latex DL450 | binder (styrene-acrylic) | 18.7 |
| Dowanol DPnB | coalescent (dipropylene glycol n-butyl ether) | 1 |
| OMYACARB 5TN | filler (CaCO3) | 48.5 |
| Cellosize HEC | thickener (hydroxyethyl cellulose) | 10.6 |
| Photocatalyst | Absorption Peaks (nm) | α/β Q-Band Intensity Ratio | Width of Q-Band Absorption Peaks (nm) | Energy Band Gap (eV) | |
|---|---|---|---|---|---|
| α-Q-Band | β-Q-Band | ||||
| P25 | – | – | – | – | 3.38 |
| P25-NiPcTC | 607 | 668 | 1.25 | 150 | 3.34 |
| P25-AlPcTC | 637 | 694 | 1.01 | 183 | 3.17 |
| P25-CoPcTC | 610 | 670 | 1.2 | 165 | 3.32 |
| P25-ZnPcTC | 637 | 686 | 0.97 | 148 | 3.18 |
| P25-Fe(III)PcTC | 650 | 703 | 1.17 | 174 | 3.34 |
| P25-CuPcTC | 609 | 678 | 1.25 | 165 | 2.74 |
| P25-MnPcTC | 639 | 674 | 1.01 | 149 | 3.34 |
| Photocatalyst | BET Surface Area (m2/g) | Pores Volume (BJH) (cm3/g) | Micropores Diameter (BJH) (nm) |
|---|---|---|---|
| P25 | 57 | 0.43 | 24 |
| P25-NiPcTC | 55 | 0.33 | 24 |
| P25-AlPcTC | 56 | 0.34 | 24 |
| P25-CoPcTC | 53 | 0.30 | 24 |
| P25-ZnPcTC | 50 | 0.31 | 34 |
| P25-Fe(III)PcTC | 54 | 0.23 | 31 |
| P25-CuPcTC | 54 | 0.38 | 33 |
| P25-MnPcTC | 53 | 0.44 | 34 |
| Photocatalyst | Gloss (Gloss Units) | ||
|---|---|---|---|
| Initial | LED Light | Xenon Light | |
| – | 21.2 | 19.4 | 9.8 |
| P25 | 16.8 | 14.5 | 11.9 |
| P25-NiPcTC | 22.6 | 16.9 | 14.7 |
| P25-AlPcTC | 23.7 | 17.8 | 13.8 |
| P25-CoPcTC | 22.6 | 17.8 | 19.1 |
| P25-ZnPcTC | 19.6 | 16.6 | 18.7 |
| P25-Fe(III)PcTC | 17.3 | 10.8 | 14.3 |
| P25-CuPcTC | 20.2 | 18.7 | 18.5 |
| P25-MnPcTC | 18.1 | 16.8 | 11.6 |
| Photocatalyst | Total Color Changes | |||||
|---|---|---|---|---|---|---|
| ∆E* | ∆a* | ∆b* | ∆L* | ∆C* | ∆H* | |
| – | 1.65 | −0.11 | 0.53 | −1.56 | −0.51 | 0.2 |
| P25 | 2.57 | −0.06 | 2.02 | 1.58 | −1.86 | 0.81 |
| P25-NiPcTC | 21.38 | 15.31 | 10.34 | 10.76 | −18.41 | 1.58 |
| P25-AlPcTC | 23.24 | 18.31 | 10.51 | 9.72 | −20.92 | 2.87 |
| P25-CoPcTC | 16.60 | 8.61 | 10.64 | 9.39 | −13.68 | 0.47 |
| P25-ZnPcTC | 18.80 | 17.07 | 3.81 | 6.89 | −16.52 | 5.75 |
| P25-Fe(III)PcTC | 15.02 | 9.86 | 2.34 | 11.08 | −9.16 | 4.34 |
| P25-CuPcTC | 21.98 | 14.11 | 11.92 | 11.91 | −18.45 | 1.01 |
| P25-MnPcTC | 12.15 | 10.61 | 2.01 | 5.58 | −10.63 | 1.88 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raditoiu, V.; Raditoiu, A.; Raduly, M.F.; Amariutei, V.; Gifu, I.C.; Anastasescu, M. Photocatalytic Behavior of Water-Based Styrene-Acrylic Coatings Containing TiO2 Sensitized with Metal-Phthalocyanine Tetracarboxylic Acids. Coatings 2017, 7, 229. https://doi.org/10.3390/coatings7120229
Raditoiu V, Raditoiu A, Raduly MF, Amariutei V, Gifu IC, Anastasescu M. Photocatalytic Behavior of Water-Based Styrene-Acrylic Coatings Containing TiO2 Sensitized with Metal-Phthalocyanine Tetracarboxylic Acids. Coatings. 2017; 7(12):229. https://doi.org/10.3390/coatings7120229
Chicago/Turabian StyleRaditoiu, Valentin, Alina Raditoiu, Monica Florentina Raduly, Viorica Amariutei, Ioana Catalina Gifu, and Mihai Anastasescu. 2017. "Photocatalytic Behavior of Water-Based Styrene-Acrylic Coatings Containing TiO2 Sensitized with Metal-Phthalocyanine Tetracarboxylic Acids" Coatings 7, no. 12: 229. https://doi.org/10.3390/coatings7120229






