3. Results and Discussion
The experimental data recorded by the TOF and SCLC measurements are summarised in
Figure 1 and
Figure 2, respectively.
Figure 1.
Hole mobility data recorded for a series of DEH MDP films that have been subjected to the UV exposure periods indicated. The solid lines show the optimised fit of Equation (1) to selected samples using adjustable values of μ0.
Figure 1.
Hole mobility data recorded for a series of DEH MDP films that have been subjected to the UV exposure periods indicated. The solid lines show the optimised fit of Equation (1) to selected samples using adjustable values of μ0.
Figure 2.
SCLC J(V) data recorded for a series of DEH MDP films that have been subjected to the UV exposure periods indicated. The solid lines show the optimised fit of Equation (3) for selected UV exposures: 400 min, θ = 0.15; 4000 min, θ = 0.015.
Figure 2.
SCLC J(V) data recorded for a series of DEH MDP films that have been subjected to the UV exposure periods indicated. The solid lines show the optimised fit of Equation (3) for selected UV exposures: 400 min, θ = 0.15; 4000 min, θ = 0.015.
The TOF mobility data are observed to be consistent with Poole-Frenkel type behaviour that is characteristic of hopping transport in MDPs such that [
16,
17]:
In Equation (1),
F (=
V/
L) represents the static electric field across the MDP layer, and β
PF depends upon the energetic and positional disorder that are associated with the MDP compositional landscape through which hole carriers hop [
5]. For electric fields lower than about 9 × 10
4 V·cm
−1 the mobility values are observed to increasingly deviate from data fitted to Equation (1) at higher fields. This phenomenon is attributed to delayed hole injection across the CG-MDP interface and these low field mobility data have consequently been ignored when applying Equation (1) to determine how μ
0 and β evolve with UV exposure. It is then evident from
Figure 1 that by increasing the UV exposure the zero-field mobility μ
0 is slowly reduced but this does not appear to be accompanied by a systematic change in β
PF. Similar behaviour has previously been reported for DEH doped polycarbonate films [
13] with the μ
0 reduction being attributed to the progressive reduction of available hopping states in the highest occupied molecular orbital (HOMO) of the DEH molecule. Dilution of HOMO states results in an increase in the average hole hopping distance (ρ) and a diminished wavefunction overlap probability so that for a characteristic DEH localisation length (ρ
0) [
6,
18]:
The effect of UV exposure is also apparent in the SCLC data which show a continuous reduction of the
J(
V) response as the exposure period is increased. For MDP hopping transport characterised by Equation (1) the associated
J(
V) response may then be approximated by [
19]:
In Equation (3), ε represents the permittivity of the MDP and θ denotes a constant (≤1) whose magnitude reflects departures from the maximum space-charge injection current due to the presence of traps. Under steady-state trap-limited hopping conditions where the density (
Nt) of iso-energetic traps remains non-saturated, the magnitude of θ is given by:
The quantities Ct and Rl in Equation (4) respectively denote the volume capture rate and release rate of holes between the trapping centres and the DEH HOMO. Assuming that Ct and Rl represent well-defined quantities that are characteristic of the fundamental chemical nature of the trap, the trap concentration Nt may consequently be deduced from Equation (4) by noting the relative reduction in SCLC current response relative to the trap-free (Nt = 0, θ = 1) J(V) reference.
A consistent interpretation of both the TOF and SCLC data thus emerges if under UV exposure a proportion of the DEH dopant molecules in the MDP layer are converted to a different chemical species which function as hole traps. The progressive conversion of DEH as the UV exposure period is increased provides the necessary dilution effect to account for the observed μ
0 reduction via Equation (2), whilst the accompanying decrease of SCLC
J(
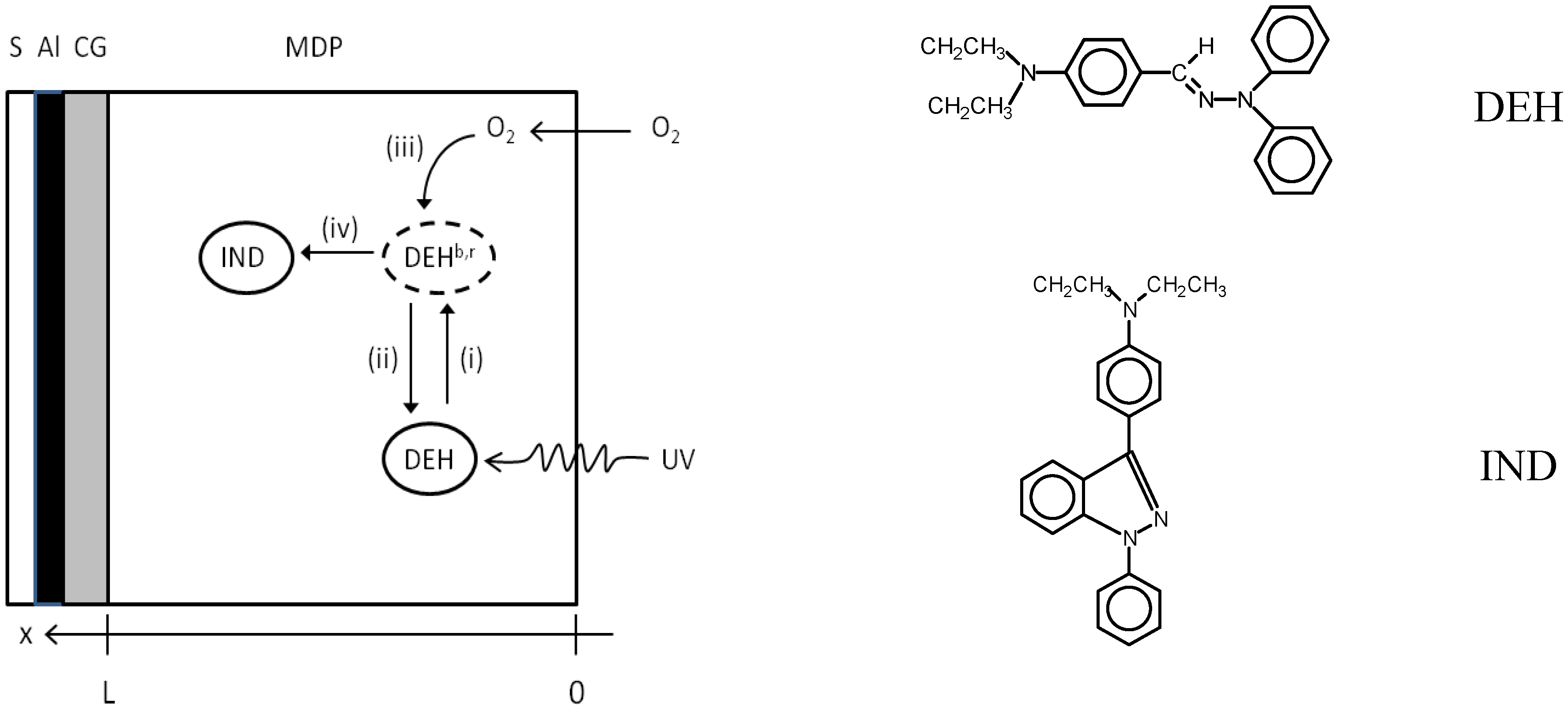
V) data may be explained via Equation (3) and (4) through the associated creation of traps. The proposed chemical conversion mechanism involves the photo-cyclic oxidation of DEH to form IND molecules [
11] which function as hole traps according to the scheme depicted in
Figure 3.
Figure 3.
Photo-cyclic oxidation model for DEH MDP film structures according to processes (i)–(iv) described in the text. The chemical structures of the DEH and IND molecules are indicated. S = substrate.
Figure 3.
Photo-cyclic oxidation model for DEH MDP film structures according to processes (i)–(iv) described in the text. The chemical structures of the DEH and IND molecules are indicated. S = substrate.
The conversion of DEH to IND involves several processes, which are labeled (i)–(iv) in
Figure 3 and may be summarised as follows:
absorption of UV photons by DEH to either form: (a) bulk excited states (DEHb) which may relax back to DEH before reaction with oxygen; (b) reactive excited states (DEHr) which are created close to soluble oxygen and rapidly react to form indazole molecules.
relaxation of un-reacted DEHb states to DEH
diffusion of soluble oxygen within reaction radius of DEHb.
reaction of soluble oxygen with DEHb to form indazole molecules
The time (
t) dynamics of these internal processes may be concisely incorporated into a set of coupled partial differential rate equations for the volume concentrations of DEH (
M), DEH
b (
B), DEH
r (
R), oxygen (
C) and bulk indazole (
I). For uniform UV exposure across the film surface, these concentrations are only expected to vary one-dimensionally (x) along the normal axis between the film surface (
x = 0) and
x =
L so that:
In the above rate equations φ represents the proportion of DEH molecules which form reactive DEH
r states upon UV absorption, β gives the relaxation rate of un-reacted DEH
b to DEH, D is the diffusion coefficient for soluble oxygen, ρ
R denotes the reaction radius for DEH
b, and G gives the UV photo-generation rate of DEH to the excited DEH
b,r states. An appropriate model for G is complicated by the presence of the MDP polymer binder but is expected to be proportional to the incident UV intensity (
GUV), and also to depend upon the local instantaneous DEH absorption coefficient α(
x,
t). A possible form for α(
x,
t) might reasonably assume that, compared to the absorption coefficient α
0 prior to UV exposure,
where M
0 is the initial DEH concentration and γ is an adjustable parameter. The photo-generation rate
G(
x,
t) in Equation (5) is accordingly represented as:
The solution of the coupled rate equations, using Equation (6), may then proceed subject to appropriate initial conditions [
M(
x,0) =
M0;
B(
x,0) = 0;
R(
x,0) = 0,
I(
x,0) = 0;
C(
x,0) = MDP oxygen solubility limit
Csol] and the boundary condition
C(0,
t) = atmospheric oxygen concentration. Many of the rate equation parameters are either determined experimentally (
GUV, α
0,
M0), or may be estimated from known literature values (
D, ρ
R,
Csol). A relatively small number of parameters (φ, β, γ and (
Rl/
Ct)) are therefore in practice available to fit the generated trap generation dynamics to the TOF and SCLC data. To investigate whether the modeled dynamics are capable of providing an acceptable fit to both data sets using a consistent set of parameters, an analysis strategy that focuses upon the total indazole concentration IND(
t) was adopted. At any time during UV exposure IND(
t) may be determined using the separate reaction contributions from DEH
b states (Equation (5e)) and DEH
r states (Equation (5c) where all DEH
r states are assumed to react with oxygen to produce indazole) so that:
Such a strategy proposes that IND(
t) may then be used to determine both the total concentration of generated traps (
Nt(
t) = IND(
t)) and the remaining concentration of un-reacted DEH sites
M(
t) that are available for hole hopping (
M(
t) =
M0 – IND(
t)). However, the simple use of IND(
t) to permit these calculations demands that two important assumptions are fulfilled. It should first be noted that the rate equation model involves DEH
b states and thus at any time during UV exposure there will also exist total populations
and
so that for overall bulk molecular conservation
. Under experimental conditions a population of states
B(
t) will consequently exist when UV exposure is terminated (
G = 0) and these may either relax back to DEH states, or react with oxygen to form IND according to Equation (5e). For IND(
t) to accurately represent the total bulk IND concentration no further DEH
b reactions should therefore occur after UV exposure has been terminated which demands that the relaxation rate β is significantly greater than the oxygen reaction rate 4πρ
RDC. The further assumption that must be addressed is whether the use of total bulk quantities IND(
t) and
M(
t) is appropriate to model the experimental data given that implicit spatial variations exist across the film thickness due to the fundamental nature of the UV absorption process (Equation (6)). Previous TOF work [
13] has endeavored to achieve a homogeneous distribution of IND throughout the film bulk by applying a post-exposure annealing procedure but this was not possible in the present work due to the use of Melinex substrates. Redistribution of IND throughout the film bulk by diffusion may nevertheless be inadvertently accomplished in the present case due to elevation of the film surface during thermal evaporation of the final encapsulating aluminium contacts. Further support for the applicability of bulk IND(
t) and
M(
t) model results to the present experimental data is provided through calculation of SCLC
J(
V) characteristics which indicate that trapping becomes significantly less effective (with θ → 1) if the total concentration of generated traps become confined to an increasingly narrow region in the vicinity of
x = 0 where UV absorption is strongest.
An illustrative set of normalised dynamical curves for
M(
t),
B(
t),
I(
t) and
R(
t) is given in
Figure 4 using experimentally determined values for
GUV = 4 × 10
−2 s
−1, α
0 = 5 × 10
4 cm
−1,
M0 = 1 × 10
21 cm
−3, ρ
R = 4 × 10
−8 cm (
molecular radius of DEH), and selected parameter values φ = 0.01, β = 0.01, γ = 1, and a relaxation to reaction ratio ε (=β/4πρ
RDC(x,0)) = 2.5 × 10
6.
Figure 4.
Modeled dynamics of DEH photo-oxidation with UV exposure for φ = 0.01, β = 0.01 and ε = 2.5 × 106. The output concentrations are normalised to the initial DEH concentration M0.
Figure 4.
Modeled dynamics of DEH photo-oxidation with UV exposure for φ = 0.01, β = 0.01 and ε = 2.5 × 106. The output concentrations are normalised to the initial DEH concentration M0.
From the dynamical output it is observed that the anticipated reduction in
M(
t) is accompanied by an increase of indazole from both
I(
t) and
R(
t) routes with
R(
t) providing the dominant contribution to overall IND(
t) production for the selected magnitude of φ. The rate of increase of
I(
t) depends upon the available supply of DEH
b states and is approximately linear over the exposure interval (10
0 min <
t < 10
2 min), where
B(
t) remains fairly constant, before approaching a final saturated value when B(
t) becomes depleted. The interval over which
B(
t) remains approximately constant, and hence
I(
t) increases linearly from Equation (5e), may be extended by increasing the relaxation to reaction ratio ε. Spreading the availability of DEH
b states across a longer exposure interval via relaxation control is accompanied by a smaller overall conversion to IND traps for equivalent overall exposure times. The dynamical output shown in
Figure 4 suggests that a final equilibrium situation will only be attained for very long exposure periods when
I +
R =
M0,
M = 0 and
B = 0.
The influence of γ from Equation (6) upon the dynamics was found to be insignificant for 0 ≤ γ ≤ 1 and for convenience was accordingly fixed at unity in all subsequent analysis. The only adjustable parameters that are available to control the dynamical output are therefore ε and φ. Initial estimates for these parameters may be obtained by considering how the hopping distance ρ(
t) increases as the average DEH concentration
M(
t) diminishes with UV exposure, and using ρ(
t) to fit the experimental variation of μ
0 observed in the TOF results (
Figure 1). Using a cubic lattice approximation [
6] ρ(
t) may be calculated from IND(
t) as:
In Equation (8),
Mw is the DEH molecular weight (343g),
d is the DEH density (1.12 g·cm
−3),
Av is Avogadro’s constant and
Cw (=0.5) is the concentration by weight of DEH to polymer. Using ρ
0 = 1.6 × 10
−8 cm for the DEH localisation length [
20] the variation of μ
0 may thus be found using Equation (2), Equation (8) and generated IND(
t) dynamics from Equation (7). To assist in the determination of an estimate for φ it is initially useful to consider that IND(
t) is dominated by the
R(
x,
t) integral term in Equation (7) for which a spatially-averaged analytical expression
R(
t) may obtained from Equation 5c by letting
M = (
M0 –
R) and setting the spatial average of
G(
x,
t) in Equation (6) to
. From Equation (5c) it is then found that:
The increase of IND(
t) under these circumstances is expected to be linear in time for short UV exposure periods such that
so that by using Equation (9) in Equation (8) ρ(
t) has a similar linear dependence with
. The use of this approximate ρ(
t) expression in Equation (2) finally permits an initial estimate for φ to be obtained. Results generated by this procedure are presented in
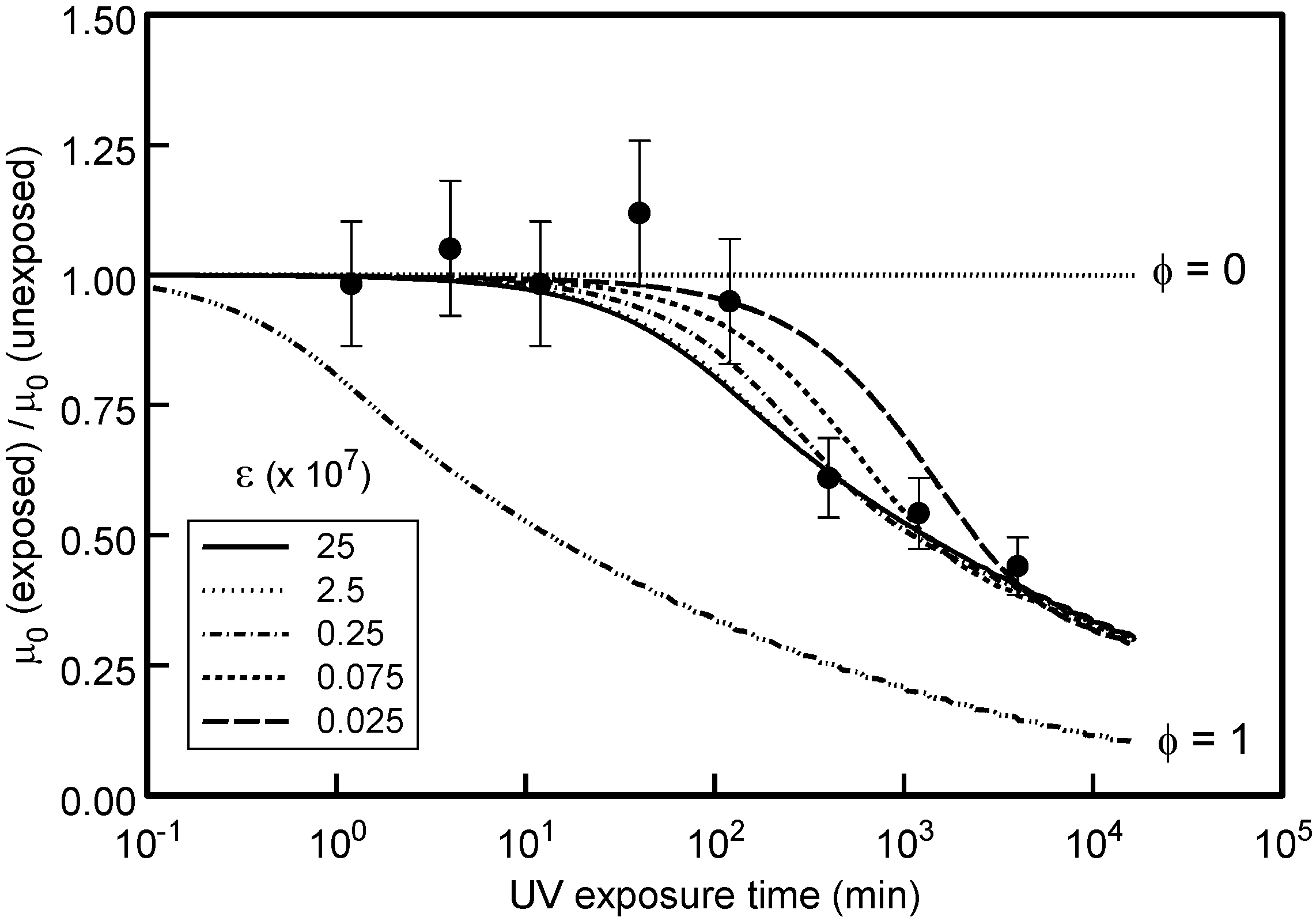
Figure 5 where the μ
0 data are plotted in a normalised format relative to the zero-field mobility magnitude for a reference un-exposed sample.
The model curves shown in
Figure 5 suggest that acceptable fitting of μ
0 requires that φ ≈ 0.01 ± 0.005 and 7.5 × 10
5 < ε < 2.5 × 10
7 with an optimum ε ≈ 2.5 × 10
6. The influence of the selected magnitude of φ is illustrated in
Figure 5 for the extreme limits where for φ = 0 (zero highly reactive states) the rate of IND production via purely DEH
b diffusive oxygen reactions
I(
t) is undetectable, whereas for φ =1 (entirely highly reactive DEH
r states) the IND generation rate should be detectable at much shorter exposure periods.
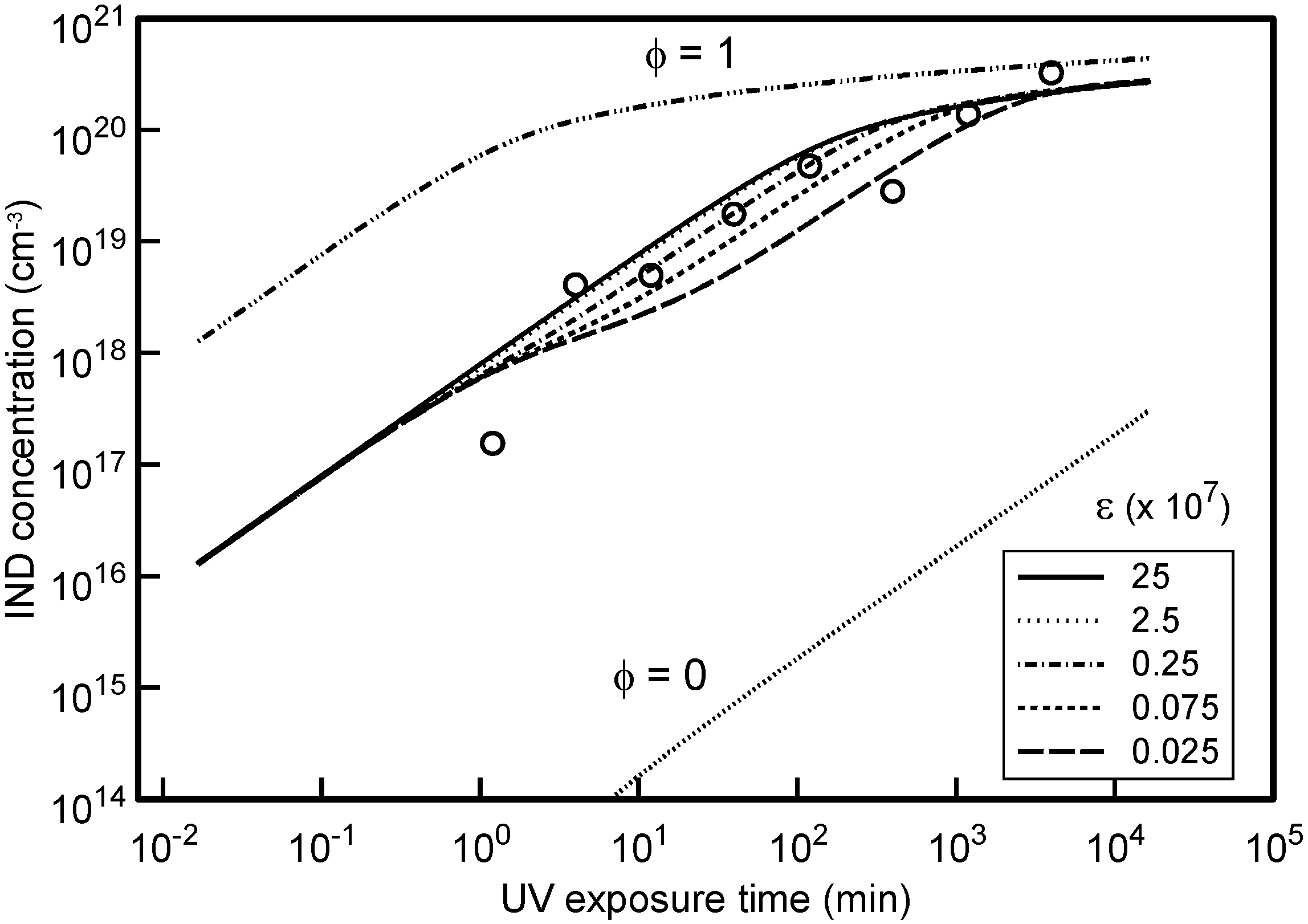
The associated IND(
t) trap generation dynamics are plotted in
Figure 6 using the corresponding φ and ε values that are inferred from the μ
0 analysis. The experimental trap densities deduced by applying Equation (3) and Equation (4) to the
Figure 2 SCLC data are shown as symbols in
Figure 6 using an adjustable scaling factor equal to (
Rl/
Ct). In practice Equation (3) was restricted to fitting
J(
V) data for
V < 250 V since there was evidence that the CG layer was unable to meet the SCLC ohmic demands for higher current densities in samples subjected to the lowest UV exposure.
Figure 5.
Variation of experimentally measured μ0 with UV exposure period. The solid curves show the expected dependence based upon the modeled DEH photo-oxidation dynamics for the ε values given. φ = 0.01 in all cases except for those labeled.
Figure 5.
Variation of experimentally measured μ0 with UV exposure period. The solid curves show the expected dependence based upon the modeled DEH photo-oxidation dynamics for the ε values given. φ = 0.01 in all cases except for those labeled.
Figure 6.
Variation of experimentally deduced IND trap concentrations with UV exposure period setting (Rl/Ct) = 5 × 1018 cm−3. The solid curves show the expected dependence based upon the modeled DEH photo-oxidation dynamics for the ε values given. φ = 0.01 in all cases except for those labeled.
Figure 6.
Variation of experimentally deduced IND trap concentrations with UV exposure period setting (Rl/Ct) = 5 × 1018 cm−3. The solid curves show the expected dependence based upon the modeled DEH photo-oxidation dynamics for the ε values given. φ = 0.01 in all cases except for those labeled.
From
Figure 6 it is found that the data points cannot in general be fitted by a linear dependence of
Nt (corresponding to higher ε values) across the entire range of UV exposures but are better described by the
Nt curves that correspond to the lower ε magnitudes as found by the μ
0 fitting procedure. Optimum agreement using ε = 2.5 × 10
6 from the μ
0 analysis requires that (
Rl/
Ct) = 5 × 10
18 cm
−3 for the IND traps. No further information may presently be deduced concerning the individual magnitudes of
Rl or
Ct for the traps although it should in principle be possible to determine these values (subject to their ratio constraint) by simulating the temporal shape of the underlying TOF photocurrent and SCLC injection responses [
5,
21]. Given that the mobility reduction would appear to be entirely attributable to an increase of ρ(
t) via hopping state dilution, however, the traps must be relatively deep such that they do not engage in multiple trapping-and-release events with DEH HOMO states during a typical hole transit time. This implies that the inverse release rate (
Rl)
−1 should be significantly less than the maximum hole transit-time across the MDP film thickness. For samples that have maximum UV exposure and are subject to the lowest experimental applied voltages the longest recorded transit times are found to be ~0.2 s so that
Rl << 5s
−1. The loss of hole carriers to IND traps under these circumstances should result in the TOF pre-extraction current signals decreasing more rapidly as N
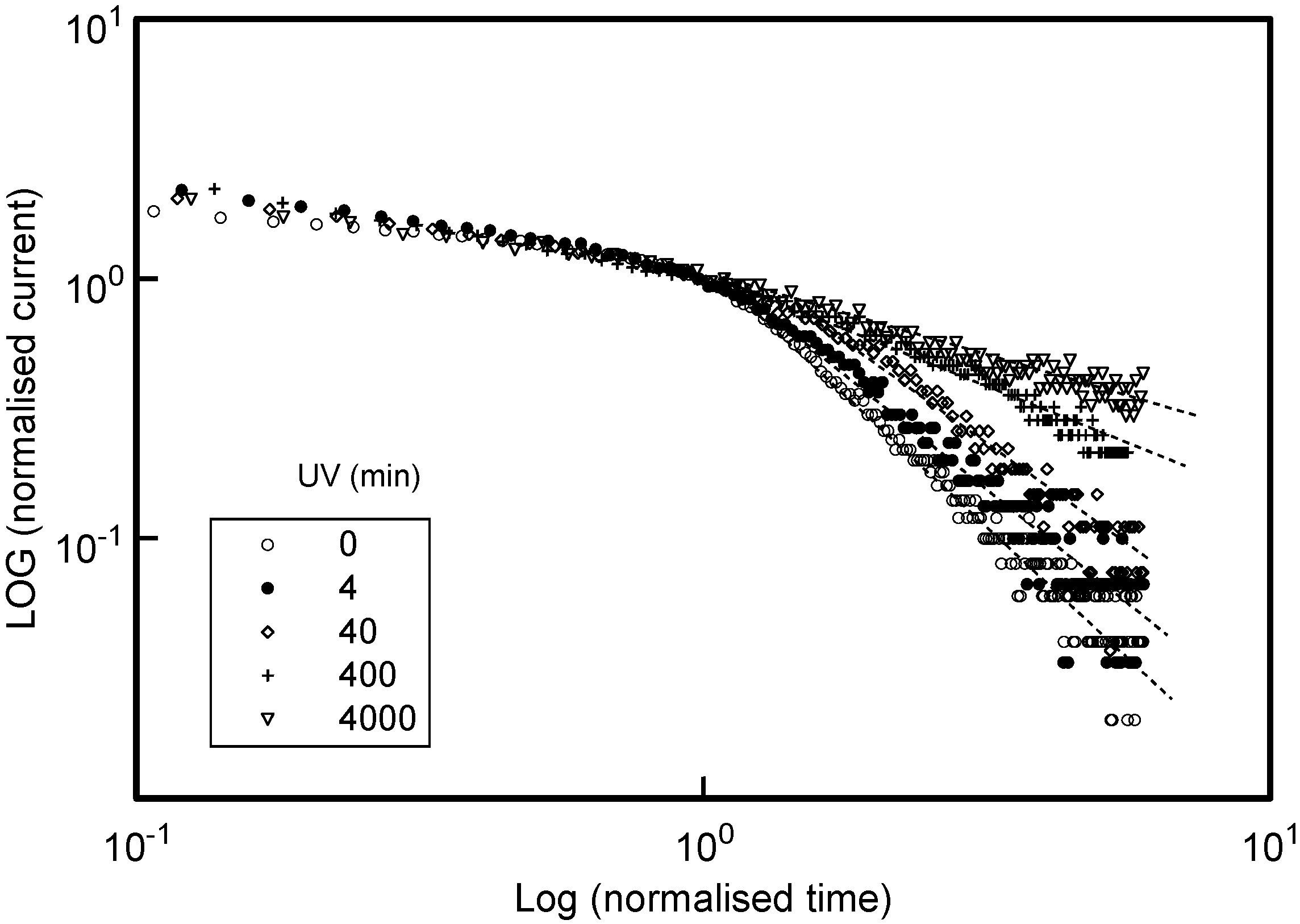
t increases, with the post-extraction currents displaying a weaker decay as trapped charge is eventually released and collected. These TOF trapping signatures are indeed evident in the normalised TOF current-time data presented in
Figure 7.
Figure 7.
TOF current-time signals recorded at V = 250 V for a series of DEH MDP films that have been subjected to the UV exposure periods indicated. The signals have been normalised to the observed hole transit-times, and corresponding currents at these transit-times, to facilitate shape comparisons. The dashed lines are an aid for the eye in the post transit-time region.
Figure 7.
TOF current-time signals recorded at V = 250 V for a series of DEH MDP films that have been subjected to the UV exposure periods indicated. The signals have been normalised to the observed hole transit-times, and corresponding currents at these transit-times, to facilitate shape comparisons. The dashed lines are an aid for the eye in the post transit-time region.
An essential requirement for the conversion of DEH to IND is the available supply of oxygen. As already noted from the analysis of the μ
0 data a small fraction φ of the DEH population must exist as rapid-reactive DEH
r states to successfully account for the deduced IND(
t) dynamics. This conclusion is again illustrated in
Figure 6 where it is observed that the absence of such DEH
r states (φ = 0) would result in an exactly linear dependence of IND(
t) (=
I(
t)) across the entire range of experimental UV exposure. Conversely, a highly non-linear IND(
t) response is expected within the experimental UV exposure period for φ = 1 as complete IND conversion becomes possible and N
t approaches saturation. The necessity to introduce rapid-reacting DEH
r states (φ ≠ 0) into the dynamic model is thus simply a consequence of the inherently low oxygen reaction rate with DEH
b states under the original assumption that ε >> 1. The reaction rate (=4πρ
RDC from Equation (5e)) is inherently controlled by the magnitude of the oxygen diffusion coefficient and the decay of induced radical states via diffusion-reaction kinetics may usefully be analysed to determine oxygen diffusion coefficients in polymer materials under specified boundary conditions [
22,
23]. The magnitude of the oxygen diffusion coefficient that is appropriate for the present MDP films is uncertain but assuming that
Csol is comparable to the pure PET magnitude (~ 5 × 10
17 cm
−3 from PET solubility coefficients [
24]) the deduced diffusion coefficient for the present MDP films is about 2 × 10
−16 cm
2·s
−1. The magnitude of this diffusion coefficient is considerably lower than that reported for pure PET (D ~ 4 × 10
−9 cm
2·s
−1 [
23,
24]) but may reflect an increase in the effective size of the penetrant O
2 molecules due to the presence of the DEH dopant. This apparently low value for
D may have associated implications for the ability of atmospheric oxygen to replenish consumed oxygen throughout the UV absorption depth since this will require a time ~ α
−2/
D = 10
6 s which is comparable to the maximum experimental exposure time. Such oxygen starvation effects do not appear to be manifest in the inferred trap generation magnitudes, however, which greatly exceeds the value for
Csol. The available concentration of soluble oxygen would therefore appear to be maintained at around the solubility limit throughout the entire exposure period but is unable to diffuse and react efficiently with excited DEH
b states before they relax to DEH. It is noted that the alternative scenario, whereby
D remains comparable to the pure PET magnitude with ε << 1, would require that the DEH
b reaction rate was highly inefficient (<10
−6) to account for the observed trap generation dynamics. This is inconsistent with independent estimates of the DEH to IND conversion efficiency which is reported to be as high as 0.4 [
13] and is in fact more typical of the envisaged DEH
r reaction process discussed below.
The preceding considerations concerning relaxation and reaction of DEH
b states afford additional insight into the possible nature of the counterpart DEH
r states. It is first noted that the DEH
r states cannot simply represent a subset of DEHb states which are effectively stable (β = 0) and subsequently react with soluble oxygen, via the same diffusive-reaction kinetics for DEH
b states, even after UV exposure has been terminated. The existence of such stable states would imply that the eventual conversion to indazole, and its experimental detection, might be sensitive to the timescale when post-exposure TOF and SCLC measurements are performed but such evidence is absent from a survey of the sample handling history. The DEH
r states must consequently represent sites within the MDP where oxygen is immediately available to initiate the indazole reaction. Such sites could therefore represent a proportion of micro-void regions within the polymer where the movement of oxygen is unrestricted by diffusion. However, for the present MDP films the interpretation of the fitted parameter φ (= 0.01) would then require that the observed saturated indazole concentration should not exceed φ
M0 = 10
19 cm
−3 which is clearly violated from the data in
Figure 5. The parameter φ would thus appear to represent the fraction of DEH states which, upon excitation by UV irradiation, lie within the reaction radius of soluble oxygen molecules and are immediately converted to indazole. For this scenario, φ may be estimated by considering the ratio of the average volume
V(DEH) that is occupied by an excited DEH state to the average volume
V(O
2) ~ C
sol−1 occupied by a soluble oxygen molecule. For small φ
V(DEH) is dominated by DEH
b states so that V(DEH) ~ B
-1 which changes dynamically with UV exposure time (
Figure 4). Restricting attention to the time regime where
B(
t) is approximately constant
from Equation (5b) and the quantity φ may thus be expressed as:
For the parameters employed to generate the model curves in
Figure 4,
Figure 5 and
Figure 6, Equation (10) produces an a priori calculated value for φ = 0.004 which shows reasonable agreement with the (average) 0.01 value deduced by data fitting across the entire exposure period.
Using the insight gained regarding the apparent dominance of DEH
r states in generating IND traps it is finally possible to consider what restrictions should be imposed upon the supply of oxygen in the MDP bulk to minimise degradation of the electronic performance of the MDP films over operationally long UV exposure periods. It is helpful in this respect to realise that the φ expression in Equation (10) may be used in Equation (9) to produce a revised expression for IND(
t) whereby:
This expression embodies the principal IND trap generation kinetics via DEHr states and confirms a number of key characteristics that have emerged from the more detailed modeling using the partial differential rate equations. A perhaps surprising parameter to appear in Equation 11 is β as this would intuitively be associated with DEHb processes (Equation (5b)). However, β also influences the concentration of DEH states according to Equation (5a) which in turn controls the concentration of UV excited DEHr states via Equation (5c). The overall rate of IND(t) production should thus be enhanced for larger β as confirmed in Equation (11). The rate of IND(t) production is also predicted to depend upon the ratio of Csol to M0 which is again physically compatible with the proposed DEHr reaction model which should become more efficient as the availability of soluble oxygen becomes comparable to the overall DEH concentration.
For any given MDP film, Equation (11) suggests that there are consequently very few experimental parameters that may be engineered to slow the rate of IND(t) trap generation. M0 is specified by the selected Cw for the MDP doping, and β is presumably characteristic of molecular electronic interactions between the dopant-polymer constituents. Only Csol may therefore be experimentally controlled which in practice would require the use of a vacuum coating system to initially desorb soluble oxygen from the films before an encapsulation layer is deposited to restrict re-absorption of oxygen upon return to atmospheric pressure. Such an encapsulation strategy would be critically dependent upon the encapsulation layer preventing the soluble oxygen concentration from re-attaining its pre-desorption level. The ability to maintain desorbed oxygen levels at a fractional level of their initial Csol magnitude not only reduces the rate of IND(t) increase as indicated by Equation (11) but significantly would also restrict the maximum IND concentration that may eventually be generated as the finite supply of soluble oxygen is entirely consumed in photo-cyclic reactions. Significant extension of the operational lifetime of the MDP films may therefore be possible but difficult to achieve using standard thin-film coating technologies.
It is curious to note that UV shielding of the exposed MDP surface is not apparently predicted to afford better protection against DEHr photo-degradation effects according to Equation (11). This incorrect interpretation arises through the substitution of the product in the exponent term of Equation 9 with the constant parameters from Equation (10). As the UV generation rate is reduced, however, φ will increase but is restricted to a maximum value of unity. A reduction in the rate of IND(t) production will consequently be achieved for UV generation rates that fall below a magnitude βCsol/M0 which for the present MDP parameters would require a reduction in the UV incident photon flux by a factor >100. Shielding may not therefore be pragmatic if the MDP layer is to be deployed in an optoelectronic application which demands a UV spectral response.