Understanding Antibiotic Use in Minya District, Egypt: Physician and Pharmacist Prescribing and the Factors Influencing Their Practices
Abstract
:1. Introduction
2. Methods
2.1. Study Location
2.2. Study Population
2.3. Survey
2.4. Qualitative Study
3. Results and Discussion
3.1. Results
3.1.1. Questionnaire
| Characteristic | Physicians | Pharmacists | ||
|---|---|---|---|---|
| No. of participants | 236 | 483 | ||
| Female | 102 (43%) | 211 (44%) | ||
| Median age (range) | 42 (23–71) | 27 (18–68) | ||
| Employer | ||||
| Government only | 150 | 80 | ||
| Private only | 14 | 348 | ||
| Both government and private | 73 | 55 | ||
| Median patient visits/day | ||||
| Government clinic | 55 (1–150) | 50 (1–1000) | ||
| Private clinic | 5 (1–50) | 50 (2–600) | ||
| Training/Education | Internal | 63 (27%) | Secondary | 89 (18%) |
| General | 64 (27%) | University | ||
| Pediatrics | 59 (25%) | Pharmacy | 305 (63%) | |
| Other | 50 (21%) | Other | 85 (18%) | |
| Prescribing practice | Colds | Bronchitis | Sinusitis | Pneumonia | |
|---|---|---|---|---|---|
| Pharmacists * | Physicians | Physicians | |||
| Prescribing frequency † | |||||
| Most times | 44 (11%) | 22 (9%) | 156 (66%) | 103 (44%) | 209 (89%) |
| Sometimes | 282 (70%) | 128 (54%) | 71 (30%) | 117 (50%) | 9 (4%) |
| Never | 78 (19%) | 84 (36%) | 6 (3%) | 12 (5%) | 2 (1%) |
| Antibiotic choice ‡ | |||||
| β-Lactam | 327 (81%) | 122 (81%) | 183 (81%) | 123 (56%) | 167 (77%) |
| Tetracycline | 4 (1%) | 4 (3%) | 4 (2%) | 14 (6%) | 1 (1%) |
| Quinolone | 16 (4%) | 3 (2%) | 21 (9%) | 34 (16%) | 38 (17%) |
| Macrolide | 12 (3%) | 20 (13%) | 19 (8%) | 49 (22%) | 10 (5%) |
| Other | 45 (11%) | 1 (1%) | 0 | 0 | 1 (1%) |
| Days of treatment | 3–5 (60%) | 3–5 (60%) | 5–7 (60%) | 5–7 (55%) | >7 (48%) |
| No. who prescribe >1 Abx | NA | 14 (9%) | 85 (37%) | 54 (25%) | 184 (85%) |
| Factors contributing to resistance | Physicians (n = 236) | Pharmacists (n = 437) | ||
|---|---|---|---|---|
| N (%) | Rank | N (%) | Rank | |
| Patient self-medication with antibiotics | 202 (86) | 1 | 310 (71) | 1 |
| Incorrect duration of treatment | 156 (66) | 2 | 304 (70) | 2 |
| Incorrect choice of antibiotics | 130 (55) | 4 | 228 (52) | 3 |
| Patient non-compliance | 109 (46) | 5 | 220 (50) | 4 |
| Inappropriate prescription by physicians | 139 (59) | 3 | 192 (44) | 5 |
| Inappropriate dispensing by pharmacist | 102 (43) | 6 | 117 (27) | 6 |
| Physician Characteristics | % Prescribe antibiotics | χ2p | Pharmacist characteristics | % Prescribe antibiotics | χ2p |
|---|---|---|---|---|---|
| Specialty | Training | ||||
| Pediatrics | 46 | <0.001 | <post-secondary | 92 | 0.01 |
| Adult medicine | 70 | ≥post-secondary | 82 | ||
| Age | Age | ||||
| <40 years | 72 | 0.03 | <30 years | 82 | 0.30 |
| >40 years | 58 | >30 years | 87 | ||
| Sex | Sex | ||||
| Female | 59 | 0.14 | Female | 85 | 0.61 |
| Male | 68 | Male | 83 | ||
| No. patients visits/week | No. patients visits/day | ||||
| <100 | 64 | 0.98 | <50 | 82 | 0.42 |
| ≥100 | 64 | ≥50 | 85 | ||
| Continuing education * | Continuing education | ||||
| Y | 65 | 0.92 | Y | 84 | 0.67 |
| N | 64 | N | 83 |
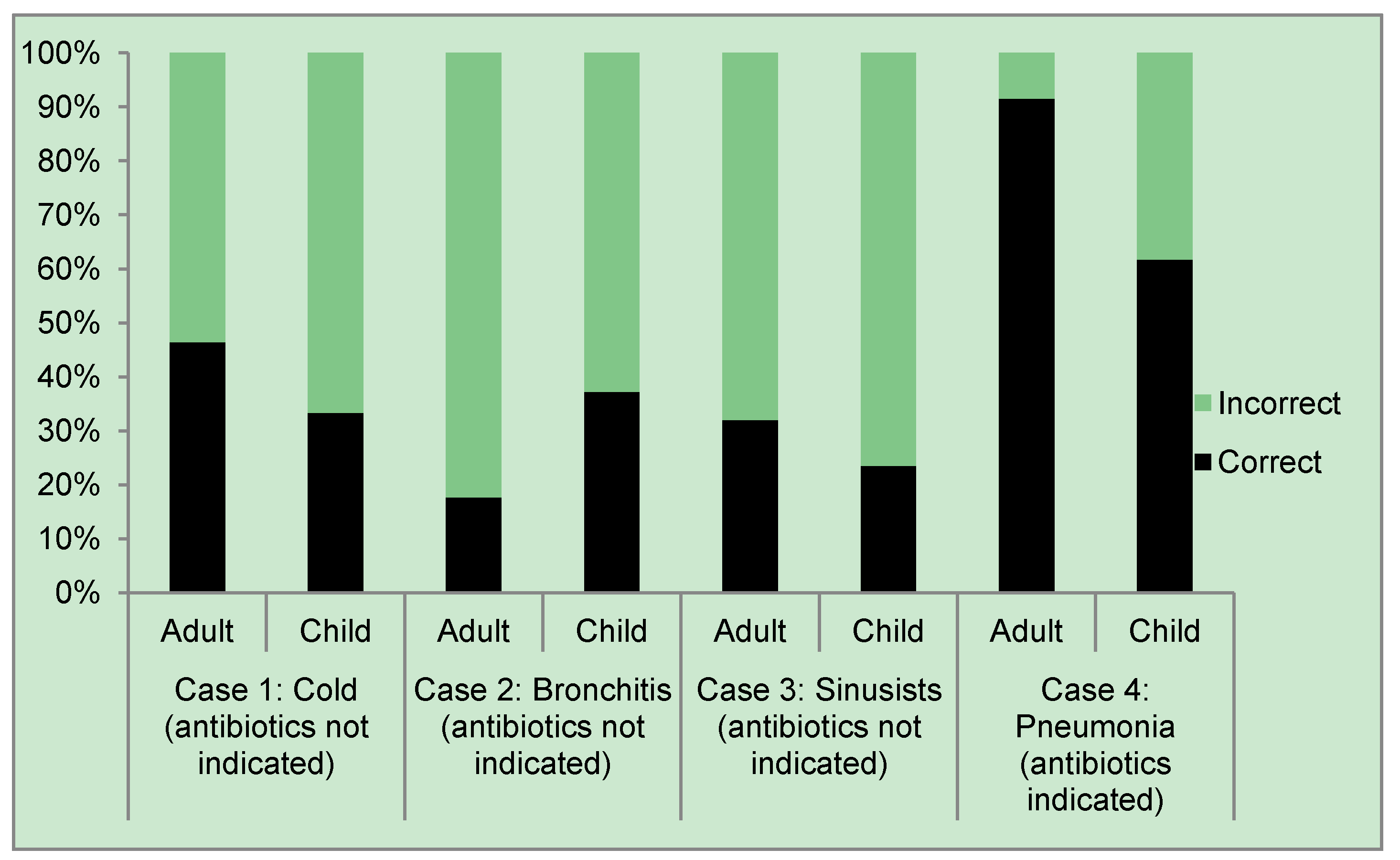
| Clinical case | Patient population | Correct | Incorrect |
|---|---|---|---|
| Case 1: Cold (antibiotics not indicated) | Adult | 46.41 | 53.59 |
| Case 2: Bronchitis (antibiotics not indicated) | Adult | 17.65 | 82.35 |
| Case 3: Sinusitis (antibiotics not indicated) | Adult | 32.03 | 67.97 |
| Case 4: Pneumonia (antibiotics indicated) | Adult | 91.5 | 8.5 |
3.1.2. Qualitative Study
3.1.2.1. Physicians
3.1.2.2. Pharmacists
3.2. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seppala, H.; Klaukka, T.; Vuopio-Varikila, J.; Muotiala, A.; Helenius, H.; Lager, K.; Huovinen, P. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N. Engl. J. Med. 1997, 337, 441–446. [Google Scholar]
- Albrich, W.C.; Monnet, D.L.; Harbarth, S. Antibiotic selection pressure and resistance in Streptococcus pneumoniae and Streptococcus pyogenes. Emerg. Infect. Dis. 2004, 10, 514–517. [Google Scholar] [CrossRef]
- Livermore, D.M. Bacterial resistance: Origins, epidemiology, and impact. Clin. Infect. Dis. 2003, 36, S11–S23. [Google Scholar] [CrossRef]
- Scicluna, E.A.; Borg, M.A.; Gür, D.; Rasslan, O.; Taher, I.; Redjeb, S.B.; Elnassar, Z.; Bagatzouni, D.P.; Daoud, Z. Self-medication with antibiotics in the ambulatory care setting within the Euro-Mediterranean region; results from the ARMed project. J. Infect. Public Health 2009, 2, 189–197. [Google Scholar] [CrossRef]
- Grigoryan, L.; Haaijer-Ruskamp, F.M.; Burgerhof, J.G.; Mechtler, R.; Deschepper, R. Self-medication with antimicrobial drugs in Europe. Emerg. Infect. Dis. 2006, 12, 452–459. [Google Scholar] [CrossRef]
- Grijalva, C.G.; Nuorti, J.P.; Griffin, M.R. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA 2009, 302, 758–766. [Google Scholar] [CrossRef]
- Borg, M.A.; Tiemersma, E.; Scicluna, E.; van de Sande-Bruinsma, N.; de Kraker, M.; Monen, J.; Grundmann, H. Prevalence of penicillin and erythromycin resistance amongst Streptococcus pneumonia from invasive isolates reported by laboratories in the Southern and Eastern Mediterranean. Clin. Microbiol. Infect. 2009, 15, 232–237. [Google Scholar]
- Okeke, I.N.; Laxminarayan, R.; Bhutta, Z.A.; Duse, A.G.; Jenkins, P.; O’Brien, T.F.; Pablos-Mendez, A.; Klugman, K.P. Antimicrobial resistance in developing countries. Part 1: Recent trends and current status. Lancet Infect. Dis. 2005, 5, 481–493. [Google Scholar] [CrossRef]
- Saied, T.; Elkholy, A.; Hafez, S.F.; Basim, H.; Wasfy, M.O.; El-Shoubary, W.; Samir, A.; Pimentel, G.; Talaat, M. Antimicrobial resistance in pathogens causing nosocomial bloodstream infections in university hospitals in Egypt. Am. J. Infect. Control 2011, 39, 61–65. [Google Scholar]
- McCaig, L.F.; Hughes, J.M. Trends in antimicrobial drug prescribing among office-based physicians in the United States. JAMA 1995, 273, 214–219. [Google Scholar] [CrossRef]
- Brown, V.; Clarke, V. Using thematic analysis is psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- The World Health Organization Maternal, Newborn, Child and Adolescent Health Documents on the Integrated Management of Childhood Illness. Available online: http://www.who.int/maternal_child_adolescent/documents/imci/en/ (accessed on 1 June 2012).
- Gouws, E.; Bryce, J.; Habicht, J.P.; Amaral, J.; Pariyo, G.; Schellenberg, J.A.; Fontaine, O. Improving antimicrobial use among health workers in first-level facilities: Results from the multi-country evaluation of the integrated management of childhood illness strategy. Bull. World Health Organ. 2004, 82, 509–515. [Google Scholar]
- James, C.; Hanson, K.; Solon, O.; Whitty, C.J.; Peabody, J. Do doctors under-provide, over-provide or both? Exploring the quality of treatment in the Philipines. Int. J. Qual. Health Care 2011, 23, 445–455. [Google Scholar]
- Wardlaw, T.; Salama, P.; Johansson, E.W.; Mason, E. Pneumonia: The leading killer of children. Lancet 2006, 1048–1050. [Google Scholar]
- The Global Burden of Disease: 2004 Update; World Health Organization: Geneva, Switzerland, 2008.
- Velasco, E.; Ziegelmann, A.; Eckmanns, T.; Krause, G. Eliciting views on antibiotics prescribing and resistance among hospital and outpatient care physicians in Berlin, Germany: Results of a qualitative study. Br. Med. J. Open 2012, 2, e000398. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dooling, K.L.; Kandeel, A.; Hicks, L.A.; El-Shoubary, W.; Fawzi, K.; Kandeel, Y.; Etman, A.; Lohiniva, A.L.; Talaat, M. Understanding Antibiotic Use in Minya District, Egypt: Physician and Pharmacist Prescribing and the Factors Influencing Their Practices. Antibiotics 2014, 3, 233-243. https://doi.org/10.3390/antibiotics3020233
Dooling KL, Kandeel A, Hicks LA, El-Shoubary W, Fawzi K, Kandeel Y, Etman A, Lohiniva AL, Talaat M. Understanding Antibiotic Use in Minya District, Egypt: Physician and Pharmacist Prescribing and the Factors Influencing Their Practices. Antibiotics. 2014; 3(2):233-243. https://doi.org/10.3390/antibiotics3020233
Chicago/Turabian StyleDooling, Kathleen L., Amr Kandeel, Lauri A. Hicks, Waleed El-Shoubary, Khaled Fawzi, Yasser Kandeel, Ahmad Etman, Anna Leena Lohiniva, and Maha Talaat. 2014. "Understanding Antibiotic Use in Minya District, Egypt: Physician and Pharmacist Prescribing and the Factors Influencing Their Practices" Antibiotics 3, no. 2: 233-243. https://doi.org/10.3390/antibiotics3020233




