An Electrocatalytic Screen-Printed Amperometric Sensor for the Selective Measurement of Thiamine (Vitamin B1) in Food Supplements
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Chemicals and Reagents
2.3. Voltammetric and Amperometric Procedures
2.4. Sample Preparation and Analysis
3. Results and Discussion
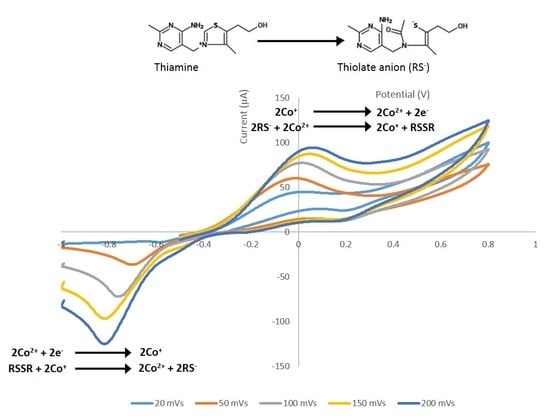
3.1. Scanning Electron Microscopy and EDX Surface Characterisation and Cyclic Voltammetric Behaviour and Optimisation of Measurement Conditions

3.2. Hydrodynamic Voltammetry and Amperometry in Stirred Solution
3.3. Interference Studies
3.4. Calibration Study
3.5. Analytical Application
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lương, K.V.Q.; Nguyễn, L.T.H. The role of thiamine in autism. J. Psychiatry Neurosci. 2013, 1, 22–37. [Google Scholar] [CrossRef][Green Version]
- Lương, K.V.Q.; Nguyễn, L.T.H. Role of thiamine in Alzheimer’s disease. Am. J. Alzheimer’s Dis. Other Dement. 2011, 26, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Lương, K.V.Q.; Nguyễn, L.T.H. The role of thiamine in cancer: Possible genetic and cellular signalling mechanisms. Cancer Genom. Proteom. 2013, 10, 169–185. [Google Scholar]
- Rodriguez, R.; Fernández-Ruiz, V.; Cámara, M.; Sánchez-Mata, M.C. Simultaneous determination of vitamin B1 and B2 in complex cereal foods, by reverse phase isocratic HPLC-UV. J. Cereal Sci. 2012, 55, 293–299. [Google Scholar] [CrossRef]
- Arella, F.; Lahely, S.; Bourguignon, J.B.; Hasselmann, C. Liquid chromatographic determination of vitamins B1 and B2 in foods. A collaborative study. Food Chem. 1996, 56, 81–86. [Google Scholar] [CrossRef]
- Aboul-Kasim, E. Anodic adsorptive voltammetric determination of the vitamin B1 (thiamine). J. Pharm. Biomed. Anal. 2000, 22, 1047–1054. [Google Scholar] [CrossRef]
- Akyilmaz, E.; Yaşa, İ.; Dinçkaya, E. Whole cell immobilized amperometric biosensor based on Saccharomyces cerevisiae for selective determination of vitamin B1 (thiamine). Anal. Biochem. 2006, 354, 78–84. [Google Scholar] [CrossRef]
- Westmacott, K.L.; Crew, A.; Doran, O.; Hart, J.P. A novel electroanalytical approach to the measurement of B vitamins in food supplements based on screen-printed carbon sensors. Talanta 2018, 181, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Wring, S.A.; Hart, J.P.; Birch, B.J. Voltammetric behaviour of screen-printed carbon electrodes, chemically modified with selected mediators, and their application as sensors for the determination of reduced glutathione. Analyst 1991, 116, 123–129. [Google Scholar] [CrossRef]
- Honeychurch, K.C.; Hart, J.P. The chronoamperometric and voltammetric behaviour of glutathione at screen-printed carbon micro-band electrodes modified with cobalt phthalocyanine. Adv. Anal. Chem. 2012, 2, 46–52. [Google Scholar]
- Hart, J.P.; Abass, A.K. A disposable amperometric gas sensor for sulphur-containing compounds based on a chemically modified screen-printed carbon electrode coated with a hydrogel. Anal. Chim. Acta. 1997, 342, 199–206. [Google Scholar] [CrossRef]
- Abass, A.K.; Hart, J.P. Electrocatalytic, diffusional and analytical characteristics of a cobalt phthalocyanine modified, screen-printed, amperometric gas sensor for propanethiol. Sens. Actuators B 1997, 41, 169–175. [Google Scholar] [CrossRef]
- Napier, A.; Hart, J.P. Voltammetric and amperometric studies of selected thiols and dimethyldisulphide using a screen-printed carbon electrode modified with cobalt phthalocyanine: Studies towards a gas sensor. Electroanal 1996, 8, 1006–1013. [Google Scholar] [CrossRef]
- Hart, J.P.; Hartley, I.C. Voltammetric and amperometric studies of thiocholine at screen-printed carbon electrode chemically modified with cobalt phthalocyanine: Studies towards a pesticide sensor. Analyst 1994, 119, 259. [Google Scholar] [CrossRef]
- Crew, A.; Hart, J.P.; Wedge, R.C.; Marty, J.L.; Fournier, D. A screen-printed, amperometric, biosensor array for the detection of organophosphate pesticides based on inhibition of wild type, and mutant acetylcholinesterases, from Drosophila melanogaster. Anal. Lett. 2004, 37, 1601–1610. [Google Scholar] [CrossRef]
- Hart, J.P.; Crew, A.; Crouch, E.; Honeychurch, K.C.; Pemberton, R.M. Some Recent Designs and Developments of Screen-Printed Carbon Electrochemical Sensors/Biosensors for Biomedical, Environmental, and Industrial Analyses. Anal. Lett. 2004, 37, 789–830. [Google Scholar] [CrossRef]
- Hart, J.P.; Crew, A.; Crouch, E.; Honeychurch, K.C.; Pemberton, R.M. Chapter 23 Screen-printed electrochemical (bio)sensors in biomedical, environmental and industrial applications. Compr. Anal. Chem. 2007, 49, 497–557. [Google Scholar]
- Hughes, G.; Westmacott, K.; Honeychurch, K.C.; Crew, A.; Pemberton, R.M.; Hart, J.P. Recent advances in the fabrication and application of screen-printed electrochemical (bio)sensors based on carbon materials for biomedical, agri-food and environmental analyses. Biosensors 2016, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Oni, J.; Westbroek, P.; Nyokong, T. Voltammetric Detection of Vitamin B1 at Carbon Paste Electrodes and Its Determination in Tablets. Electroanal 2002, 14, 1165–1168. [Google Scholar] [CrossRef]
- Hart, J.P.; Norman, M.; Tsang, S. Voltammetric behaviour of vitamin B1 (thiamine) at a glassy carbon electrode and its determination in multivitamin tablets using anion-exchange liquid chromatography with amperometric detection under basic conditions. Analyst 1995, 120, 1059–1064. [Google Scholar] [CrossRef]
- Wopschall, R.H.; Shain, I. Effects of adsorption of electroactive species in stationary electrode polarography. Anal. Chem. 1967, 39, 1514–1527. [Google Scholar] [CrossRef]
- Hart, J.P. Electroanalysis of Biologically Important Compounds; Ellis Horwood Limited: Hemel Hempstead, UK, 1990; Chapter 4; pp. 137–191. [Google Scholar]






| Element (% by Weight) | CoPC-SPCE 1 | SD | CoPC-SPCE 2 | SD | CoPC-SPCE 3 | SD |
|---|---|---|---|---|---|---|
| C | 88.423 | 0.340 | 88.143 | 0.331 | 88.020 | 0.466 |
| O | 2.917 | 0.059 | 3.043 | 0.090 | 3.127 | 0.154 |
| S | 0.187 | 0.023 | 0.193 | 0.021 | 0.187 | 0.006 |
| Cl | 8.097 | 0.326 | 8.233 | 0.370 | 8.297 | 0.329 |
| Co | 0.317 | 0.012 | 0.327 | 0.025 | 0.307 | 0.006 |
| Br | 0.057 | 0.006 | 0.060 | 0.017 | 0.063 | 0.006 |
| Scan Rate (mVs−1) | αna Forward Peak | αna Reverse Peak |
|---|---|---|
| 20 | 0.221 | −0.440 |
| 50 | 0.207 | −0.351 |
| 100 | 0.213 | −0.340 |
| 150 | 0.213 | −0.363 |
| 200 | 0.202 | −0.297 |
| Compound | Response |
|---|---|
| B1 (thiamine) | Yes |
| B2 (riboflavin) | No |
| B3 (niacin) | No |
| B5 (pantothenic acid) | No |
| B6 (pyridoxine) | No |
| B7 (biotin) | No |
| B9 (folic acid) | No |
| B12 (cobalamin) | No |
| Malic acid | No |
| Citric acid | No |
| Sample | Declared (mg/Tablet) | Measured (mg/Tablet) | Recovered (%) |
|---|---|---|---|
| 1 | 5 | 4.804 | 96.1 |
| 2 | 5 | 5.065 | 101.3 |
| 3 | 5 | 5.722 | 114.4 |
| 4 | 5 | 4.603 | 92.1 |
| Average recovery (%) | 101.0 | ||
| Standard deviation | 9.7 | ||
| Coefficient of variation (%) | 9.6 | ||
| Sample | Declared (mg/100 mL) | Measured (mg/100 mL) | Recovered (%) |
|---|---|---|---|
| 1 | 0.22 | 0.198 | 90.1 |
| 2 | 0.22 | 0.198 | 90.1 |
| 3 | 0.22 | 0.218 | 99.1 |
| 4 | 0.22 | 0.188 | 85.6 |
| 5 | 0.22 | 0.223 | 101.4 |
| Average recovery (%) | 93.3 | ||
| Standard deviation | 9.7 | ||
| Coefficient of variation (%) | 7.2 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smart, A.; Westmacott, K.L.; Crew, A.; Doran, O.; Hart, J.P. An Electrocatalytic Screen-Printed Amperometric Sensor for the Selective Measurement of Thiamine (Vitamin B1) in Food Supplements. Biosensors 2019, 9, 98. https://doi.org/10.3390/bios9030098
Smart A, Westmacott KL, Crew A, Doran O, Hart JP. An Electrocatalytic Screen-Printed Amperometric Sensor for the Selective Measurement of Thiamine (Vitamin B1) in Food Supplements. Biosensors. 2019; 9(3):98. https://doi.org/10.3390/bios9030098
Chicago/Turabian StyleSmart, Amy, Kelly L. Westmacott, Adrian Crew, Olena Doran, and John P. Hart. 2019. "An Electrocatalytic Screen-Printed Amperometric Sensor for the Selective Measurement of Thiamine (Vitamin B1) in Food Supplements" Biosensors 9, no. 3: 98. https://doi.org/10.3390/bios9030098
APA StyleSmart, A., Westmacott, K. L., Crew, A., Doran, O., & Hart, J. P. (2019). An Electrocatalytic Screen-Printed Amperometric Sensor for the Selective Measurement of Thiamine (Vitamin B1) in Food Supplements. Biosensors, 9(3), 98. https://doi.org/10.3390/bios9030098