Surface-Enhanced Raman Scattering-Based Immunoassay Technologies for Detection of Disease Biomarkers
Abstract
:1. Introduction
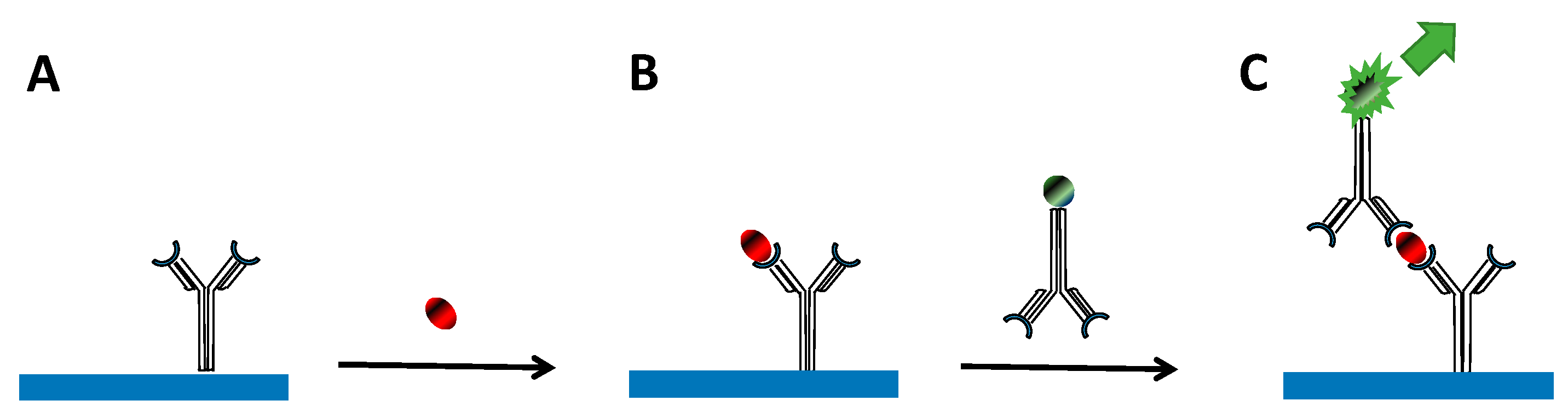
2. Sandwich Immunoassay Format
3. Surface-Enhanced Raman Scattering as Readout Strategy—Mechanism and Advantage
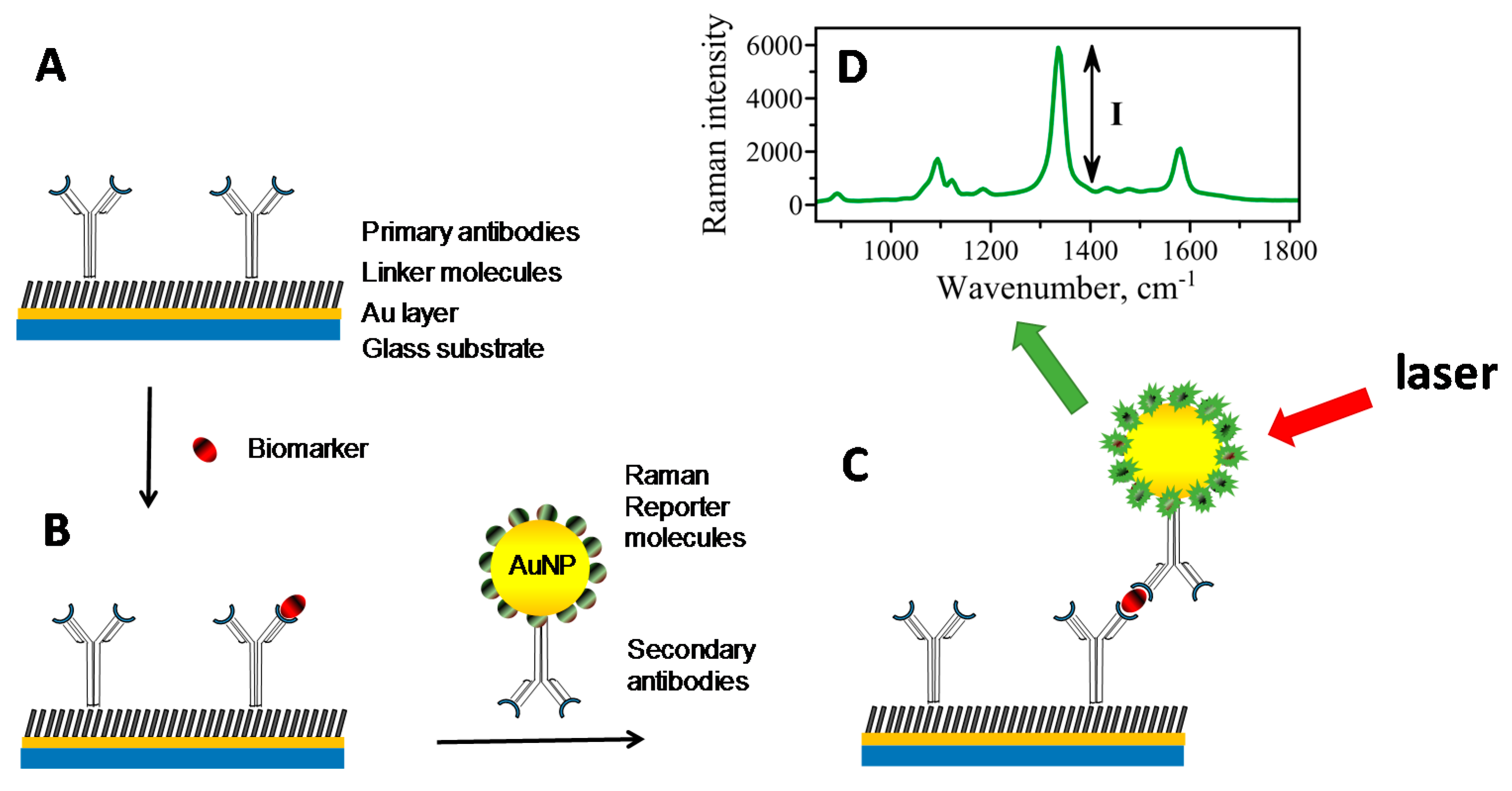
4. SERS-Based Immunoassay Sensors
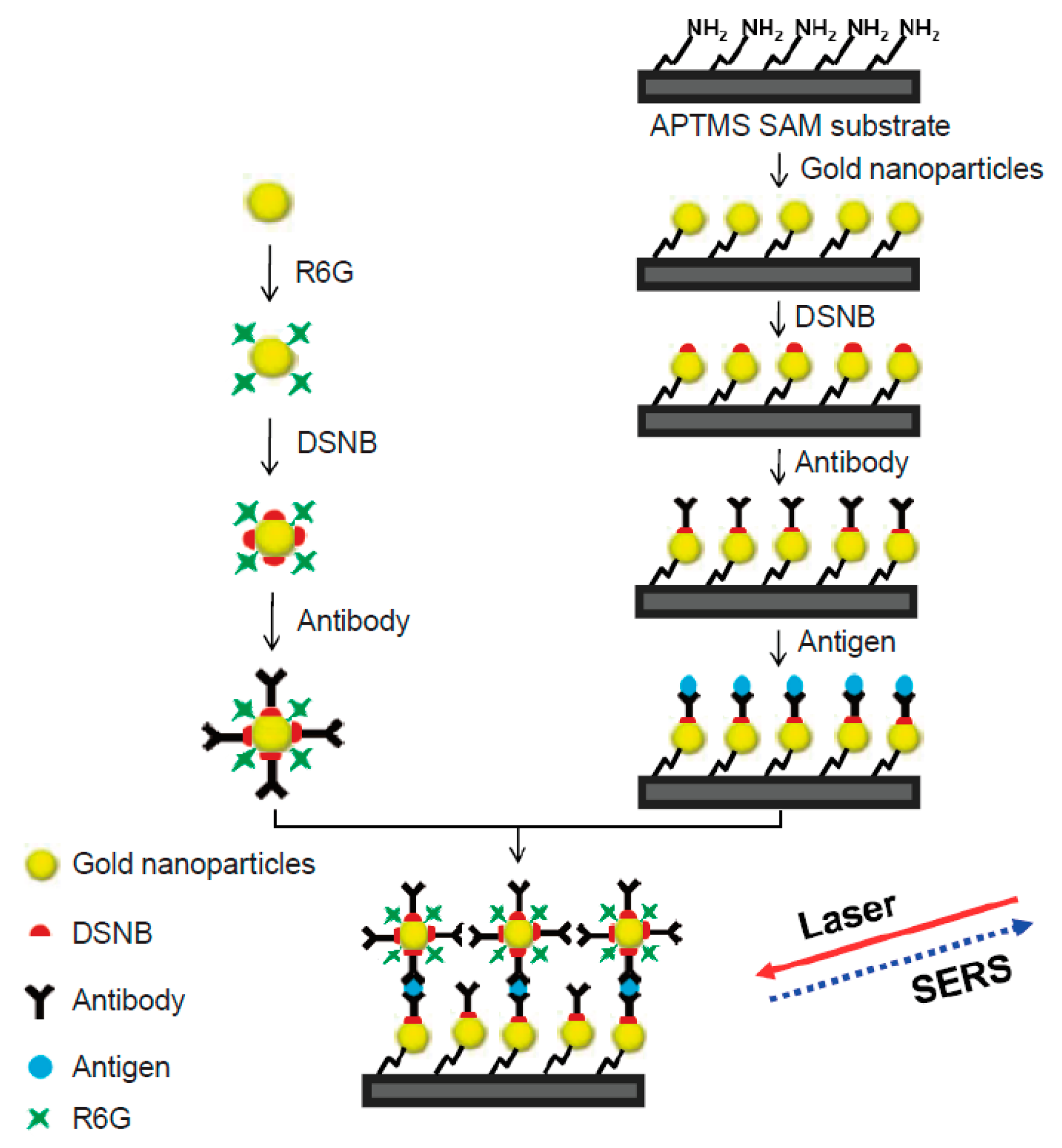
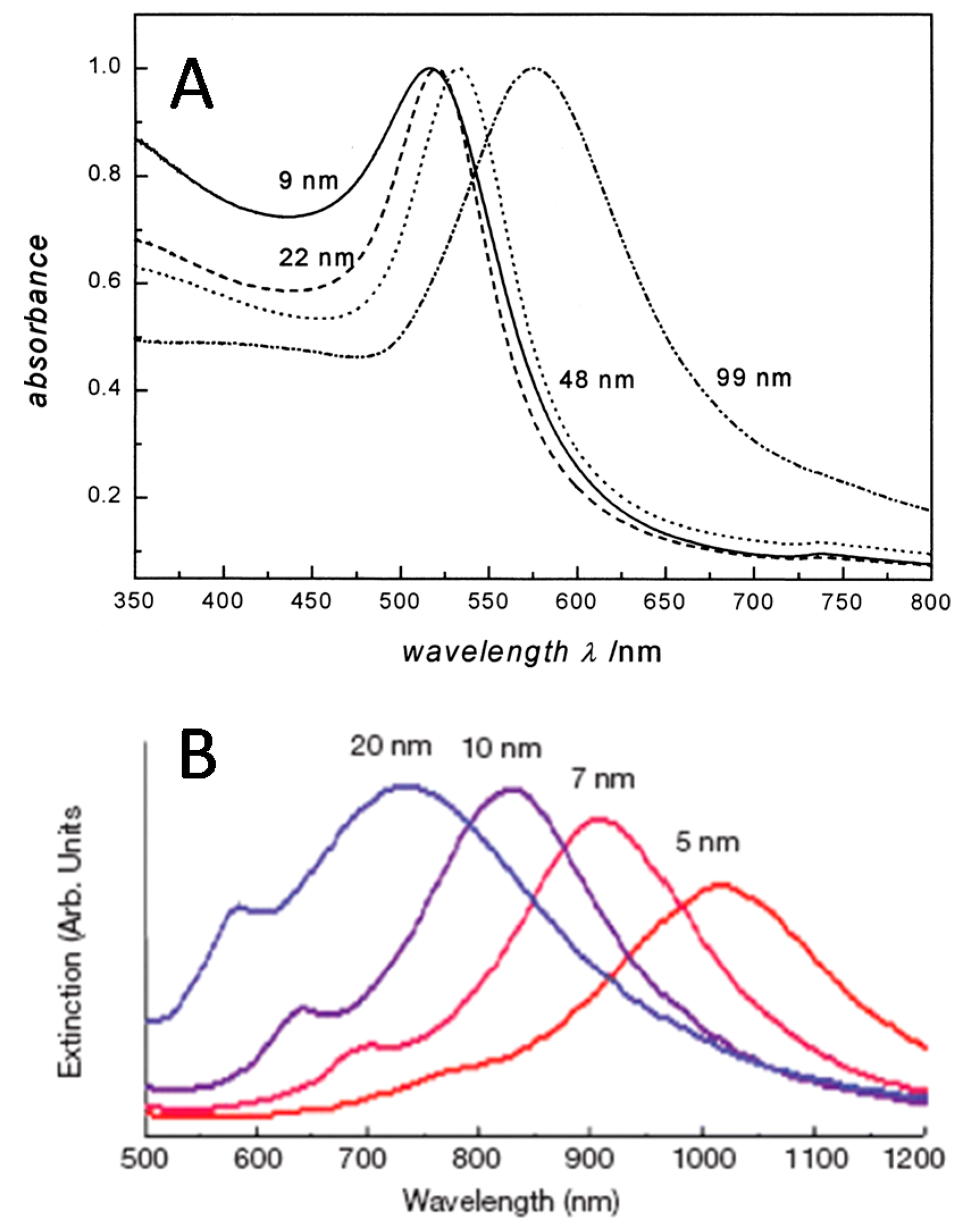
5. Capture Substrate
6. SERS Nanotags
7. Raman Reporter Molecules (RRM)
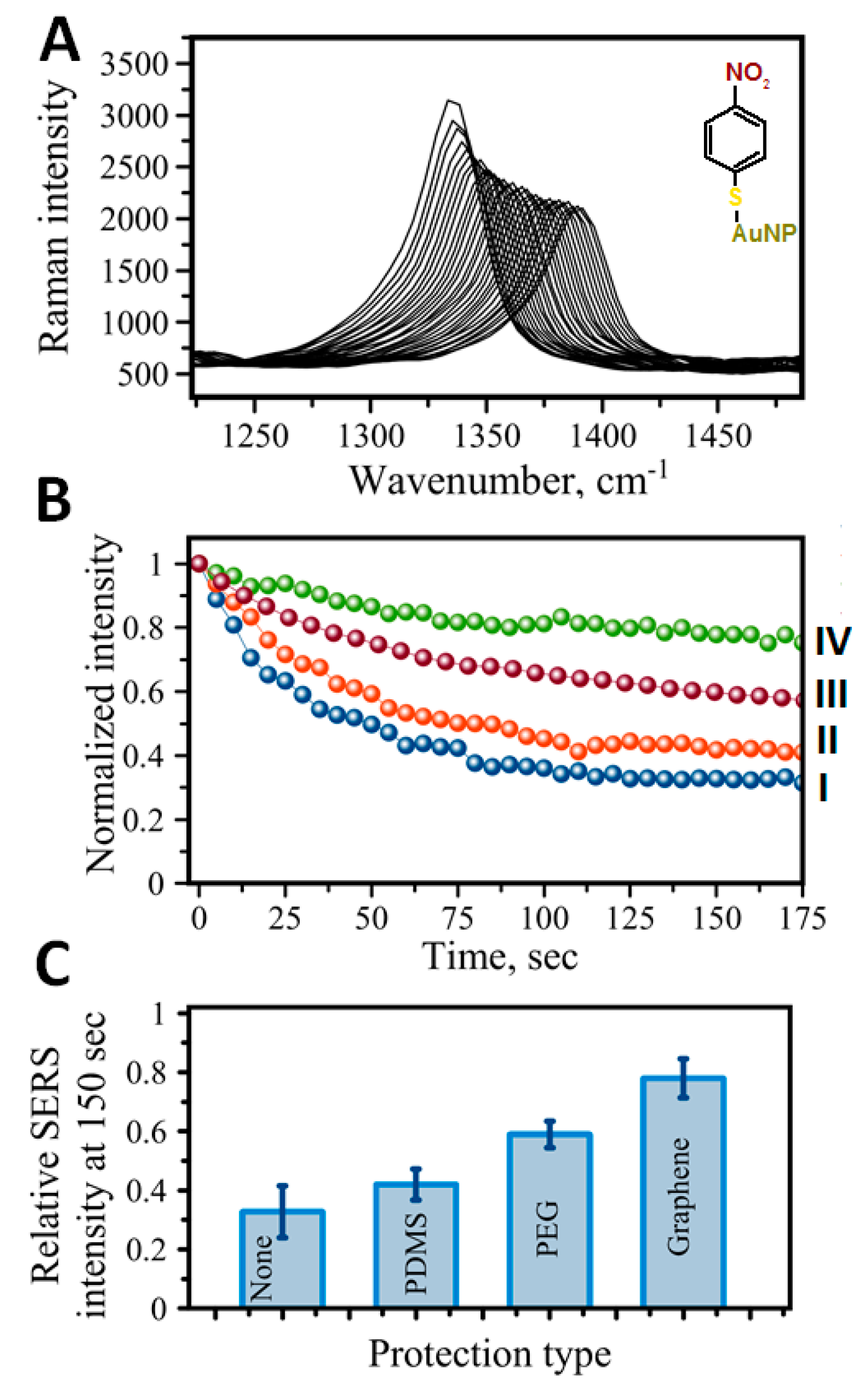
8. Protection Strategies
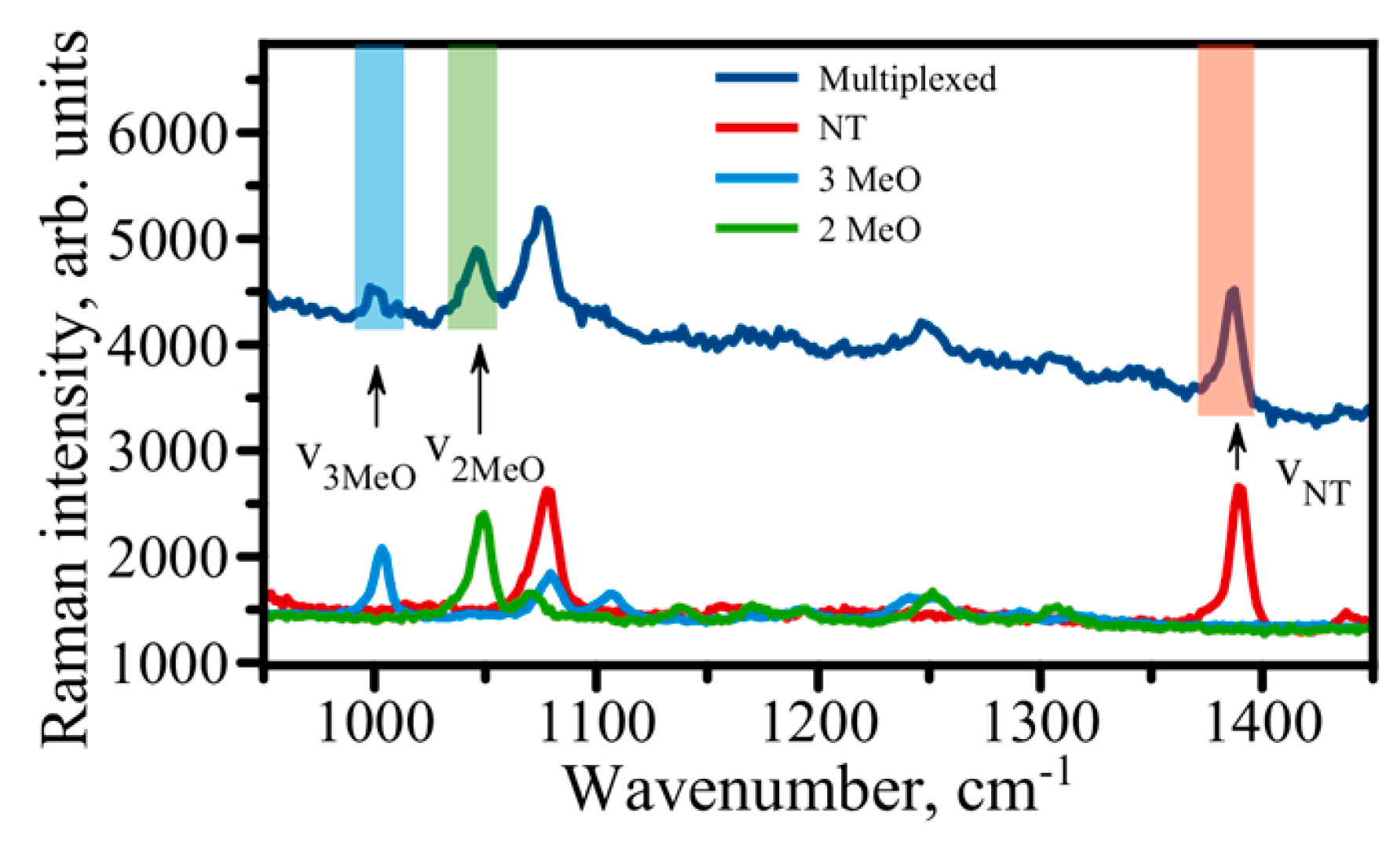
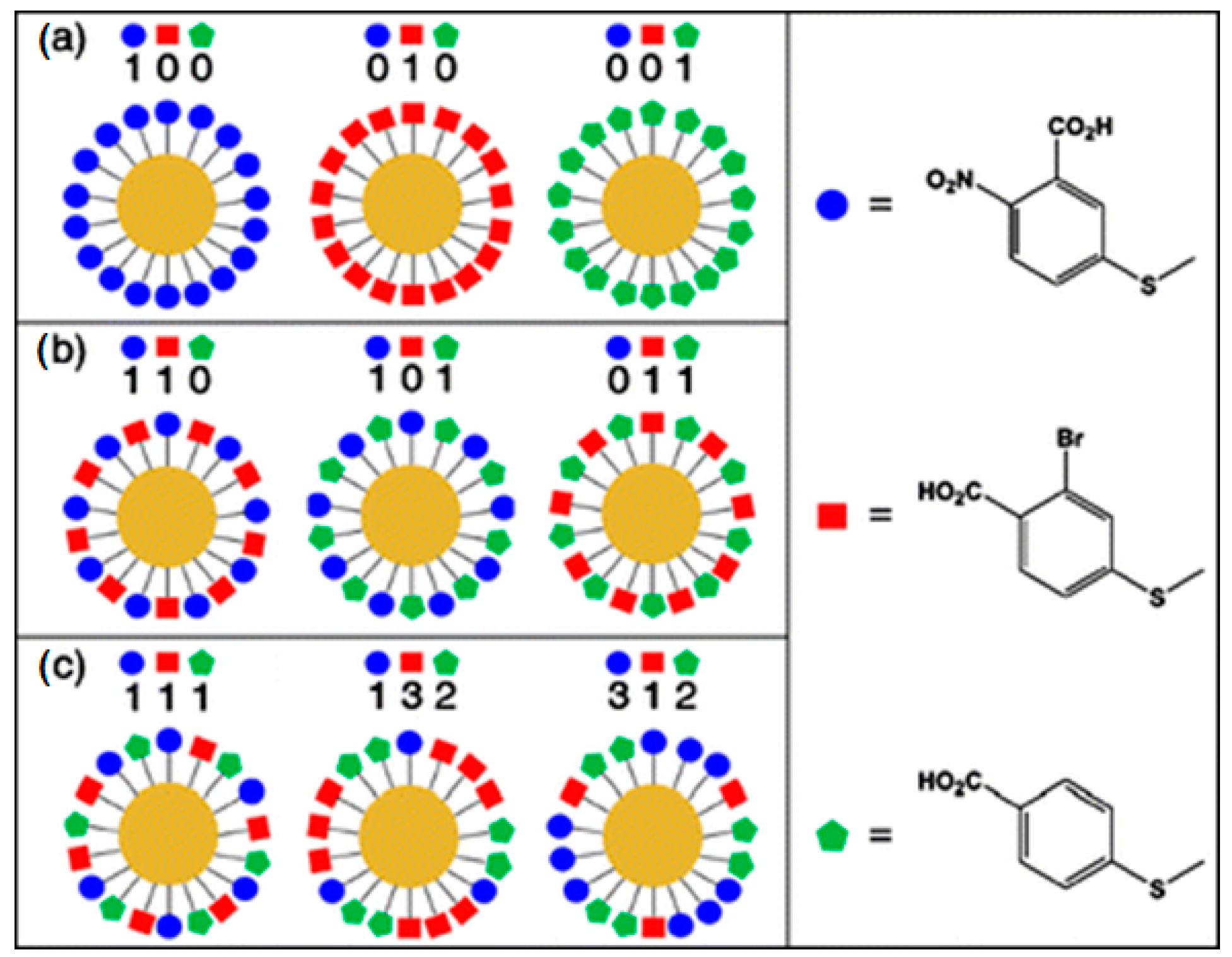
9. Multiplexing with SERS Immunoassay
- (1)
- The availability of reliable detection platforms with high sensitivity.
- (2)
- The narrow width of the characteristic Raman bands observed in a spectrum for individual RRM.
- (3)
- Single laser wavelength allows for concurrent excitation of many different Raman active reporter molecules.
10. Multiplex Detection in Vivo
11. Concluding Remarks
Financial Support
Conflicts of Interest
References
- Davies, C. Principles of competitive and immunometric assays (including ELISA). In The Immunoassay Handbook, 4th ed.; Elsevier: Oxford, UK, 2013; pp. 29–59. [Google Scholar]
- Klee, G.G.; Post, G. Effect of counting errors on immunoassay precision. Clin. Chem. 1989, 35, 1362. [Google Scholar] [PubMed]
- Hicks, J.M. Fluorescence immunoassay. Hum. Pathol. 1984, 15, 112–116. [Google Scholar] [CrossRef]
- Brown, C.R.; Higgins, K.W.; Frazer, K.; Schoelz, L.K.; Dyminski, J.W.; Marinkovich, V.A.; Miller, S.P.; Burd, J.F. Simultaneous determination of total ige and allergen-specific ige in serum by the mast chemiluminescent assay system. Clin. Chem. 1985, 31, 1500. [Google Scholar] [PubMed]
- Hayes, F.J.; Halsall, H.B.; Heineman, W.R. Simultaneous immunoassay using electrochemical detection of metal ion labels. Anal. Chem. 1994, 66, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-J.; Liu, Y.; Hu, L.-H.; Jiang, L.-P.; Zhu, J.-J. “Proof-of-principle” concept for ultrasensitive detection of cytokines based on the electrically heated carbon paste electrode. Chem. Commun. 2011, 47, 6551–6553. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.E. Solid supports in enzyme-linked immunosorbent assay and other solid-phase immunoassays. Methods 2000, 22, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Xie, W.; Yi, G.; Chen, D.; Zhou, Y.; Cheng, J. Microminiaturized immunoassays using quantum dots as fluorescent label by laser confocal scanning fluorescence detection. J. Immunol. Methods 2001, 249, 85–89. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Zheng, T.-T.; Cheng, F.-F.; Zhu, J.-J. Electrochemical sensing for caspase 3 activity and inhibition using quantum dot functionalized carbon nanotube labels. Chem. Commun. 2011, 47, 1178–1180. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.C.; Bertolucci, M.D. Symmetry and Spectroscopy: An Introduction to Vibrational and Electronic Spectroscopy; Oxford University Press: New York, NY, USA, 1978. [Google Scholar]
- McCreery, R.L. Introduction and scope. In Raman Spectroscopy for Chemical Analysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000; pp. 1–14. [Google Scholar]
- Ni, J.; Lipert, R.J.; Dawson, G.B.; Porter, M.D. Immunoassay readout method using extrinsic raman labels adsorbed on immunogold colloids. Anal. Chem. 1999, 71, 4903–4908. [Google Scholar] [CrossRef] [PubMed]
- Grubisha, D.S.; Lipert, R.J.; Park, H.Y.; Driskell, J.; Porter, M.D. Femtomolar detection of prostate-specific antigen: An immunoassay based on surface-enhanced raman scattering and immunogold labels. Anal. Chem. 2003, 75, 5936–5943. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Kneipp, H.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Ultrasensitive chemical analysis by raman spectroscopy. Chem. Rev. 1999, 99, 2957–2976. [Google Scholar] [CrossRef] [PubMed]
- Etchegoin, P.G.; Le Ru, E.C. A perspective on single molecule sers: Current status and future challenges. Phys. Chem. Chem. Phys. 2008, 10, 6079–6089. [Google Scholar] [CrossRef] [PubMed]
- Pieczonka, N.P.W.; Aroca, R.F. Single molecule analysis by surfaced-enhanced raman scattering. Chem. Soc. Rev. 2008, 37, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hutter, T.; Steiner, U.; Mahajan, S. Single molecule sers and detection of biomolecules with a single gold nanoparticle on a mirror junction. Analyst 2013, 138, 4574–4578. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Irudayaraj, J. Surface-enhanced raman spectroscopy at single-molecule scale and its implications in biology. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J.K.; Chumanov, G. Nanoparticle-mirror sandwich substrates for surface-enhanced raman scattering. J. Phys. Chem. B 2005, 109, 17936–17942. [Google Scholar] [CrossRef] [PubMed]
- Schatz, G.C.; Young, M.A.; Van Duyne, R.P. Electromagnetic mechanism of sers. In Surface-Enhanced Raman Scattering: Physics and Applications; Kneipp, K., Moskovits, M., Kneipp, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 19–45. [Google Scholar]
- Rohr, T.E.; Cotton, T.; Fan, N.; Tarcha, P.J. Immunoassay employing surface-enhanced raman spectroscopy. Anal. Biochem. 1989, 182, 388–398. [Google Scholar] [CrossRef]
- Graham, D.; Mallinder, B.J.; Smith, W.E. Surface-enhanced resonance raman scattering as a novel method of DNA discrimination. Angew. Chem. Int. Ed. 2000, 39, 1061–1063. [Google Scholar] [CrossRef]
- Faulds, K.; Littleford, R.E.; Graham, D.; Dent, G.; Smith, W.E. Comparison of surface-enhanced resonance raman scattering from unaggregated and aggregated nanoparticles. Anal. Chem. 2004, 76, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-enhanced raman spectroscopy. Annu. Rev. Anal. Chem. (Palo Alto, CA) 2008, 1, 601–626. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.M.; Ewusi-Annan, E.; Jensen, L. Controlling the non-resonant chemical mechanism of sers using a molecular photoswitch. Phys. Chem. Chem. Phys. 2009, 11, 7424–7429. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Frontiera, R.R.; Henry, A.-I.; Ringe, E.; Van Duyne, R.P. Sers: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Shen, A.G.; Chen, L.F.; Xie, W.; Hu, J.C.; Zeng, A.; Richards, R.; Hu, J.M. Triplex au-ag-c core shell nanoparticles as a novel raman label. Adv. Funct. Mater. 2010, 20, 969–975. [Google Scholar] [CrossRef]
- Yoon, K.J.; Seo, H.K.; Hwang, H.; Pyo, D.; Eom, I.Y.; Hahn, J.H.; Jung, Y.M. Bioanalytical application of sers immunoassay for detection of prostate-specific antigen. Bull. Korean Chem. Soc. 2010, 31, 1215–1218. [Google Scholar] [CrossRef]
- Han, X.X.; Cai, L.J.; Guo, J.; Wang, C.X.; Ruan, W.D.; Han, W.Y.; Xu, W.Q.; Zhao, B.; Ozaki, Y. Fluorescein isothiocyanate linked immunoabsorbent assay based on surface-enhanced resonance raman scattering. Anal. Chem. 2008, 80, 3020–3024. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.; Mitchelson, K.; Yu, Z.; Cheng, J. Analysis of the sensitivity and frequency characteristics of coplanar electrical cell-substrate impedance sensors. Biosens. Bioelectr. 2008, 24, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.H.; Hong, W.; Sa, Y.; Chen, L.; Jung, Y.-J.; Wang, X.; Zhao, B.; Jung, Y.M. Multiple detection of proteins by sers-based immunoassay with core shell magnetic gold nanoparticles. Vib. Spectrosc. 2014, 72, 44–49. [Google Scholar] [CrossRef]
- Driskell, J.D.; Kwarta, K.M.; Lipert, R.J.; Porter, M.D.; Neill, J.D.; Ridpath, J.F. Low-level detection of viral pathogens by a surface-enhanced raman scattering based immunoassay. Anal. Chem. 2005, 77, 6147–6154. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Yang, Y.-M.; Chen, Y.-F.; Yang, T.-S.; Chang, H.-C. A new protein a assay based on raman reporter labeled immunogold nanoparticles. Biosens. Bioelectr. 2008, 24, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ji, X.; Xu, W.; Li, X.; Wang, L.; Bai, Y.; Zhao, B.; Ozaki, Y. Immunoassay using probe-labelling immunogold nanoparticles with silver staining enhancement via surface-enhanced raman scattering. Analyst 2004, 129, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Yakes, B.J.; Lipert, R.J.; Bannantine, J.P.; Porter, M.D. Detection of mycobacterium avium subsp. Paratuberculosis by a sonicate immunoassay based on surface-enhanced raman scattering. Clin. Vaccine Immunol. 2008, 15, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, K.; Kim, K.H.; Oh, K.W.; Choo, J. Sers-based immunoassay using a gold array-embedded gradient microfluidic chip. Lab Chip 2012, 12, 3720–3727. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Li, W.; Lu, D.; Chen, K.; He, Q.; Han, H.; Zou, M. A sers-based immunoassay for porcine circovirus type 2 using multi-branched gold nanoparticles. Microchim. Acta 2013, 180, 1501–1507. [Google Scholar] [CrossRef]
- Wang, G.; Lipert, R.J.; Jain, M.; Kaur, S.; Chakraboty, S.; Torres, M.P.; Batra, S.K.; Brand, R.E.; Porter, M.D. Detection of the potential pancreatic cancer marker muc4 in serum using surface-enhanced raman scattering. Anal. Chem. 2011, 83, 2554–2561. [Google Scholar] [CrossRef] [PubMed]
- Granger, J.H.; Granger, M.C.; Firpo, M.A.; Mulvihill, S.J.; Porter, M.D. Toward development of a surface-enhanced raman scattering (sers)-based cancer diagnostic immunoassay panel. Analyst 2013, 138, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Krasnoslobodtsev, A.V.; Torres, M.P.; Kaur, S.; Vlassiouk, I.V.; Lipert, R.J.; Jain, M.; Batra, S.K.; Lyubchenko, Y.L. Nano-immunoassay with improved performance for detection of cancer biomarkers. Nanomedicine 2015, 11, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Zengin, A.; Tamer, U.; Caykara, T. A sers-based sandwich assay for ultrasensitive and selective detection of alzheimer’s tau protein. Biomacromolecules 2013, 14, 3001–3009. [Google Scholar] [CrossRef] [PubMed]
- Boca, S.; Farcau, C.; Baia, M.; Astilean, S. Metanephrine neuroendocrine tumor marker detection by sers using au nanoparticle/au film sandwich architecture. Biomed. Microdevices 2016, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Dong, J.; Xie, W.; Tao, L.; Lu, W.; Wang, Y.; Qian, W. Sers tags-based novel monodispersed hollow gold nanospheres for highly sensitive immunoassay of cea. J. Mater. Sci. 2015, 50, 3329–3336. [Google Scholar] [CrossRef]
- Song, C.; Wang, Z.; Yang, J.; Zhang, R.; Cui, Y. Effects of solid substrate on the sers-based immunoassay: A comparative study. J. Raman Spectrosc. 2011, 42, 313–318. [Google Scholar] [CrossRef]
- Khan, I.; Polwart, E.; McComb, D.W.; Smith, W.E. Correlation of optical properties with structure of immobilised nanoparticles—A method for probing the mechanism of serrs. Analyst 2004, 129, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Driskell, J.D.; Lipert, R.J.; Porter, M.D. Labeled gold nanoparticles immobilized at smooth metallic substrates: Systematic investigation of surface plasmon resonance and surface-enhanced raman scattering. J. Phys. Chem. B 2006, 110, 17444–17451. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Yamaguchi, I. Optical absorption study of the surface plasmon resonance in gold nanoparticles immobilized onto a gold substrate by self-assembly technique. J. Phys. Chem. B 2003, 107, 10321–10324. [Google Scholar] [CrossRef]
- Porter, M.D.; Lipert, R.J.; Siperko, L.M.; Wang, G.; Narayanan, R. Sers as a bioassay platform: Fundamentals, design, and applications. Chem. Soc. Rev. 2008, 37, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Hutter, T.; Huang, F.M.; Elliott, S.R.; Mahajan, S. Near-field plasmonics of an individual dielectric nanoparticle above a metallic substrate. J. Phys. Chem. C 2013, 117, 7784–7790. [Google Scholar] [CrossRef]
- Yockell-Lelièvre, H.; Lussier, F.; Masson, J.F. Influence of the particle shape and density of self-assembled gold nanoparticle sensors on lspr and sers. J. Phys. Chem. C 2015, 119, 28577–28585. [Google Scholar] [CrossRef]
- Liberman, V.; Yilmaz, C.; Bloomstein, T.M.; Somu, S.; Echegoyen, Y.; Busnaina, A.; Cann, S.G.; Krohn, K.E.; Marchant, M.F.; Rothschild, M. A nanoparticle convective directed assembly process for the fabrication of periodic surface enhanced raman spectroscopy substrates. Adv. Mater. 2010, 22, 4298–4302. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.A.; Wu, C.; Bao, K.; Bao, J.; Bardhan, R.; Halas, N.J.; Manoharan, V.N.; Nordlander, P.; Shvets, G.; Capasso, F. Self-assembled plasmonic nanoparticle clusters. Science 2010, 328, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Theiss, J.; Pavaskar, P.; Echternach, P.M.; Muller, R.E.; Cronin, S.B. Plasmonic nanoparticle arrays with nanometer separation for high-performance sers substrates. Nano Lett. 2010, 10, 2749–2754. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Meng, G.; Huang, Q.; Huang, Z.; Xu, Q.; Zhu, C.; Qian, Y.; Ding, Y. Green synthesis of large-scale highly ordered core@shell nanoporous au@ag nanorod arrays as sensitive and reproducible 3D SERS substrates. ACS Appl. Mater. Interfaces 2014, 6, 15667–15675. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhan, Z.; Dou, J.; Zheng, X.; Xu, R.; Wu, M.; Lei, Y. Highly reproducible and sensitive sers substrates with ag inter-nanoparticle gaps of 5 nm fabricated by ultrathin aluminum mask technique. ACS Appl. Mater. Interfaces 2015, 7, 13322–13328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lee, Y.H.; Phang, I.Y.; Lee, C.K.; Ling, X.Y. Hierarchical 3D SERS substrates fabricated by integrating photolithographic microstructures and self-assembly of silver nanoparticles. Small 2014, 10, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Lesuffleur, A.; Kumar, L.K.S.; Brolo, A.G.; Kavanagh, K.L.; Gordon, R. Apex-enhanced raman spectroscopy using double-hole arrays in a gold film. J. Phys. Chem. C 2007, 111, 2347–2350. [Google Scholar] [CrossRef]
- Yokota, Y.; Ueno, K.; Misawa, H. Essential nanogap effects on surface-enhanced raman scattering signals from closely spaced gold nanoparticles. Chem. Commun. 2011, 47, 3505–3507. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Hu, H.; Kumar, K.; Shen, Z.; Yang, J.K. Direct and reliable patterning of plasmonic nanostructures with sub-10-nm gaps. ACS Nano 2011, 5, 7593–7600. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Aaron, J.; Sokolov, K. Directional conjugation of antibodies to nanoparticles for synthesis of multiplexed optical contrast agents with both delivery and targeting moieties. Nat. Protoc. 2008, 3, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface raman spectroelectrochemistry. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Kreibig, U.; Genzel, L. Optical absorption of small metallic particles. Surf. Sci. 1985, 156, 678–700. [Google Scholar] [CrossRef]
- Mie, G. Beiträge zur optik trüber medien, speziell kolloidaler metallösungen. Ann. Phys. 1908, 330, 377–445, In German. [Google Scholar] [CrossRef]
- Papavassiliou, G.C. Optical properties of small inorganic and organic metal particles. Prog. Solid State Chem. 1979, 12, 185–271. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and scattering by a sphere. In Absorption and Scattering of Light by Small Particles; Wiley-VCH Verlag GmbH: Weinheim, Germany, 1998; pp. 82–129. [Google Scholar]
- Link, S.; El-Sayed, M.A. Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Loo, C.; Lin, A.; Hirsch, L.; Lee, M.-H.; Barton, J.; Halas, N.; West, J.; Drezek, R. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol. Cancer Res. Treat. 2004, 3, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, S.; Chen, L.; Lee, E.K.; Shin, S.Y.; Lee, Y.H.; Son, S.W.; Oh, C.H.; Song, J.M.; Kang, S.H.; et al. Sers imaging of HER2-overexpressed MCF7 cells using antibody-conjugated gold nanorods. Phys. Chem. Chem. Phys. 2009, 11, 7444–7449. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.K.; Dinish, U.S.; Samanta, A.; Vendrell, M.; Soh, K.-S.; Park, S.-J.; Olivo, M.; Chang, Y.-T. Multiplex targeted in vivo cancer detection using sensitive near-infrared sers nanotags. Nano Today 2012, 7, 85–93. [Google Scholar] [CrossRef]
- Lal, S.; Clare, S.E.; Halas, N.J. Nanoshell-enabled photothermal cancer therapy: Impending clinical impact. Acc. Chem. Res. 2008, 41, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.M.; Cao, Y.; Urban, C.; Neumann, O.; Ayala-Orozco, C.; Knight, M.W.; Joshi, A.; Nordlander, P.; Halas, N.J. The surprising in vivo instability of near-IR-absorbing hollow Au-Ag nanoshells. ACS Nano 2014, 8, 3222–3231. [Google Scholar] [CrossRef] [PubMed]
- Schutz, M.; Steinigeweg, D.; Salehi, M.; Kompe, K.; Schlücker, S. Hydrophilically stabilized gold nanostars as SERS labels for tissue imaging of the tumor suppressor p63 by immuno-sers microscopy. Chem. Commun. 2011, 47, 4216–4218. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, L.; Mourdikoudis, S.; Pastoriza-Santos, I.; Perez-Juste, J. Nanocrystal engineering of noble metals and metal chalcogenides: Controlling the morphology, composition and crystallinity. CrystEngComm 2015, 17, 3727–3762. [Google Scholar] [CrossRef]
- Schlücker, S. SERS microscopy: Nanoparticle probes and biomedical applications. Chemphyschem 2009, 10, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, Y.; Fales, A.M.; Li, Y.L.; Liu, J.; Vo-Dinh, T. Quantitative surface-enhanced resonant raman scattering multiplexing of biocompatible gold nanostars for in vitro and ex vivo detection. Anal. Chem. 2013, 85, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Fu, T.; Chen, K.; Han, H.; Zou, M. Synthesis of multi-branched gold nanoparticles by reduction of tetrachloroauric acid with tris base, and their application to SERS and cellular imaging. Microchim. Acta 2011, 175, 55–61. [Google Scholar] [CrossRef]
- Khoury, C.G.; Vo-Dinh, T. Gold nanostars for surface-enhanced raman scattering: Synthesis, characterization and optimization. J. Phys. Chem. C Nanomater. Interfaces 2008, 112, 18849–18859. [Google Scholar] [CrossRef]
- Niu, W.; Chua, Y.A.A.; Zhang, W.; Huang, H.; Lu, X. Highly symmetric gold nanostars: Crystallographic control and surface-enhanced raman scattering property. J. Am. Chem. Soc. 2015, 137, 10460–10463. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sun, M.; Ma, W.; Kuang, H.; Xu, C. Self-assembled nanoparticle dimers with contemporarily relevant properties and emerging applications. Mater. Today 2016, 19, 595–606. [Google Scholar] [CrossRef]
- Yang, J.-K.; Kang, H.; Lee, H.; Jo, A.; Jeong, S.; Jeon, S.-J.; Kim, H.-I.; Lee, H.-Y.; Jeong, D.H.; Kim, J.-H.; et al. Single-step and rapid growth of silver nanoshells as SERS-active nanostructures for label-free detection of pesticides. ACS Appl. Mater. Interfaces 2014, 6, 12541–12549. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Puebla, R.A.; Ross, D.J.; Nazri, G.A.; Aroca, R.F. Surface-enhanced raman scattering on nanoshells with tunable surface plasmon resonance. Langmuir 2005, 21, 10504–10508. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Sikdar, D.; Huang, X.; Si, K.J.; Xiong, W.; Gong, S.; Yap, L.W.; Premaratne, M.; Cheng, W. Plasmonic core-shell nanoparticles for SERS detection of the pesticide thiram: Size- and shape-dependent raman enhancement. Nanoscale 2015, 7, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Kim, K.H.; Choi, N.; Chon, H.; Lee, S.; Choo, J. Preparation of silica-encapsulated hollow gold nanosphere tags using layer-by-layer method for multiplex surface-enhanced raman scattering detection. Langmuir 2011, 27, 10228–10233. [Google Scholar] [CrossRef] [PubMed]
- Barbara, A.; Dubois, F.; Ibanez, A.; Eng, L.M.; Quémerais, P. SERS correlation spectroscopy of silver aggregates in colloidal suspension: Quantitative sizing down to a single nanoparticle. J. Phys. Chem. C 2014, 118, 17922–17931. [Google Scholar] [CrossRef]
- Chen, J.-W.; Lei, Y.; Liu, X.-J.; Jiang, J.-H.; Shen, G.-L.; Yu, R.-Q. Immunoassay using surface-enhanced raman scattering based on aggregation of reporter-labeled immunogold nanoparticles. Anal. Bioanal. Chem. 2008, 392, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.M.; Waldeisen, J.R.; Wang, T.; Lee, L.P. Strategies for nanoplasmonic core-satellite biomolecular sensors: Theory-based design. Appl. Phys. Lett. 2009, 95, 193112. [Google Scholar] [CrossRef] [PubMed]
- Gellner, M.; Steinigeweg, D.; Ichilmann, S.; Salehi, M.; Schütz, M.; Kömpe, K.; Haase, M.; Schlücker, S. 3D self-assembled plasmonic superstructures of gold nanospheres: Synthesis and characterization at the single-particle level. Small 2011, 7, 3445–3451. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Herrmann, C.; Kömpe, K.; Haase, M.; Schlücker, S. Synthesis of bifunctional Au/Pt/Au core/shell nanoraspberries for in situ SERS monitoring of platinum-catalyzed reactions. J. Am. Chem. Soc. 2011, 133, 19302–19305. [Google Scholar] [CrossRef] [PubMed]
- Meneghetti, M.; Scarsi, A.; Litti, L.; Marcolongo, G.; Amendola, V.; Gobbo, M.; Di Chio, M.; Boscaini, A.; Fracasso, G.; Colombatti, M. Serrs: Plasmonic nanostructures for SERRS multiplexed identification of tumor-associated antigens (small 24/2012). Small 2012, 8, 3860. [Google Scholar] [CrossRef]
- Bastús, N.G.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of highly monodisperse citrate-stabilized silver nanoparticles of up to 200 nm: Kinetic control and catalytic properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- Aswathy Aromal, S.; Philip, D. Facile one-pot synthesis of gold nanoparticles using tannic acid and its application in catalysis. Phys. E Low-Dimens. Syst. Nanostruct. 2012, 44, 1692–1696. [Google Scholar] [CrossRef]
- Benkovičová, M.; Végsö, K.; Šiffalovič, P.; Jergel, M.; Majková, E.; Luby, Š.; Šatka, A. Preparation of sterically stabilized gold nanoparticles for plasmonic applications. Chem. Pap. 2013, 67, 1225–1230. [Google Scholar] [CrossRef]
- Teranishi, T.; Kiyokawa, I.; Miyake, M. Synthesis of monodisperse gold nanoparticles using linear polymers as protective agents. Adv. Mater. 1998, 10, 596–599. [Google Scholar] [CrossRef]
- Wuelfing, W.P.; Gross, S.M.; Miles, D.T.; Murray, R.W. Nanometer gold clusters protected by surface-bound monolayers of thiolated poly(ethylene glycol) polymer electrolyte. J. Am. Chem. Soc. 1998, 120, 12696–12697. [Google Scholar] [CrossRef]
- Mandal, T.K.; Fleming, M.S.; Walt, D.R. Preparation of polymer coated gold nanoparticles by surface-confined living radical polymerization at ambient temperature. Nano Lett. 2002, 2, 3–7. [Google Scholar] [CrossRef]
- Jager, M.; Schubert, S.; Ochrimenko, S.; Fischer, D.; Schubert, U.S. Branched and linear poly(ethylene imine)-based conjugates: Synthetic modification, characterization, and application. Chem. Soc. Rev. 2012, 41, 4755–4767. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Sung, K.-B.; Dentinger, C.; Lutz, B.; Nguyen, L.; Zhang, J.; Qin, H.; Yamakawa, M.; Cao, M.; Lu, Y.; et al. Composite organic-inorganic nanoparticles as raman labels for tissue analysis. Nano Lett. 2007, 7, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Morrow, B.J.; Matijevic, E.; Goia, D.V. Preparation and stabilization of monodisperse colloidal gold by reduction with aminodextran. J. Colloid Interface Sci. 2009, 335, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Salsamendi, M.; Cormack, P.A.G.; Graham, D. Template-directed synthesis of uniformly-sized silver nanoparticles with high colloidal stability. New J. Chem. 2013, 37, 3591–3594. [Google Scholar] [CrossRef]
- Cormack, P.A.; Hernandez-Santana, A.; Prasath, R.A.; McKenzie, F.; Graham, D.; Smith, W.E. Multidentate macromolecules for functionalisation, passivation and labelling of metal nanoparticles. Chem. Commun. 2008, 22, 2517–2519. [Google Scholar] [CrossRef] [PubMed]
- Jehn, C.; Kustner, B.; Adam, P.; Marx, A.; Strobel, P.; Schmuck, C.; Schlücker, S. Water soluble SERS labels comprising a sam with dual spacers for controlled bioconjugation. Phys. Chem. Chem. Phys. 2009, 11, 7499–7504. [Google Scholar] [CrossRef] [PubMed]
- Küstner, B.; Gellner, M.; Schütz, M.; Schöppler, F.; Marx, A.; Ströbel, P.; Adam, P.; Schmuck, C.; Schlücker, S. SERS labels for red laser excitation: Silica-encapsulated SAMs on tunable gold/silver nanoshells. Angew. Chem. Int. Ed. 2009, 48, 1950–1953. [Google Scholar] [CrossRef] [PubMed]
- Schutz, M.; Kustner, B.; Bauer, M.; Schmuck, C.; Schlücker, S. Synthesis of glass-coated SERS nanoparticle probes via SAMs with terminal SiO2 precursors. Small 2010, 6, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Yeo, B.S.; Schmid, T.; Zhang, W.; Zenobi, R. A strategy to prevent signal losses, analyte decomposition, and fluctuating carbon contamination bands in surface-enhanced raman spectroscopy. Appl. Spectrosc. 2008, 62, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Fedoruk, M.; Meixner, M.; Carretero-Palacios, S.; Lohmuller, T.; Feldmann, J. Nanolithography by plasmonic heating and optical manipulation of gold nanoparticles. ACS Nano 2013, 7, 7648–7653. [Google Scholar] [CrossRef] [PubMed]
- Thakor, A.S.; Paulmurugan, R.; Kempen, P.; Zavaleta, C.; Sinclair, R.; Massoud, T.F.; Gambhir, S.S. Oxidative stress mediates the effects of raman-active gold nanoparticles in human cells. Small 2011, 7, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Ahn, Y.-H.; Maiti, K.K.; Dinish, U.S.; Fu, C.Y.; Thoniyot, P.; Olivo, M.; Chang, Y.-T. Combinatorial synthesis of a triphenylmethine library and their application in the development of surface enhanced raman scattering (SERS) probes. Chem. Commun. 2010, 46, 722–724. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.K.; Dinish, U.S.; Fu, C.Y.; Lee, J.-J.; Soh, K.-S.; Yun, S.-W.; Bhuvaneswari, R.; Olivo, M.; Chang, Y.-T. Development of biocompatible SERS nanotag with increased stability by chemisorption of reporter molecule for in vivo cancer detection. Biosens. Bioelectr. 2010, 26, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Sonawane, M.D.; Song, K.S.; Kim, T. Biomarker detection technologies and future directions. Analyst 2016, 141, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Garzon, R.; Marcucci, G.; Croce, C.M. Targeting micrornas in cancer: Rationale, strategies and challenges. Nat. Rev. Drug Discov. 2010, 9, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Price, C.; Chen, J. Micrornas in cancer biology and therapy: Current status and perspectives. Genes Dis. 2014, 1, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Gellner, M.; Kömpe, K.; Schlücker, S. Multiplexing with SERS labels using mixed SAMs of raman reporter molecules. Anal. Bioanal. Chem. 2009, 394, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Schlücker, S. Rational design and synthesis of SERS labels. Analyst 2013, 138, 2224–2238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, D.; Mallinder, B.J.; Smith, W.E. Detection and identification of labeled DNA by surface enhanced resonance raman scattering. Biopolymers 2000, 57, 85–91. [Google Scholar] [CrossRef]
- Faulds, K.; McKenzie, F.; Smith, W.E.; Graham, D. Quantitative simultaneous multianalyte detection of DNA by dual-wavelength surface-enhanced resonance raman scattering. Angew. Chem. Int. Ed. 2007, 46, 1829–1831. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.C.; Jin, R.; Mirkin, C.A. Nanoparticles with raman spectroscopic fingerprints for DNA and RNA detection. Science 2002, 297, 1536. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.K.; Samanta, A.; Vendrell, M.; Soh, K.S.; Olivo, M.; Chang, Y.T. Multiplex cancer cell detection by sers nanotags with cyanine and triphenylmethine raman reporters. Chem. Commun. 2011, 47, 3514–3516. [Google Scholar] [CrossRef] [PubMed]
- Dinish, U.S.; Balasundaram, G.; Chang, Y.T.; Olivo, M. Actively targeted in vivo multiplex detection of intrinsic cancer biomarkers using biocompatible SERS nanotags. Sci. Rep. 2014, 4, 4075. [Google Scholar] [CrossRef] [PubMed]
- Neng, J.; Harpster, M.H.; Wilson, W.C.; Johnson, P.A. Surface-enhanced raman scattering (SERS) detection of multiple viral antigens using magnetic capture of SERS-active nanoparticles. Biosens. Bioelectr. 2013, 41, 316–321. [Google Scholar] [CrossRef] [PubMed]
| Biological Antigen | Limit of Detection (LOD) | Reference |
|---|---|---|
| Alzheimer’s Tau Protein | <25 fM | [41] |
| Prostate-specific antigen (PSA) | 1 pg/mL (~30 fM) | [13,28] |
| Immunoglobulin (IgG) antigens | 300 pg/mL | [12,29,30,31] |
| Metanephrine | <10 µM | [42] |
| Mucin 4 (MUC4) | 33 ng/mL | [38,40] |
| Mycobacterium avium subsp. Paratuberculosis (MAP) | 1000 MAP/mL | [35] |
| Hepatitis B virus | 0.5 µg/mL | [34] |
| Feline calicivirus (FCV) | 106 FCV/mL | [32] |
| Carcinoembryonic antigen (CEA) | 10 pg/mL | [43] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolsky, J.; Kaur, S.; Hayashi, C.; Batra, S.K.; Krasnoslobodtsev, A.V. Surface-Enhanced Raman Scattering-Based Immunoassay Technologies for Detection of Disease Biomarkers. Biosensors 2017, 7, 7. https://doi.org/10.3390/bios7010007
Smolsky J, Kaur S, Hayashi C, Batra SK, Krasnoslobodtsev AV. Surface-Enhanced Raman Scattering-Based Immunoassay Technologies for Detection of Disease Biomarkers. Biosensors. 2017; 7(1):7. https://doi.org/10.3390/bios7010007
Chicago/Turabian StyleSmolsky, Joseph, Sukhwinder Kaur, Chihiro Hayashi, Surinder K. Batra, and Alexey V. Krasnoslobodtsev. 2017. "Surface-Enhanced Raman Scattering-Based Immunoassay Technologies for Detection of Disease Biomarkers" Biosensors 7, no. 1: 7. https://doi.org/10.3390/bios7010007







