Integrating Nanostructured Artificial Receptors with Whispering Gallery Mode Optical Microresonators via Inorganic Molecular Imprinting Techniques
Abstract
:1. Introduction
2. Materials and Methods
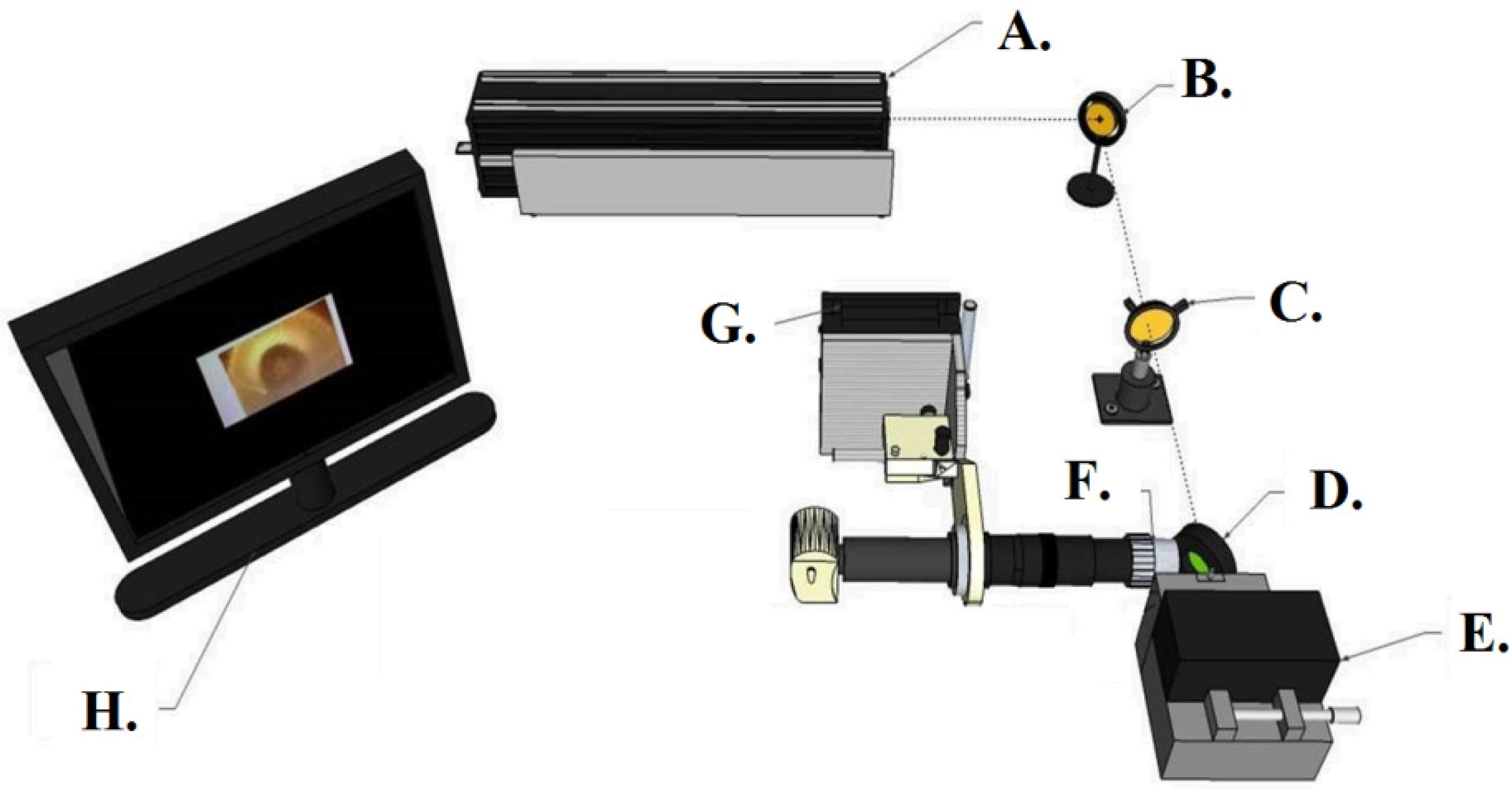
2.1. Preparation of Whispering Gallery Mode Optical Microresonators and Control Surfaces
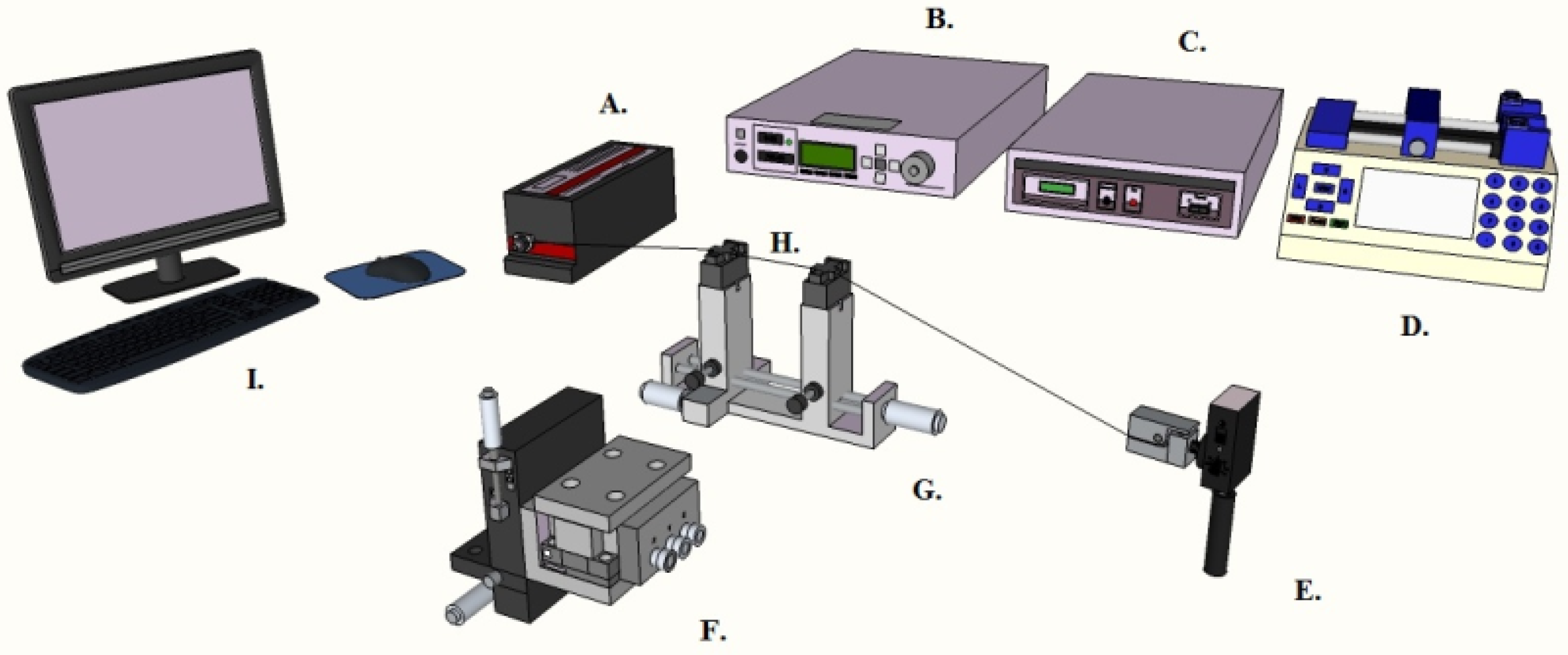
2.2. Characterization of the Optical Devices
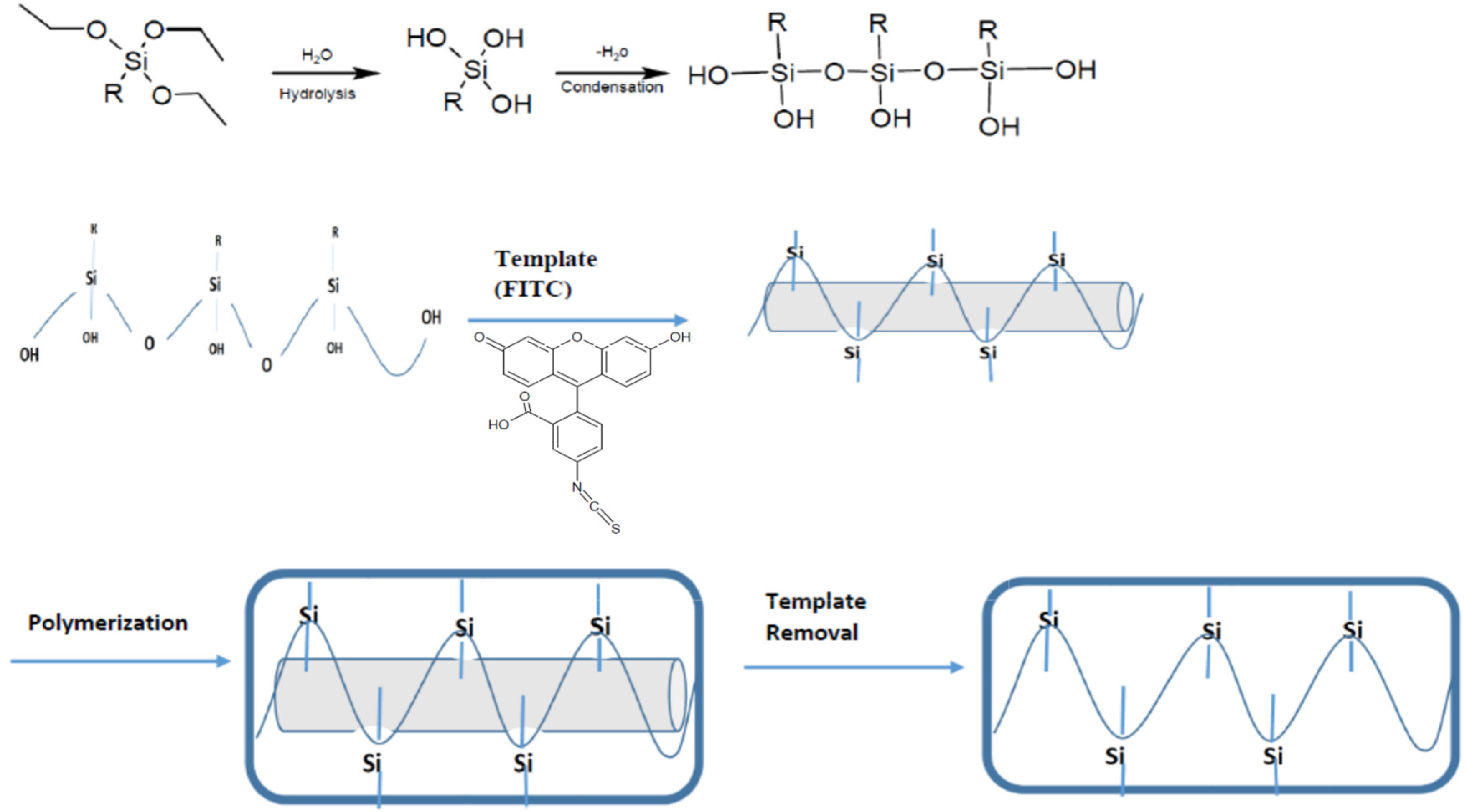
2.3. Synthesis of MIPs
2.4. Optimization of Microspheres
2.5. Extraction Procedure
2.6. Characterization of the Surface Coatings
3. Results
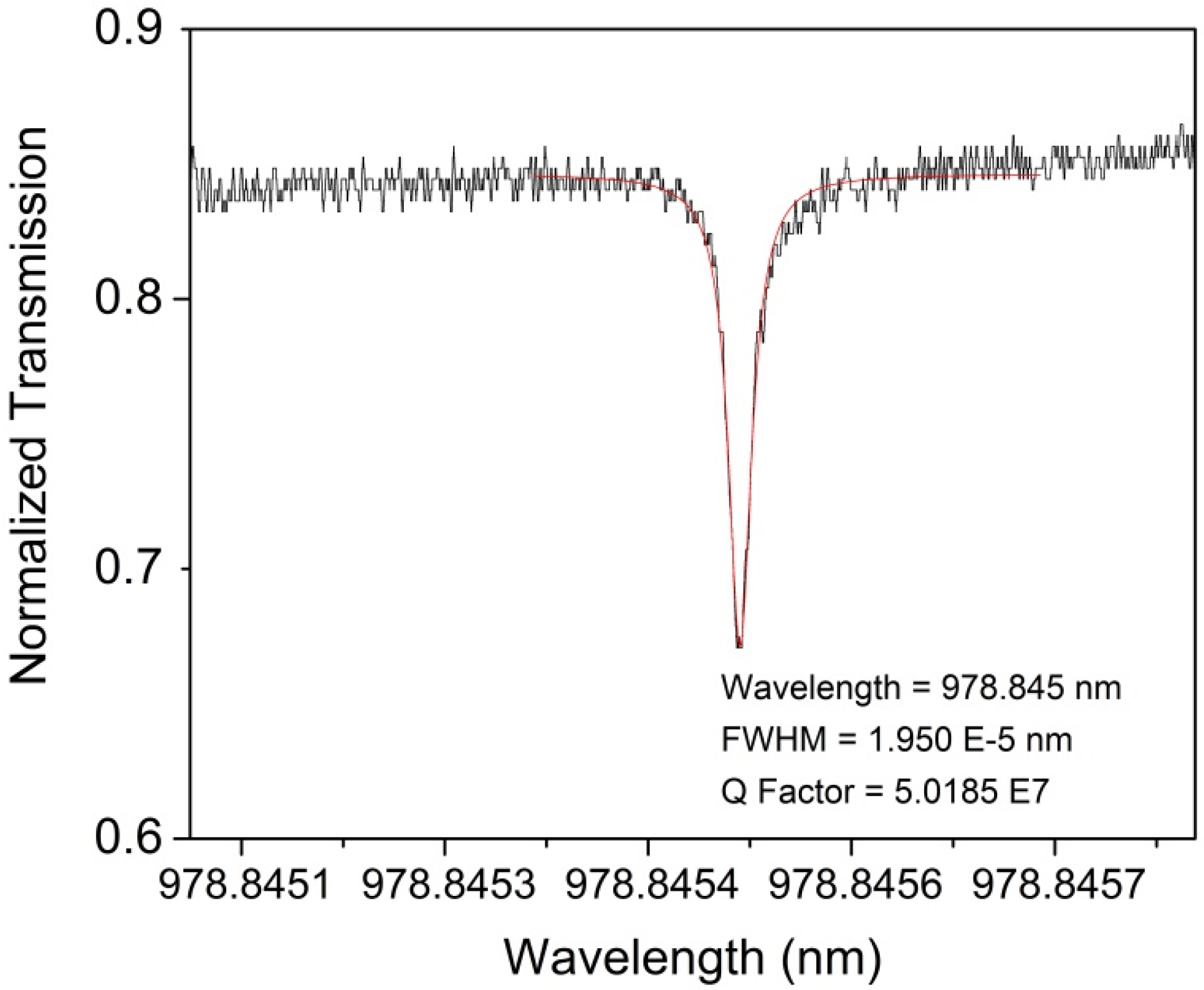
3.1. Characterization of the Uncoated Optical Devices
3.2. Optimization of Microsphere Coating
3.2.1. Coating Methods
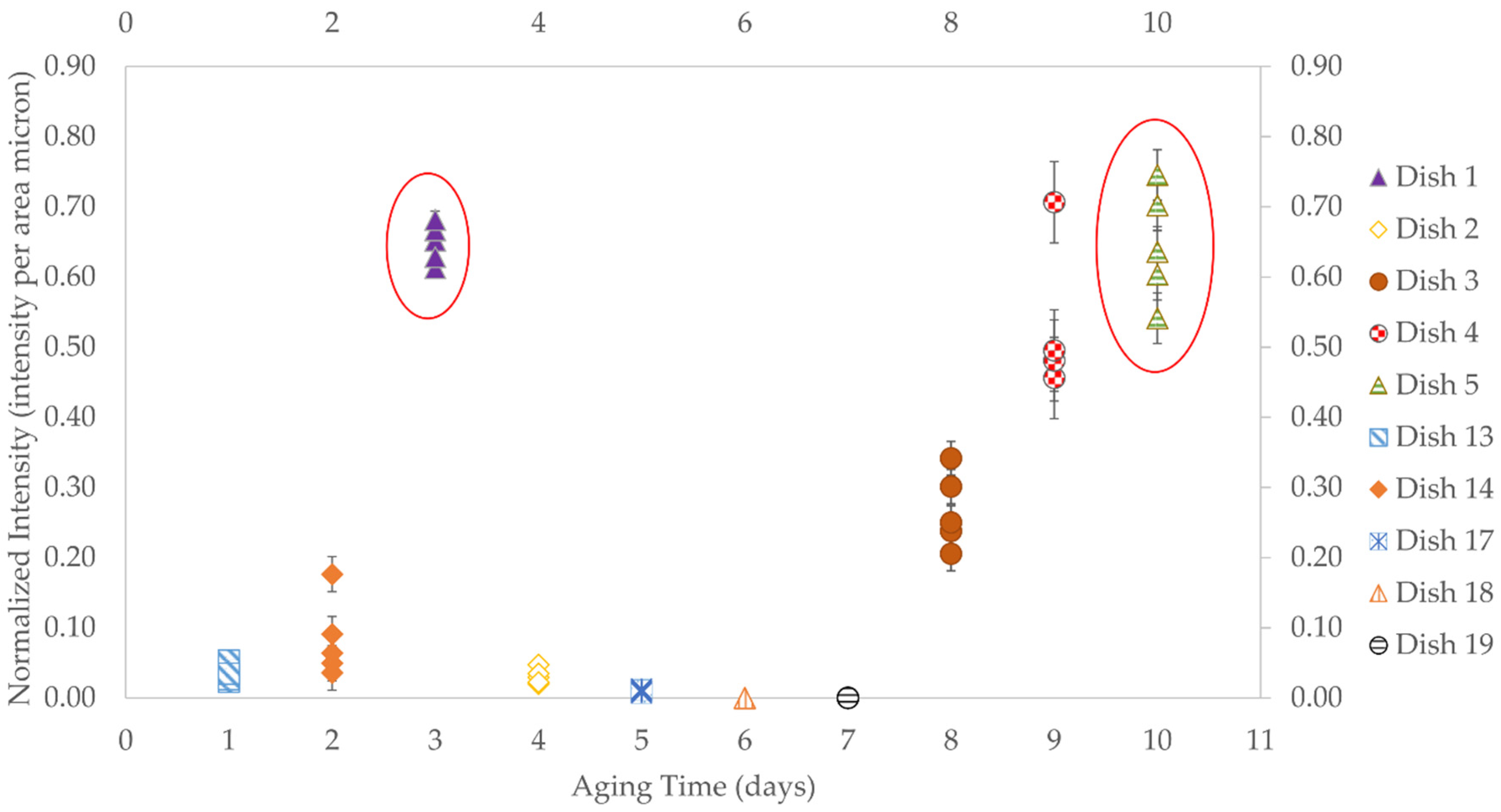
3.2.2. Aging Times
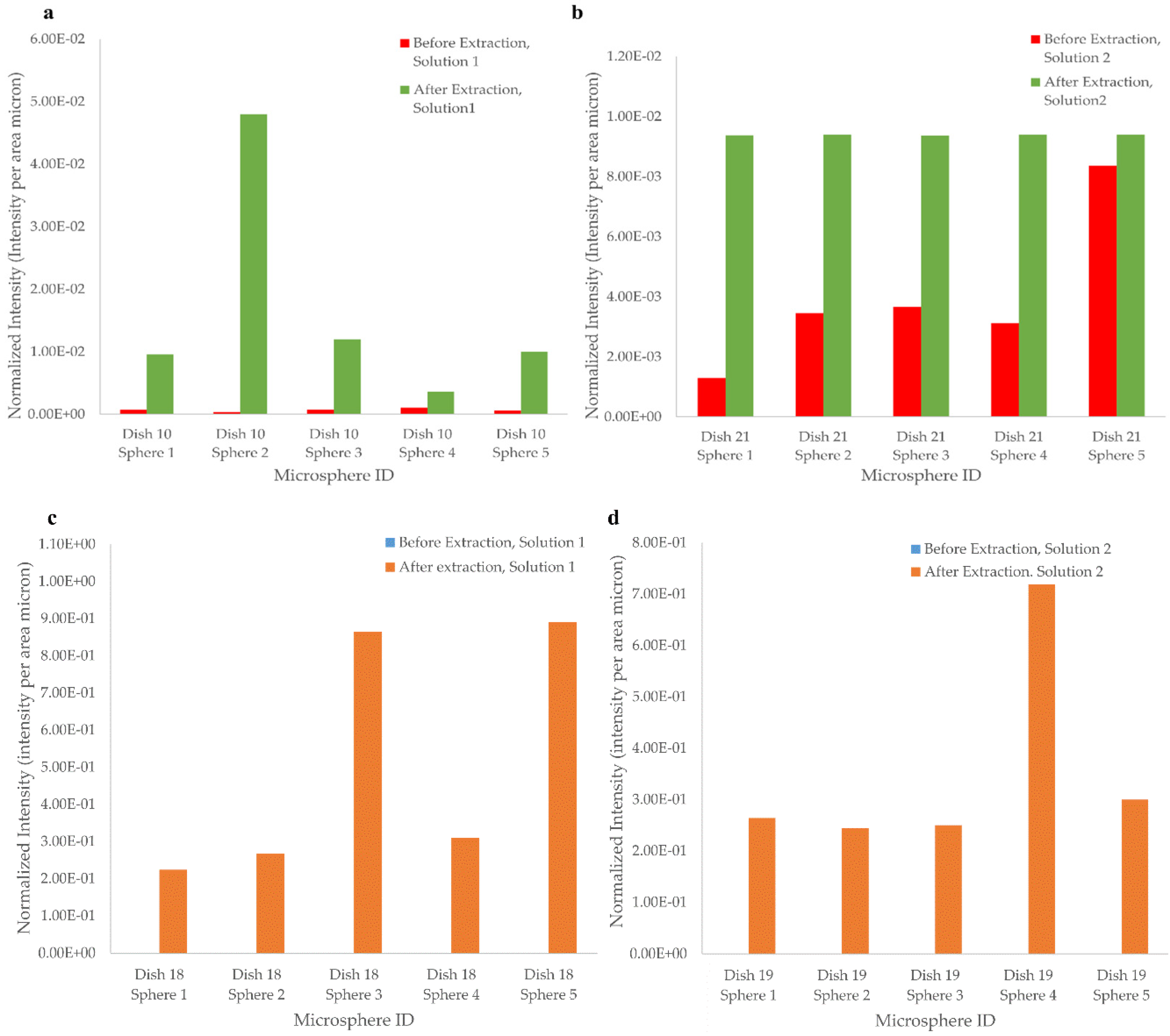
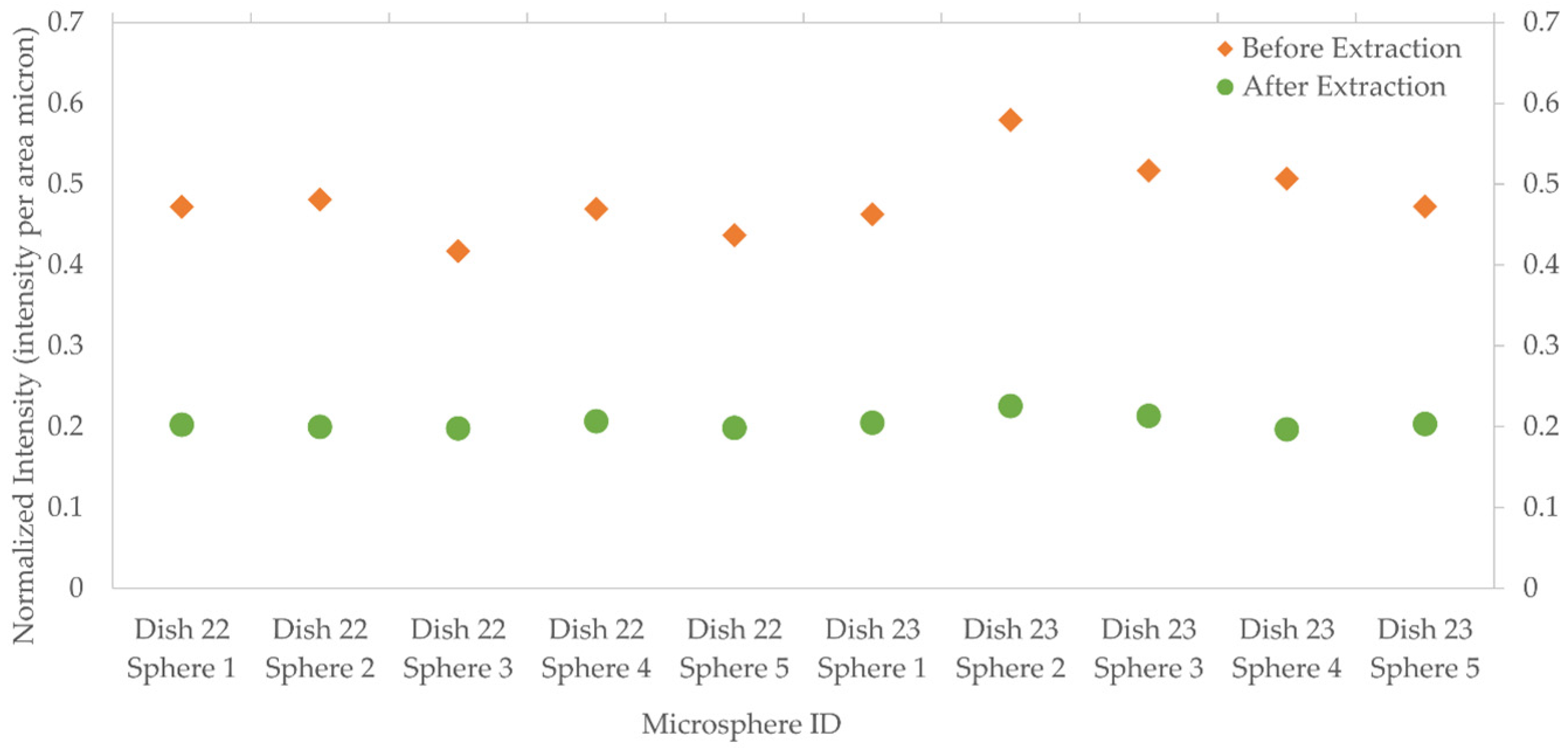
3.2.3. Template Removal Procedure
3.2.4. Q Factor Measurements
3.3. UV-VIS Analysis
3.4. Control Groups—Surface Quality of the Conformal Coatings
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MIP | Molecularly Imprinted Polymer |
| WGM | Whispering Gallery Mode |
| FITC | Fluorescein isothiocyanate |
| HCL | Hydrochloric Acid |
| DMSO | Dimethyl Sulfoxide |
References
- Barceló, D. Environmental protection agency and other methods for the determination of priority pesticides and their transformation products in water. J. Chromatogr. A 1993, 643, 117–143. [Google Scholar] [CrossRef]
- Simcox, N.J.; Fenske, R.A.; Wolz, S.A.; Lee, I.C.; Kalman, D.A. Pesticides in household dust and soil: Exposure pathways for children of agricultural families. Environ. Health Perspect. 1995, 103, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. Streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Dich, J.; Zahm, S.H.; Hanberg, A.; Adami, H.-O. Pesticied and cancer. Cancer Causes Control 1997, 8, 420–443. [Google Scholar] [CrossRef] [PubMed]
- Stackelberg, P.E.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Henderson, A.K.; Reissman, D.B. Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-water-treatment plant. Sci. Total Environ. 2004, 329, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; White, I.M.; Fan, X. An opto-fluidic ring resonator biosensor for the detection of organophosphorus pesticides. Sens. Actuators B Chem. 2008, 133, 105–112. [Google Scholar] [CrossRef]
- Frense, D.; Müller, A.; Beckmann, D. Detection of environmental pollutants using optical biosensor with immobilized algae cells. Sens. Actuators B Chem. 1998, 51, 256–260. [Google Scholar] [CrossRef]
- Mulchandani, A.; Chen, W.; Mulchandani, P.; Wang, J.; Rogers, K.R. Biosensors for direct determination of organophosphate pesticides. Biosens. Bioelectron. 2001, 16, 225–230. [Google Scholar] [CrossRef]
- Viveros, L.; Paliwal, S.; McCrae, D.; Wild, J.; Simonian, A. A fluorescence-based biosensor for the detection of organophosphate pesticides and chemical warfare agents. Sens. Actuators B Chem. 2006, 115, 150–157. [Google Scholar] [CrossRef]
- Fang, Y. Label-free cell-based assays with optical biosensors in drug discovery. Assay Drug Dev. Technol. 2006, 4, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, D.; Stadden, E.; Hashem, E.; Sparrow, I.J.G.; Emmerson, G.D. Multi-purpose optical biosensors for real-time detection of bacteria, viruses and toxins. Sens. Actuators B Chem. 2010, 149, 233–238. [Google Scholar] [CrossRef]
- Long, F.; Zhu, A.; Shi, H. Recent advances in optical biosensors for environmental monitoring and early warning. Sensors 2013, 13, 13928–13948. [Google Scholar] [CrossRef] [PubMed]
- Luppa, P.B.; Sokoll, L.J.; Chan, D.W. Immunosensors—Principles and applications to clinical chemistry. Clin. Chim. Act Int. J. Clin. Chem. 2001, 314, 1–26. [Google Scholar] [CrossRef]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Optical detection systems using immobilized aptamers. Biosens. Bioelectron. 2011, 26, 3725–3736. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Linman, M.J.; Cheng, Q. New trends in instrumental design for surface plasmon resonance-based biosensors. Biosens. Bioelectron. 2011, 26, 1815–1824. [Google Scholar] [CrossRef] [PubMed]
- Dudak, F.C.; Boyacı, İ.H. Rapid and label-free bacteria detection by surface plasmon resonance (SPR) biosensors. Biotechnol. J. 2009, 4, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Lee, H.; Chen, T.; Herchak, S.; Kim, J.-H.; Fraser, S.E.; Flagan, R.C.; Vahala, K. High sensitivity nanoparticle detection using optical microcavities. PNAS 2011, 108, 5976–5979. [Google Scholar] [CrossRef] [PubMed]
- Vahala, K.J. Optical microcavities. Nature 2003, 424, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Norwood, R.A. Label-free, single-object sensing with a microring resonator: FDTD simulation. Opt. Express 2013, 21, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, F.; Arnold, S. Whispering-gallery-mode biosensing: Label-free detection down to single molecules. Nat. Methods 2008, 5, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Hunt, H.K.; Armani, A.M. Label-free biological and chemical sensors. Nanoscale 2010, 2, 1544–1559. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, C.; Burke, C.S.; MacCraith, B.D. Optical chemical sensors. Chem. Rev. 2008, 108, 400–422. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, X. Characterization of sensing capability of optofluidic ring resonator biosensors. Appl. Phys. Lett. 2010, 97, 011105. [Google Scholar] [CrossRef]
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive optical biosensors for unlabeled targets: A review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Charlebois, M.; Paquet, A.; Verret, L.S.; Boissinot, K.; Boissinot, M.; Bergeron, M.G.; Allen, C.N. Toward automatic label-free whispering gallery modes biodetection with a quantum dot-coated microsphere population. Nanoscale Res. Lett. 2010, 5, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Himmelhaus, M.; Krishnamoorthy, S.; Francois, A. Optical sensors based on whispering gallery modes in fluorescent microbeads: Response to specific interactions. Sensors 2010, 10, 6257–6274. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, Y.; Sun, Y.; Reddy, K.; Fan, X. Analysis of single nanoparticle detection by using 3-dimensionally confined optofluidic ring resonators. Opt. Express 2010, 18, 25081–25088. [Google Scholar] [CrossRef] [PubMed]
- Swaim, J.D.; Knittel, J.; Bowen, W.P. Detection of nanoparticles with a frequency locked whispering gallery mode microresonator. Appl. Phys. Lett. 2013, 102, 183106. [Google Scholar] [CrossRef]
- Kim, K.W.; Song, J.; Kee, J.S.; Liu, Q.; Lo, G.-Q.; Park, M.K. Label-free biosensor based on an electrical tracing-assisted silicon microring resonator with a low-cost broadband source. Biosens. Bioelectron. 2013, 46, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Kirk, J.T.; Brault, N.D.; Baehr-Jones, T.; Hochberg, M.; Jiang, S.; Ratner, D.M. Zwitterionic polymer-modified silicon microring resonators for label-free biosensing in undiluted humanplasma. Biosens. Bioelectron. 2013, 42, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Knittel, J.; Chow, J.H.; Gray, M.B.; Taylor, M.A.; Bowen, W.P. Ultrasensitive real-time measurement of dissipation and dispersion in a whispering-gallery mode microresonator. Opt. Lett. 2013, 38, 1915–1917. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.; Khoshsima, M.; Teraoka, I.; Holler, S.; Vollmer, F. Shift of whispering-gallery modes in microspheres by protein adsorption. Opt. Lett. 2003, 28, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, F.; Arnold, S.; Braun, D.; Teraoka, I.; Libchaber, A. Multiplexed DNA quantification by spectroscopic shift of two microsphere cavities. Biophys. J. 2003, 85, 1974–1979. [Google Scholar] [CrossRef]
- Dantham, V.R.; Holler, S.; Barbre, C.; Keng, D.; Kolchenko, V.; Arnold, S. Label-free detection of single protein using a nanoplasmonic-photonic hybrid microcavity. Nano Lett. 2013, 13, 3347–3351. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Karube, I. Molecularly imprinted polymers for biosensor applications. TrAC Trends Anal. Chem. 1999, 18, 199–204. [Google Scholar] [CrossRef]
- Li, S.; Ge, Y.; Piletsky, S.A.; Lunec, J. Molecularly Imprinted Sensors: Overview and Applications; Elsevier: Amsterdam, The Netherlands, 2012; p. 390. [Google Scholar]
- Cheng, Z.; Wang, E.; Yang, X. Capacitive detection of glucose using molecularly imprinted polymers. Biosens. Bioelectron. 2001, 16, 179–185. [Google Scholar] [CrossRef]
- Mayes, A.; Whitcombe, M. Synthetic strategies for the generation of molecularly imprinted organic polymers. Adv. Drug Deliv. Rev. 2005, 57, 1742–1778. [Google Scholar] [CrossRef] [PubMed]
- Kryscio, D.R.; Peppas, N.A. Critical review and perspective of macromolecularly imprinted polymers. Acta Biomater. 2012, 8, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Koshland, D.E. The key–lock theory and the induced fit theory. Angew. Chem. Int. Ed. Engl. 1995, 33, 2375–2378. [Google Scholar] [CrossRef]
- Pauling, L. A theory of the structure and process of formation of antibodies. J. Am. Chem. Soc. 1940, 62, 2643–2657. [Google Scholar] [CrossRef]
- Takeuchi, T.; Haginaka, J. Separation and sensing based on molecular recognition using molecularly imprinted polymers. J. Chromatogr. B Biomed. Sci. Appl. 1999, 728, 1–20. [Google Scholar] [CrossRef]
- Owens, P.K.; Karlsson, L.; Lutz, E.S.M.; Andersson, L.I. Molecular imprinting for bio- and pharmaceutical analysis. TrAC Trends Anal. Chem. 1999, 18, 146–154. [Google Scholar] [CrossRef]
- Wei, S.; Mizaikoff, B. Recent advances on noncovalent molecular imprints for affinity separations. J. Sep. Sci. 2007, 30, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Vasapollo, G.; Sole, R.D.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly imprinted polymers: Present and future prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, M. Molecularly imprinted polymer films and membranes. In Molecularly Imprinted Materials; Yan, M., Ramström, O., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 455–490. [Google Scholar]
- Schneider, F.; Piletsky, S.; Piletska, E.; Guerreiro, A.; Ulbricht, M. Comparison of thin-layer and bulk mips synthesized by photoinitiated in situ crosslinking polymerization from the same reaction mixtures. J. Appl. Polym. Sci. 2005, 98, 362–372. [Google Scholar] [CrossRef]
- Poma, A.; Turner, A.P.F.; Piletsky, S.A. Advances in the manufacture of mip nanoparticles. Trends Biotechnol. 2010, 28, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ritt, M.; Sivaramakrishnan, S.; Sun, Y.; Fan, X. Optofluidic lasers with a single molecular layer of gain. Lab Chip 2014, 14, 4590–4595. [Google Scholar] [CrossRef] [PubMed]
- Wienhold, T.; Kraemmer, S.; Bacher, A.; Kalt, H.; Koos, C.; Koeber, S.; Mappes, T. Efficient free-space read-out of wgm lasers using circular micromirrors. Opt. Express 2015, 23, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.C.; Armendariz, K.P.; Dunn, R.C. Integration of microsphere resonators with bioassay fluidics for whispering gallery mode imaging. Analyst 2013, 138, 3189–3195. [Google Scholar] [CrossRef] [PubMed]
- Soteropulos, C.E.; Hunt, H.K. Attaching biological probes to silica optical biosensors using silane coupling agents. J. Vis. Exp. 2012. [Google Scholar] [CrossRef] [PubMed]
- Donskoi, A.V.; Dresvin, S.V.; Orlova, M.A.; Osovskii, B.B.; Khait, O.D.; Paushkin, E.V. Plasma polishing of surface of wares made of silicate glass of any composition. Glass Ceram. 1976, 33, 162–165. [Google Scholar] [CrossRef]
- Ljungberg, K.; Jansson, U.; Bengtsson, S.; Söderbärg, A. Modification of silicon surfaces with H2SO4:H2O2:HF and HNO3:HF for wafer bonding applications. J. Electrochem. Soc. 1996, 143, 1709–1714. [Google Scholar] [CrossRef]
- Chandekar, A.; Sengupta, S.K.; Whitten, J.E. Thermal stability of thiol and silane monolayers: A comparative study. Appl. Surf. Sci. 2010, 256, 2742–2749. [Google Scholar] [CrossRef]
- Wang, F.; Anderson, M.; Bernards, M.; Hunt, H. Peg functionalization of whispering gallery mode optical microresonator biosensors to minimize non-specific adsorption during targeted, label-free sensing. Sensors 2015, 15, 18040–18060. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Suter, J.D.; White, I.M.; Fan, X. Aptamer based microsphere biosensor for thrombin detection. Sensors 2006, 6, 785–795. [Google Scholar] [CrossRef]
- Stringer, R.C.; Gangopadhyay, S.; Grant, S.A. Detection of nitroaromatic explosives using a fluorescent-labeled imprinted polymer. Anal. Chem. 2010, 82, 4015–4019. [Google Scholar] [CrossRef] [PubMed]
- Stringer, R.C.; Gangopadhyay, S.; Grant, S.A. Comparison of molecular imprinted particles prepared using precipitation polymerization in water and chloroform for fluorescent detection of nitroaromatics. Anal. Chim. Acta 2011, 703, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Hunt, H.K.; Armani, A.M. Recycling microcavity optical biosensors. Opt. Lett. 2011, 36, 1092–1094. [Google Scholar] [CrossRef] [PubMed]
- Rahachou, A.I.; Zozoulenko, I.V. Effects of boundary roughness on a Q-factor of whispering-gallery-mode lasing microdisk cavities. J. Appl. Phys. 2003, 94, 7929–7933. [Google Scholar] [CrossRef]
- Freeman, L.M.; Li, S.; Dayani, Y.; Choi, H.-S.; Malmstadt, N.; Armani, A.M. Excitation of Cy5 in self-assembled lipid bilayers using optical microresonators. Appl. Phys. Lett. 2011, 98, 143703. [Google Scholar] [CrossRef] [PubMed]
- Hunt, H.K.; Soteropulos, C.; Armani, A.M. Bioconjugation strategies for microtoroidal optical resonators. Sensors 2010, 10, 9317–9336. [Google Scholar] [CrossRef] [PubMed]

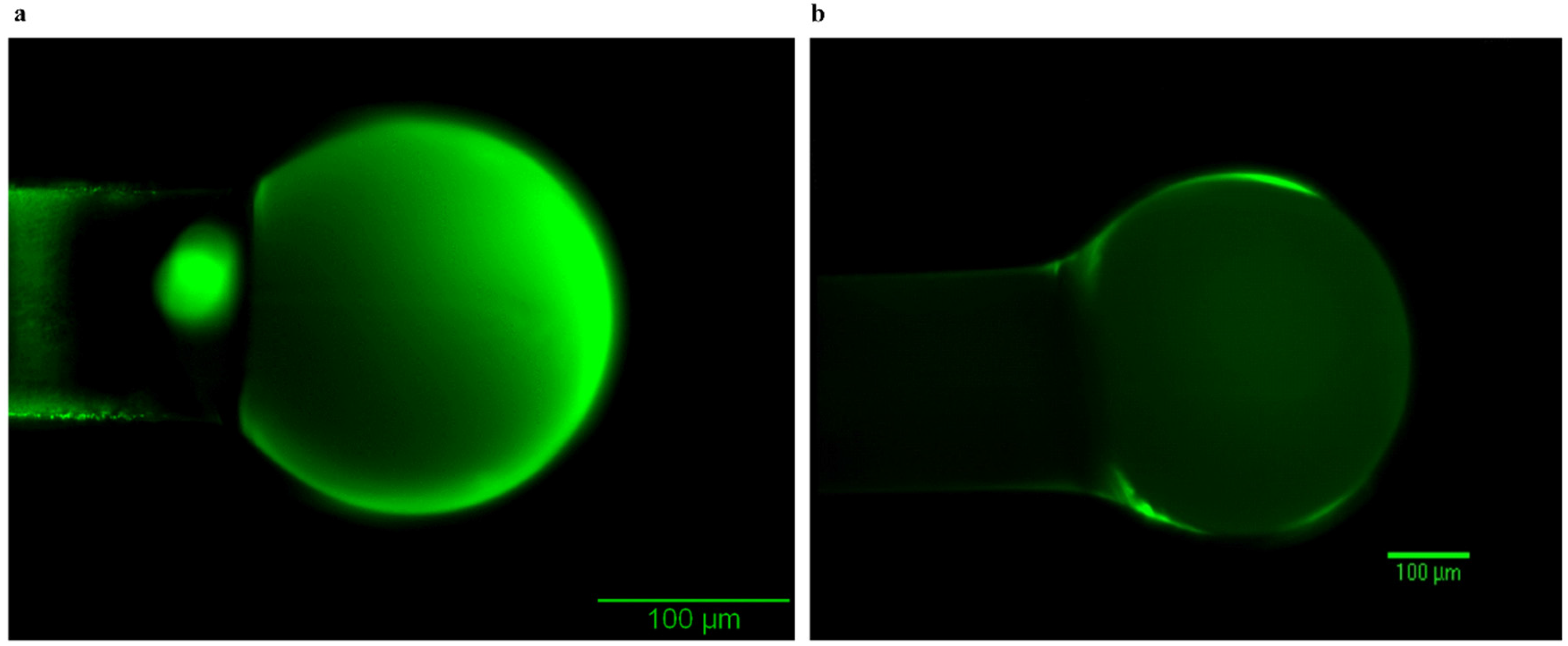
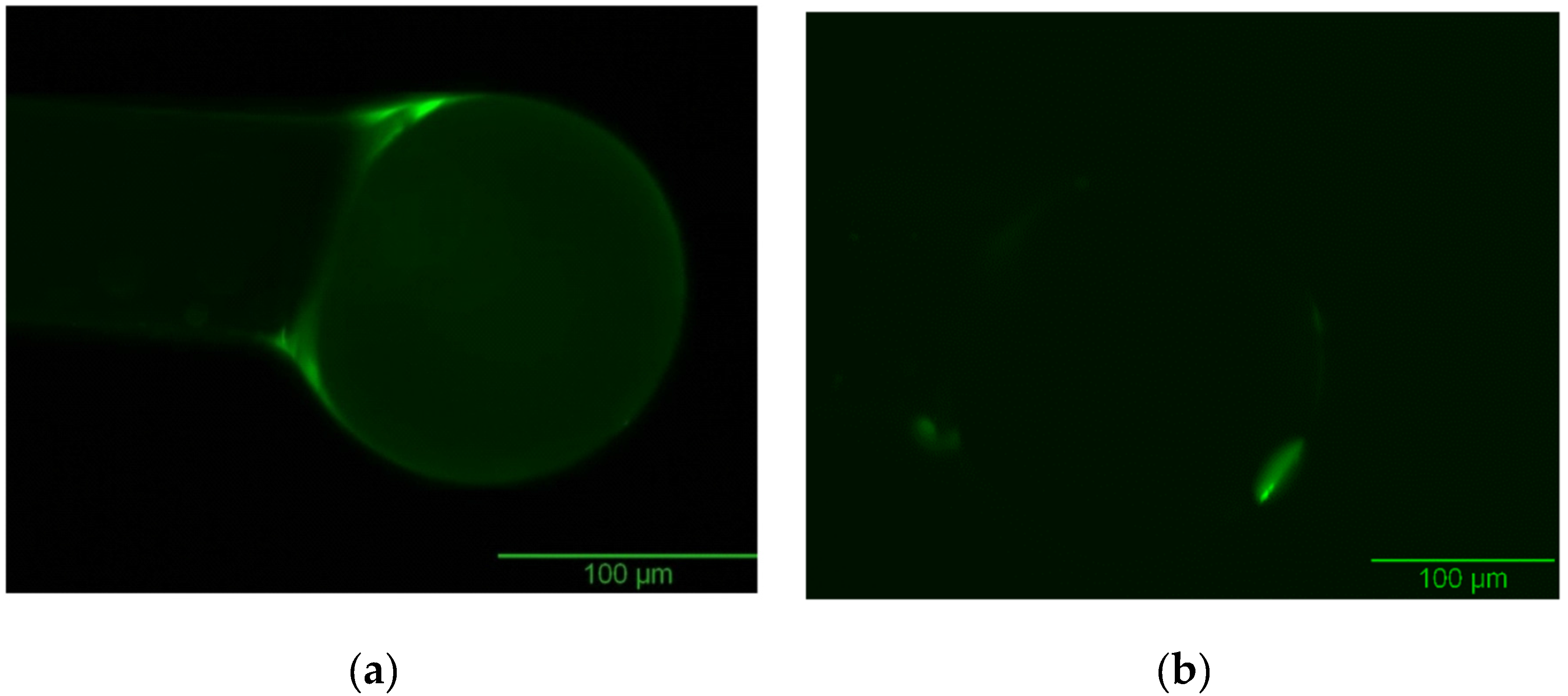
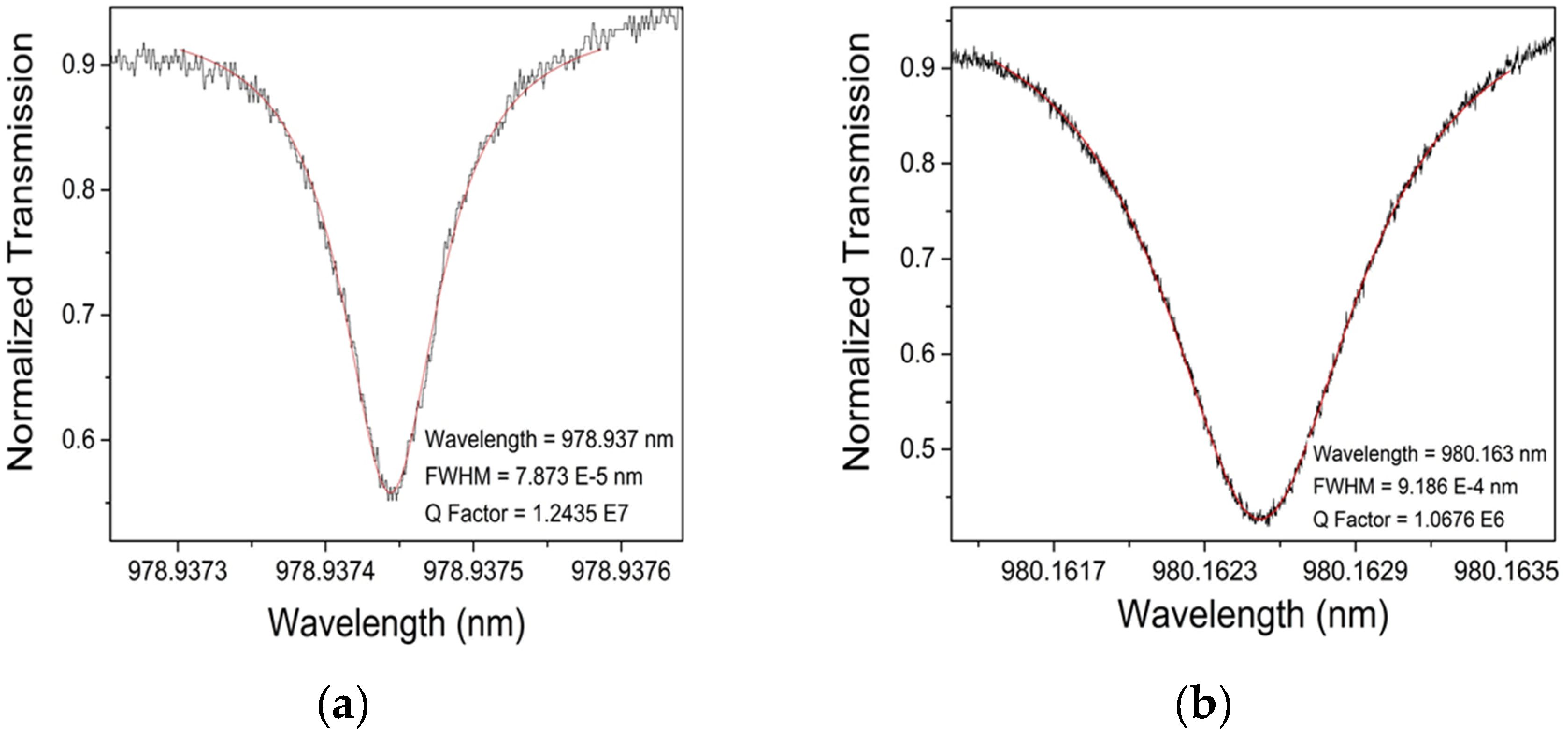
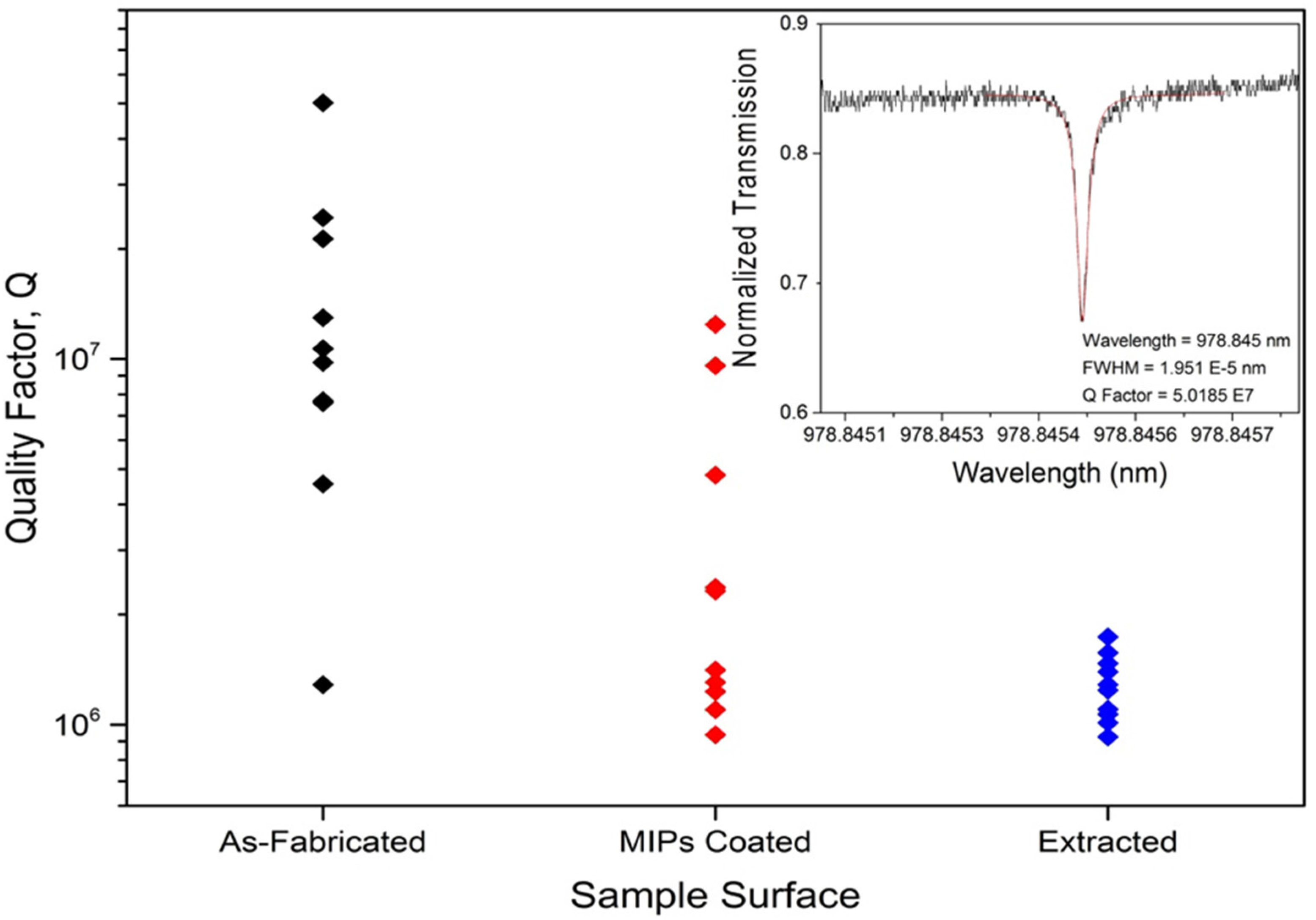
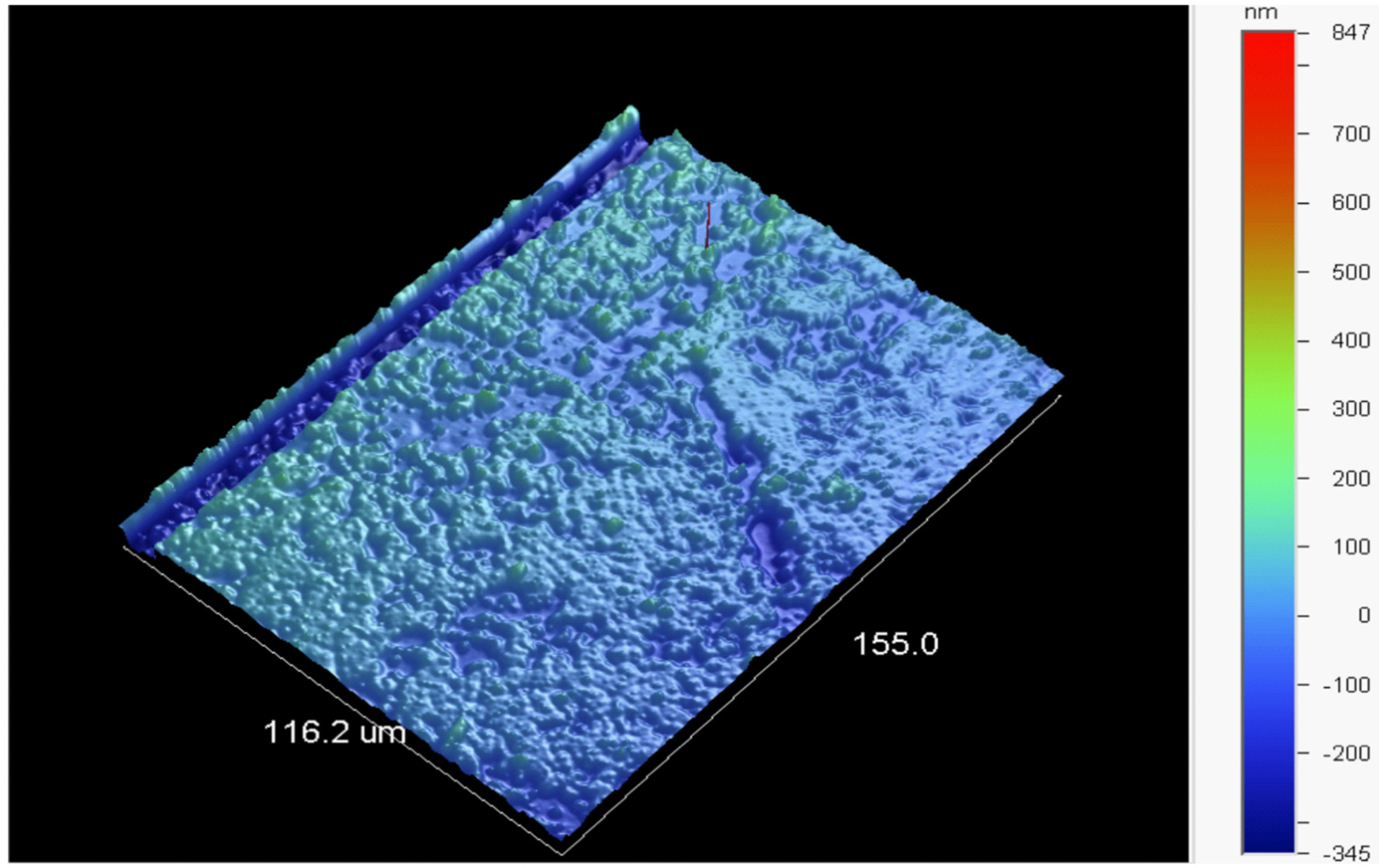
| Optimization Parameters | Tested Methods | |
|---|---|---|
| Coating | Automated dip coating | Manual dip coating |
| Aging Times | 1–3 days aging | 1–10 days aging |
| Extraction | Chemical extraction | Oxygen Plasma extraction |
| Extraction Solution | Chemicals Used | Extraction Stage | Extraction Time |
|---|---|---|---|
| Solution 1 | 4 mL Ehanol, 1 mL Chloroform, 0.5 mL Acetic Acid | Tilt-tray, Stationary | 24 h |
| Solution 2 | 8 mL Ethanol, 2 mL Acetonitrile, 1 mL Acetic Acid | Tilt-tray, Stationary | 24 h |
| Petri Dish ID | Sphere | Stage Condition | Extraction Solution |
|---|---|---|---|
| Dish 10 | 1–5 | Tilt-Tray | 1 |
| Dish 18 | 1–5 | Stationary | 2 |
| Dish 19 | 1–5 | Stationary | 2 |
| Dish 21 | 1–5 | Tilt-Tray | 1 |
| Microsphere ID | Coating Conditions | Extraction Conditions | |||||
|---|---|---|---|---|---|---|---|
| Petri Dish | Spheres | Coating Method | Aging Time | Extraction Method | Extraction Solution | Extraction Stage | Extraction Time |
| Dish 1 | 1–5 | Manual | 3 days | NA | NA | NA | NA |
| Dish 3 | 1–5 | Manual | 8 days | NA | NA | NA | NA |
| Dish 4 | 1–5 | Manual | 9 days | NA | NA | NA | NA |
| Dish 5 | 1–5 | Manual | 10 days | NA | NA | NA | NA |
| Dish 9 | 1–3 | Uncoated | NA | NA | NA | NA | NA |
| Dish 10 | 1–5 | Automated | 3 days | Chemical | Solution 1 | Tilt-Tray | 24 h |
| Dish 12 | 1–3 | Manual | 10 days | O2 Plasma | NA | NA | 2 h |
| Dish 13 | 1–5 | Automated | 1 days | NA | NA | NA | NA |
| Dish 14 | 1–5 | Automated | 1 days | NA | NA | NA | NA |
| Dish 17 | 1–5 | Manual | 5 days | NA | NA | NA | NA |
| Dish 18 | 1–5 | Manual | 6 days | Chemical | Solution 2 | Stationary | 24 h |
| Dish 19 | 1–5 | Manual | 7 days | Chemical | Solution 2 | Stationary | 24 h |
| Dish 20 | 1–5 | Manual | 3 days | NA | NA | NA | NA |
| Dish 21 | 1–5 | Manual | 3 days | Chemical | Solution 1 | Tilt-tray | 24 h |
| Dish 22 | 1–5 | Manual | 3 days | O2 Plasma | NA | NA | 15 min |
| Dish 23 | 1–5 | Manunal | 3 days | O2 Plasma | NA | NA | 30 min |
| Fluorescence Parameters | Normalized Intensity |
|---|---|
| Autofluorescence | 0.193 |
| 3 Day Aging | 0.648 |
| 10 Day Aging | 0.645 |
| Post Extraction | 0.031 |
| Reuptake | 0.332 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammond, G.D.; Vojta, A.L.; Grant, S.A.; Hunt, H.K. Integrating Nanostructured Artificial Receptors with Whispering Gallery Mode Optical Microresonators via Inorganic Molecular Imprinting Techniques. Biosensors 2016, 6, 26. https://doi.org/10.3390/bios6020026
Hammond GD, Vojta AL, Grant SA, Hunt HK. Integrating Nanostructured Artificial Receptors with Whispering Gallery Mode Optical Microresonators via Inorganic Molecular Imprinting Techniques. Biosensors. 2016; 6(2):26. https://doi.org/10.3390/bios6020026
Chicago/Turabian StyleHammond, G. Denise, Adam L. Vojta, Sheila A. Grant, and Heather K. Hunt. 2016. "Integrating Nanostructured Artificial Receptors with Whispering Gallery Mode Optical Microresonators via Inorganic Molecular Imprinting Techniques" Biosensors 6, no. 2: 26. https://doi.org/10.3390/bios6020026
APA StyleHammond, G. D., Vojta, A. L., Grant, S. A., & Hunt, H. K. (2016). Integrating Nanostructured Artificial Receptors with Whispering Gallery Mode Optical Microresonators via Inorganic Molecular Imprinting Techniques. Biosensors, 6(2), 26. https://doi.org/10.3390/bios6020026







