A Paper-Based Biomimetic Sensing Device for the Discrimination of Original and Fraudulent Cigarette Brands Using Mixtures of MoS2 Quantum Dots and Organic Dyes
Abstract
:1. Introduction
2. Experimental
2.1. Materials, Reagents, and Solutions
2.2. Instruments and Software
2.3. Synthesis of MoS2 QDs
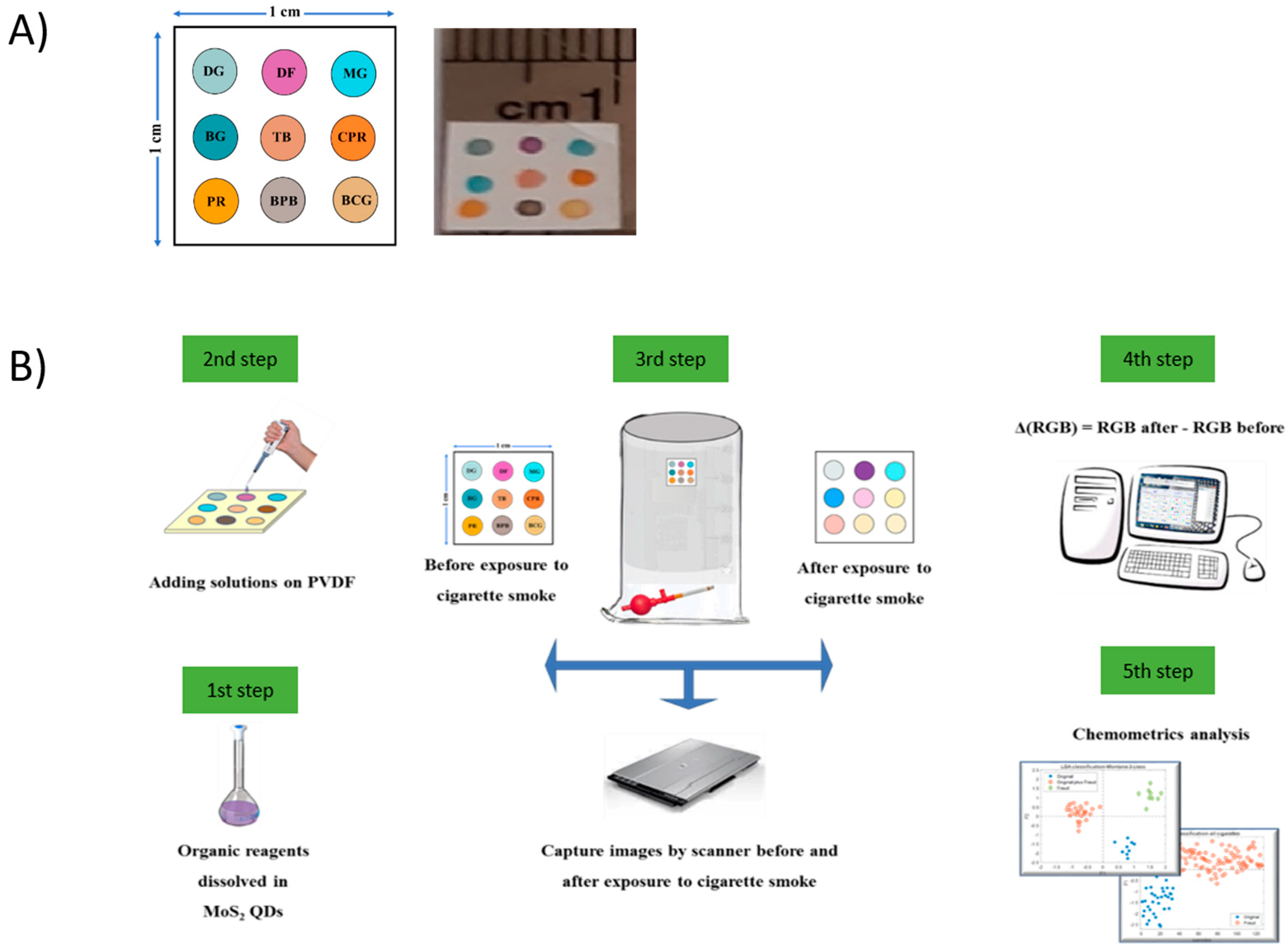
2.4. Fabrication of MoS2 QD Sensor Array
2.5. Experimental Protocol for Study of Cigarette VOCs
2.6. Cigarette Sample Preparation
2.7. Conditions of Data Acquisition for Side-Stream Cigarette Smoke
2.8. Data Collection and Analysis
3. Results and Discussion
3.1. Characterization of the Synthesized QDs
3.2. Humidity Tolerance and Durability of the Device
3.3. Device Response to Cigarette VOCs
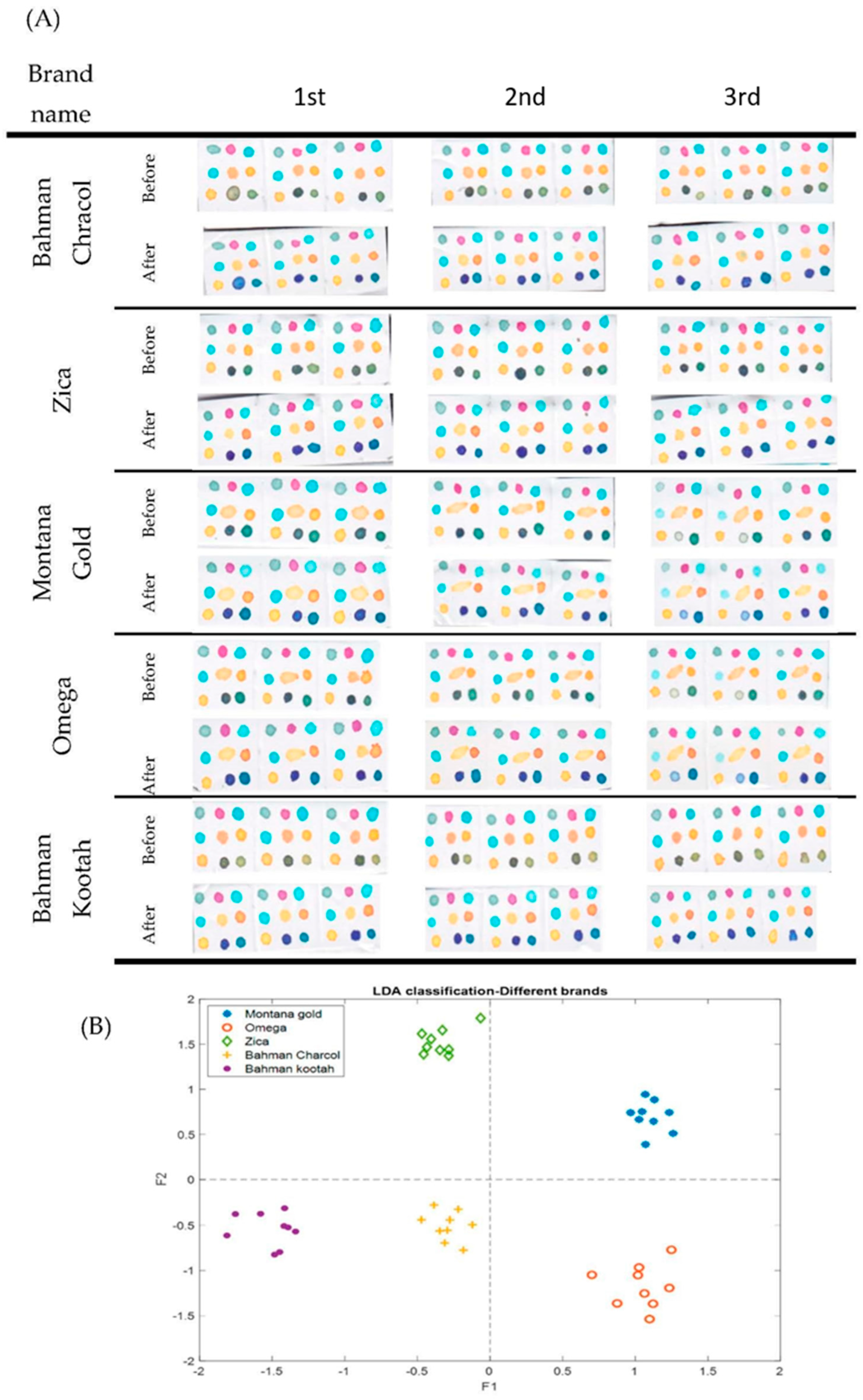
3.4. Classification of the Cigarette Brands
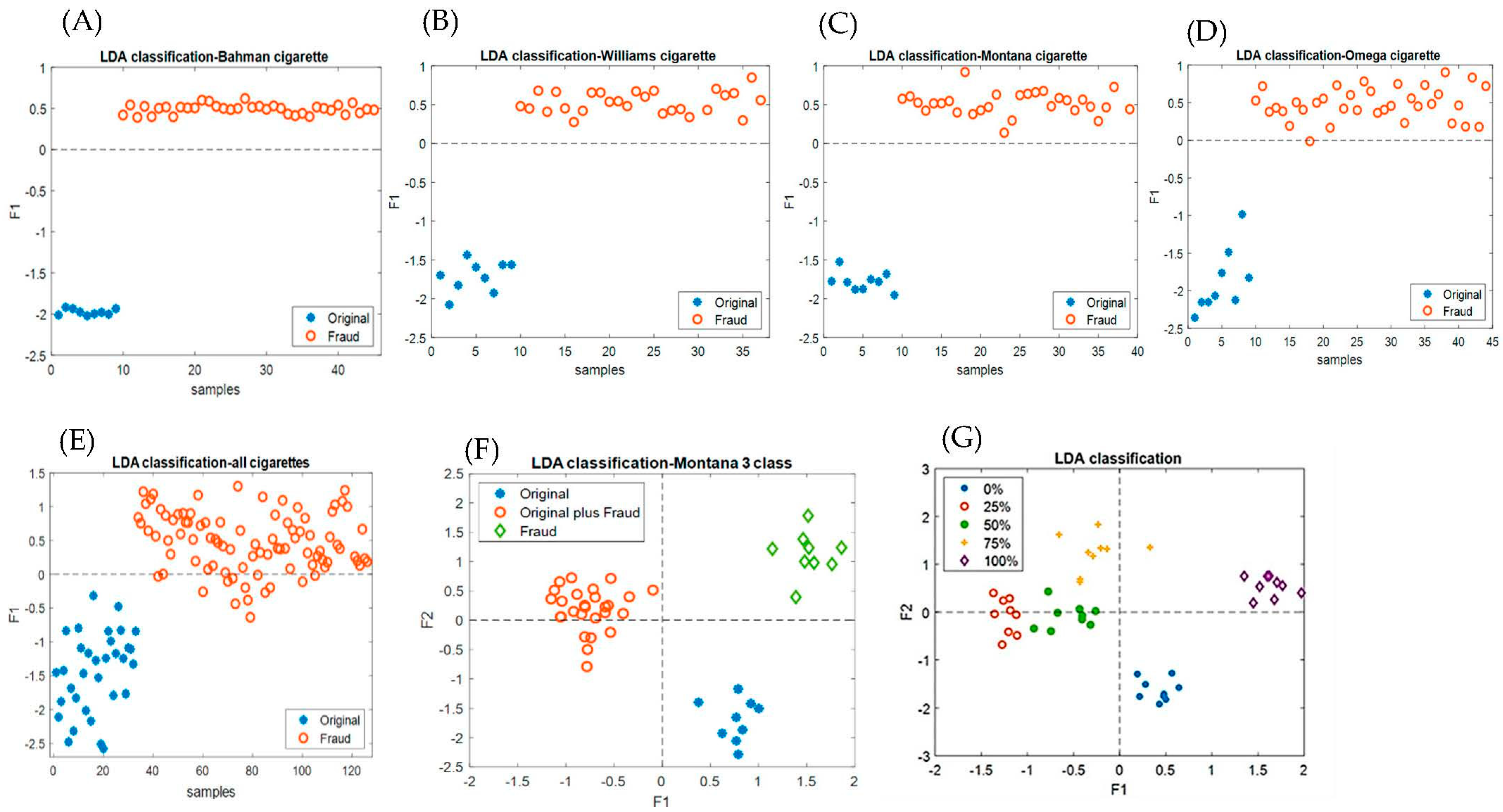
3.5. Discrimination of the Original and Fraudulent Cigarette Brands
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Staerz, A.; Roeck, F.; Weimar, U.; Barsan, N. Electronic Nose: Current Status and Future Trends. Surf. Interface Sci. Vol. 9 Appl. Surf. Sci. I 2020, 9, 335–379. [Google Scholar]
- Mittal, D.; Ali, A.; Md, S.; Baboota, S.; Sahni, J.K.; Ali, J. Insights into direct nose to brain delivery: Current status and future perspective. Drug Deliv. 2014, 21, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Lekha, S.; Suchetha, M. Recent advancements and future prospects on e-nose sensors technology and machine learning approaches for non-invasive diabetes diagnosis: A review. IEEE Rev. Biomed. Eng. 2020, 14, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.-W.; Tang, K.-T. Towards a chemiresistive sensor-integrated electronic nose: A review. Sensors 2013, 13, 14214–14247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Gu, H.; Yao, L.; Teye, E.; Wen, Y. Probing the reactions of colorimetric sensor array and volatile organic compounds using time-dependent density-functional theory. J. Comput. Theor. Nanosci. 2014, 11, 2194–2198. [Google Scholar] [CrossRef]
- Gu, H.-Y.; Sun, Y.-H.; Dai, H.; Han, F.-K.; Huang, X.-Y. The Sensor Construct of Colorimetric Sensor Array for Rapid Evaluation of Fish Freshness. Adv. J. Food Sci. Technol. 2015, 7, 631–637. [Google Scholar] [CrossRef]
- Kung, C.-T.; Hou, C.-Y.; Wang, Y.-N.; Fu, L.-M. Microfluidic paper-based analytical devices for environmental analysis of soil, air, ecology and river water. Sens. Actuators B Chem. 2019, 301, 126855. [Google Scholar] [CrossRef]
- Kawamura, K.; Miyazawa, K.; Kent, L.J.A. The past, present and future in tube-and paper-based colorimetric gas detectors. AppliedChem 2021, 1, 14–40. [Google Scholar] [CrossRef]
- Bordbar, M.M.; Tashkhourian, J.; Hemmateenejad, B. Structural elucidation and ultrasensitive analyses of volatile organic compounds by paper-based nano-optoelectronic noses. ACS Sens. 2019, 4, 1442–1451. [Google Scholar] [CrossRef]
- Bordbar, M.M.; Tashkhourian, J.; Tavassoli, A.; Bahramali, E.; Hemmateenejad, B. Ultrafast detection of infectious bacteria using optoelectronic nose based on metallic nanoparticles. Sens. Actuators B Chem. 2020, 319, 128262. [Google Scholar] [CrossRef]
- Bordbar, M.M.; Barzegar, H.; Tashkhourian, J.; Bordbar, M.; Hemmateenejad, B. A non-invasive tool for early detection of acute leukemia in children using a paper-based optoelectronic nose based on an array of metallic nanoparticles. Anal. Chim. Acta 2021, 1141, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Chaharlangi, M.; Tashkhourian, J.; Bordbar, M.M.; Brendel, R.; Weller, P.; Hemmateenejad, B. A paper-based colorimetric sensor array for discrimination of monofloral European honeys based on gold nanoparticles and chemometrics data analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119076. [Google Scholar] [CrossRef] [PubMed]
- Mostafapour, S.; Gharaghani, F.M.; Hemmateenejad, B. Converting electronic nose into opto-electronic nose by mixing MoS2 quantum dots with organic reagents: Application to recognition of aldehydes and ketones and determination of formaldehyde in milk. Anal. Chim. Acta 2021, 1170, 338654. [Google Scholar] [CrossRef] [PubMed]
- Bordbar, M.M.; Tashkhourian, J.; Hemmateenejad, B. Based Optical Nose Made with Bimetallic Nanoparticles for Monitoring Ignitable Liquids in Gasoline. ACS Appl. Mater. Interfaces 2022, 14, 8333–8342. [Google Scholar] [CrossRef]
- Pesaran, S.; Shojaeifard, Z.; Tashkhourian, J.; Hemmateenejad, B. A miniaturized all-in-one optical nose based on 3D printing technology and patterned paper substrate for discrimination of volatile organic compounds. Sens. Actuators B Chem. 2023, 375, 132889. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Ahmed, S.R.; Sherazee, M.; Margel, S.; Gedanken, A.; Srinivasan, S.; Rajabzadeh, A.R. Carbon Dot Biopolymer-Based Flexible Functional Films for Antioxidant and Food Monitoring Applications. ACS Appl. Polym. Mater. 2022, 4, 9323–9340. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Zhang, Y.; Wang, Y.; Wang, M.; Li, Z.; Wang, G.; Su, X. A pH-responsive fluorometric and colorimetric system based on silicon quantum dots and 4-nitrophenol for urease activity detection. Talanta 2022, 237, 122956. [Google Scholar] [CrossRef]
- Alossaimi, M.A.; Elmansi, H.; Alajaji, M.; Altharawi, A.; Altamimi, A.S.A.; Magdy, G. A novel quantum dots-based fluorescent sensor for determination of the anticancer dacomitinib: Application to dosage forms. Molecules 2023, 28, 2351. [Google Scholar] [CrossRef]
- Singh, A.K.; Sri, S.; Garimella, L.B.V.S.; Dhiman, T.K.; Sen, S.; Solanki, P.R. Graphene Quantum Dot-Based Optical Sensing Platform for Aflatoxin B1 Detection via the Resonance Energy Transfer Phenomenon. ACS Appl. Bio Mater. 2022, 5, 1179–1186. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Wu, Y.; Yu, X. Dual recognition strategy for selective fluorescent detection of dopamine and antioxidants based on graphite carbon nitride in human blood serum. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 265, 120385. [Google Scholar] [CrossRef]
- Xiong, J.; Zhang, H.; Qin, L.; Zhang, S.; Cao, J.; Jiang, H. Magnetic Fluorescent Quantum Dots Nanocomposites in Food Contaminants Analysis: Current Challenges and Opportunities. Int. J. Mol. Sci. 2022, 23, 4088. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Ganguly, S.; Margel, S.; Gedanken, A. Tailor made magnetic nanolights: Fabrication to cancer theranostics applications. Nanoscale Adv. 2021, 3, 6762–6796. [Google Scholar] [CrossRef]
- Geng, X.; Liu, D.; Hewa-Rahinduwage, C.C.; Brock, S.L.; Luo, L. Electrochemical Gelation of Metal Chalcogenide Quantum Dots: Applications in Gas Sensing and Photocatalysis. Acc. Chem. Res. 2023, 56, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Dashtian, K.; Shahbazi, N.; Amourizi, F.; Saboorizadeh, B.; Mousavi, A.; Astaraei, S.S.; Zare-Dorabei, R. Metal chalcogenides for sensing applications. In Fundamentals of Sensor Technology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 551–589. [Google Scholar]
- Galstyan, V. Quantum dots: Perspectives in next-generation chemical gas sensors—A review. Anal. Chim. Acta 2021, 1152, 238192. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Jang, M.; Suslick, K.S. Preoxidation for Colorimetric Sensor Array Detection of VOCs. J. Am. Chem. Soc. 2011, 133, 16786–16789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hariharan, S.; Karthikeyan, B. Optical and surface band bending mediated fluorescence sensing properties of MoS2 quantum dots. Rsc Adv. 2016, 6, 101770–101777. [Google Scholar] [CrossRef]
- Lee, K.; Gatensby, R.; McEvoy, N.; Hallam, T.; Duesberg, G.S. High-performance sensors based on molybdenum disulfide thin films. Adv. Mater. 2013, 25, 6699–6702. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.-W.; Dhenadhayalan, N.; Lee, H.-L.; Lin, Y.-T.; Lin, K.-C.; Chang, A. Fluorescence turn-on chemosensors based on surface-functionalized MoS2 quantum dots. Sens. Actuators B Chem. 2019, 281, 659–669. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, X.; Huo, D.; Zhao, Y.; Geng, X.; Fa, H.; Luo, X.; Yang, M.; Hou, C. Fast recognition of trace volatile compounds with a nanoporous dyes-based colorimetric sensor array. Talanta 2019, 192, 407–417. [Google Scholar] [CrossRef]
- Kim, J.-S.; Yoo, H.-W.; Choi, H.O.; Jung, H.-T. Tunable volatile organic compounds sensor by using thiolated ligand conjugation on MoS2. Nano Lett. 2014, 14, 5941–5947. [Google Scholar] [CrossRef]
- Pasupathi, P.; Bakthavathsalam, G.; Rao, Y.Y.; Farook, J. Cigarette smoking—Effect of metabolic health risk: A review. Diabetes Metab. Syndr. Clin. Res. Rev. 2009, 3, 120–127. [Google Scholar] [CrossRef]
- Harrison, A.; Ramo, D.; Hall, S.M.; Estrada-Gonzalez, V.; Tolou-Shams, M. Cigarette smoking, mental health, and other substance use among court-involved youth. Subst. Use Misuse 2020, 55, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.R.; da Silva, J.R.P.; Smith, G. The effect of tobacco ingredients on smoke chemistry. Part I: Flavourings and additives. Food Chem. Toxicol. 2004, 42, 3–37. [Google Scholar] [CrossRef] [PubMed]
- Mendel, J.R.; Baig, S.A.; Hall, M.G.; Jeong, M.; Byron, M.J.; Morgan, J.C.; Noar, S.M.; Ribisl, K.M.; Brewer, N.T. Brand switching and toxic chemicals in cigarette smoke: A national study. PLoS ONE 2018, 13, e0189928. [Google Scholar] [CrossRef] [Green Version]
- Esteves, C.H.A.; Iglesias, B.A.; Ogawa, T.; Araki, K.; Hoehne, L.; Gruber, J. Identification of tobacco types and cigarette brands using an electronic nose based on conductive polymer/porphyrin composite sensors. ACS Omega 2018, 3, 6476–6482. [Google Scholar] [CrossRef]
- Luo, D.; Hosseini, H.; Stewart, J.R. Application of ANN with extracted parameters from an electronic nose in cigarette brand identification. Sens. Actuators B Chem. 2004, 99, 253–257. [Google Scholar] [CrossRef]
- Du, N.; Wu, Q.; Chen, L.; Zhang, G.; Liu, X. Fluorescent carbon nanodots-based artificial tongue for determining and discriminating cigarettes. J. Hazard. Mater. 2020, 384, 121118. [Google Scholar] [CrossRef]
- Brudzewski, K.; Osowski, S.; Golembiecka, A. Differential electronic nose and support vector machine for fast recognition of tobacco. Expert Syst. Appl. 2012, 39, 9886–9891. [Google Scholar] [CrossRef]
- Ködderitzsch, P.; Bischoff, R.; Veitenhansl, P.; Lorenz, W.; Bischoff, G. Sensor array based measurement technique for fast-responding cigarette smoke analysis. Sens. Actuators B Chem. 2005, 107, 479–489. [Google Scholar] [CrossRef]
- Brudzewski, K.; Osowski, S.; Ulaczyk, J. Differential electronic nose of two chemo sensor arrays for odor discrimination. Sens. Actuators B Chem. 2010, 145, 246–249. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gharaghani, F.M.; Mostafapour, S.; Hemmateenejad, B. A Paper-Based Biomimetic Sensing Device for the Discrimination of Original and Fraudulent Cigarette Brands Using Mixtures of MoS2 Quantum Dots and Organic Dyes. Biosensors 2023, 13, 705. https://doi.org/10.3390/bios13070705
Gharaghani FM, Mostafapour S, Hemmateenejad B. A Paper-Based Biomimetic Sensing Device for the Discrimination of Original and Fraudulent Cigarette Brands Using Mixtures of MoS2 Quantum Dots and Organic Dyes. Biosensors. 2023; 13(7):705. https://doi.org/10.3390/bios13070705
Chicago/Turabian StyleGharaghani, Fereshte Mohamadi, Sara Mostafapour, and Bahram Hemmateenejad. 2023. "A Paper-Based Biomimetic Sensing Device for the Discrimination of Original and Fraudulent Cigarette Brands Using Mixtures of MoS2 Quantum Dots and Organic Dyes" Biosensors 13, no. 7: 705. https://doi.org/10.3390/bios13070705




