Antibody Profiling: Kinetics with Native Biomarkers for Diagnostic Assay and Drug Developments
Abstract
:1. Introduction
2. Material and Methods
2.1. SPR Instruments and Software
2.2. Consumables, Sensor Chips, Reagents and Buffers
2.3. Antigens
2.4. Antibodies
2.5. Functionalizing the Sensor
2.6. Interaction Analysis via SPR
2.7. Blocking
2.8. Comparing and Ranking the Epitope Accessibility of Antibody Pairs
2.9. Evaluating the SPR Data
3. Theory and Calculations
3.1. Sandwich-Assay Setup
- (1)
- Capturing
- (2)
- Target-Enrichment
- (3)
- Binding-kinetics of the 2nd binder
3.2. Target-Enrichment Step
3.3. Simulating the Targets’ Enrichment Time
4. Results and Discussion
4.1. In Silico-Determined Assay Settings Successfully Proven In Vitro
4.2. Proved Technical Reproducibility of Antibody Kinetics with Native GDF15 from Donors of Different Sources
4.3. Small Study Reveals Comparable Kinetic Constants for Native GDF15 from Different Sources
4.4. Kinetic Constants for Antibodies Binding to Native Neurofilament Light Chain from Patients’ Cerebrospinal Fluid (CSF)
4.5. Empirical Relation for Offered Amount of Native NFL and Its Surface Density
4.6. Identifying Antibodies Binding to Native NFL from CSF
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahmuda, A.; Bande, F.; Abdulhaleem, N.; Al-Zihiry, K.J.K.; Majid, R.A.; Hamat, R.A.; Abdullah, W.O.; Zasmy, N. Monoclonal antibodies in immunodiagnostic assays: A review of recent applications, Sokoto. J. Vet. Sci. 2017, 15, 1–10. [Google Scholar] [CrossRef]
- Fesseha, H. Monoclonal Antibody and its Diagnostic Application—Review. Biomed. J. Sci. Tech. Res. 2020, 30, 23645–23651. [Google Scholar] [CrossRef]
- Siddiqui, M.Z. Monoclonal Antibodies as Diagnostics; an Appraisal. Indian J. Pharm. Sci. 2010, 72, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Shakya, R.; Nguyen, T.H.; Waterhouse, N.; Khanna, R. Immune contexture analysis in immuno-oncology: Applications and challenges of multiplex fluorescent immunohistochemistry. Clin. Transl. Immunol. 2020, 9, e1183. [Google Scholar] [CrossRef] [PubMed]
- Schindler, S.E.; Gray, J.D.; Gordon, B.A.; Xiong, C.; Batrla-Utermann, R.; Quan, M.; Wahl, S.; Benzinger, T.L.S.; Holtzman, D.M.; Morris, J.C.; et al. Cerebrospinal fluid biomarkers measured by Elecsys assays compared to amyloid imaging. Alzheimer’s Dement. 2018, 14, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Assadi, A.; Zahabi, A.; Hart, R.A. GDF15, an update of the physiological and pathological roles it plays: A review, Pflügers. Arch. Eur. J. Physiol. 2020, 472, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Delaby, C.; Bousiges, O.; Bouvier, D.; Fillée, C.; Fourier, A.; Mondésert, E.; Nezry, N.; Omar, S.; Quadrio, I.; Rucheton, B.; et al. Neurofilaments contribution in clinic: State of the art. Front. Aging Neurosci. 2022, 14, 1034684. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, L.; Blennow, K.; Calabresi, P.; Filippo, M.D.; Parnetti, L.; Zetterberg, H. Neurofilament light chain as a biomarker in neurological disorders. J. Neurol. Neurosurg. Psychiatry 2019, 90, 870. [Google Scholar] [CrossRef]
- Agrawal, N.; Farhat, N.Y.; Sinaii, N.; Do, A.D.; Xiao, C.; Berry-Kravis, E.; Bianconi, S.; Masvekar, R.; Bielekova, B.; Solomon, B.; et al. Neurofilament light chain in cerebrospinal fluid as a novel biomarker in evaluating both clinical severity and therapeutic response in Niemann-Pick disease type C1. Genet. Med. Off. J. Am. Coll. Méd. Genet. 2022, 25, 100349. [Google Scholar] [CrossRef]
- Arslan, B.; Zetterberg, H. Neurofilament light chain as neuronal injury marker—What is needed to facilitate implementation in clinical laboratory practice? Clin. Chem. Lab. Med. (CCLM) 2023, 61, 1140–1149. [Google Scholar] [CrossRef]
- Rezabakhsh, A.; Rahbarghazi, R.; Fathi, F. Surface plasmon resonance biosensors for detection of Alzheimer’s biomarkers; an effective step in early and accurate diagnosis. Biosens. Bioelectron. 2020, 167, 112511. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Liu, L.; Harrington, M.G.; Wang, J.; Zhou, F. Regenerable and Simultaneous Surface Plasmon Resonance Detection of Aβ(1−40) and Aβ(1−42) Peptides in Cerebrospinal Fluids with Signal Amplification by Streptavidin Conjugated to an N-Terminus-Specific Antibody. Anal. Chem. 2010, 82, 10151–10157. [Google Scholar] [CrossRef] [PubMed]
- Lisi, S.; Scarano, S.; Fedeli, S.; Pascale, E.; Cicchi, S.; Ravelet, C.; Peyrin, E.; Minunni, M. Toward sensitive immuno-based detection of tau protein by surface plasmon resonance coupled to carbon nanostructures as signal amplifiers. Biosens. Bioelectron. 2017, 93, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Špringer, T.; Hemmerová, E.; Finocchiaro, G.; Krištofiková, Z.; Vyhnálek, M.; Homola, J. Surface plasmon resonance biosensor for the detection of tau-amyloid β complex. Sens. Actuators B Chem. 2020, 316, 128146. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.J. Direct Detection of α-1 Antitrypsin in Serum Samples using Surface Plasmon Resonance with a New Aptamer–Antibody Sandwich Assay. Anal. Chem. 2015, 87, 7235–7240. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Wark, A.W.; Lee, H.J. Femtomolar Detection of Tau Proteins in Undiluted Plasma Using Surface Plasmon Resonance. Anal. Chem. 2016, 88, 7793–7799. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, H.J. Gold Nanostar Enhanced Surface Plasmon Resonance Detection of an Antibiotic at Attomolar Concentrations via an Aptamer-Antibody Sandwich Assay. Anal. Chem. 2017, 89, 6624–6630. [Google Scholar] [CrossRef]
- Karlsson, R.; Katsamba, P.S.; Nordin, H.; Pol, E.; Myszka, D.G. Analyzing a kinetic titration series using affinity biosensors. Anal. Biochem. 2006, 349, 136–147. [Google Scholar] [CrossRef]
- Gaetani, L.; Höglund, K.; Parnetti, L.; Pujol-Calderon, F.; Becker, B.; Eusebi, P.; Sarchielli, P.; Calabresi, P.; Filippo, M.D.; Zetterberg, H.; et al. A new enzyme-linked immunosorbent assay for neurofilament light in cerebrospinal fluid: Analytical validation and clinical evaluation. Alzheimer’s Res. Ther. 2018, 10, 8. [Google Scholar] [CrossRef]
- Karlsson, R.; Fridh, V.; Frostell, Å. Surrogate potency assays: Comparison of binding profiles complements dose response curves for unambiguous assessment of relative potencies. J. Pharm. Anal. 2018, 8, 138–146. [Google Scholar] [CrossRef]
- Paiva, A.C.F.; Lemos, A.R.; Busse, P.; Martins, M.T.; Silva, D.O.; Freitas, M.C.; Santos, S.P.; Freire, F.; Barrey, E.J.; Manival, X.; et al. Extract2Chip—Bypassing Protein Purification in Drug Discovery Using Surface Plasmon Resonance. Biosensors 2023, 13, 913. [Google Scholar] [CrossRef]
- Schräml, M.; Biehl, M. Kinetic screening in the antibody development process. Methods Mol. Biol. 2012, 901, 171–181. [Google Scholar] [CrossRef]
- Löfås, S.; Lagerström, K. Solid Phase Binding. Assay. Patent US 5.716.854, 1998. [Google Scholar]


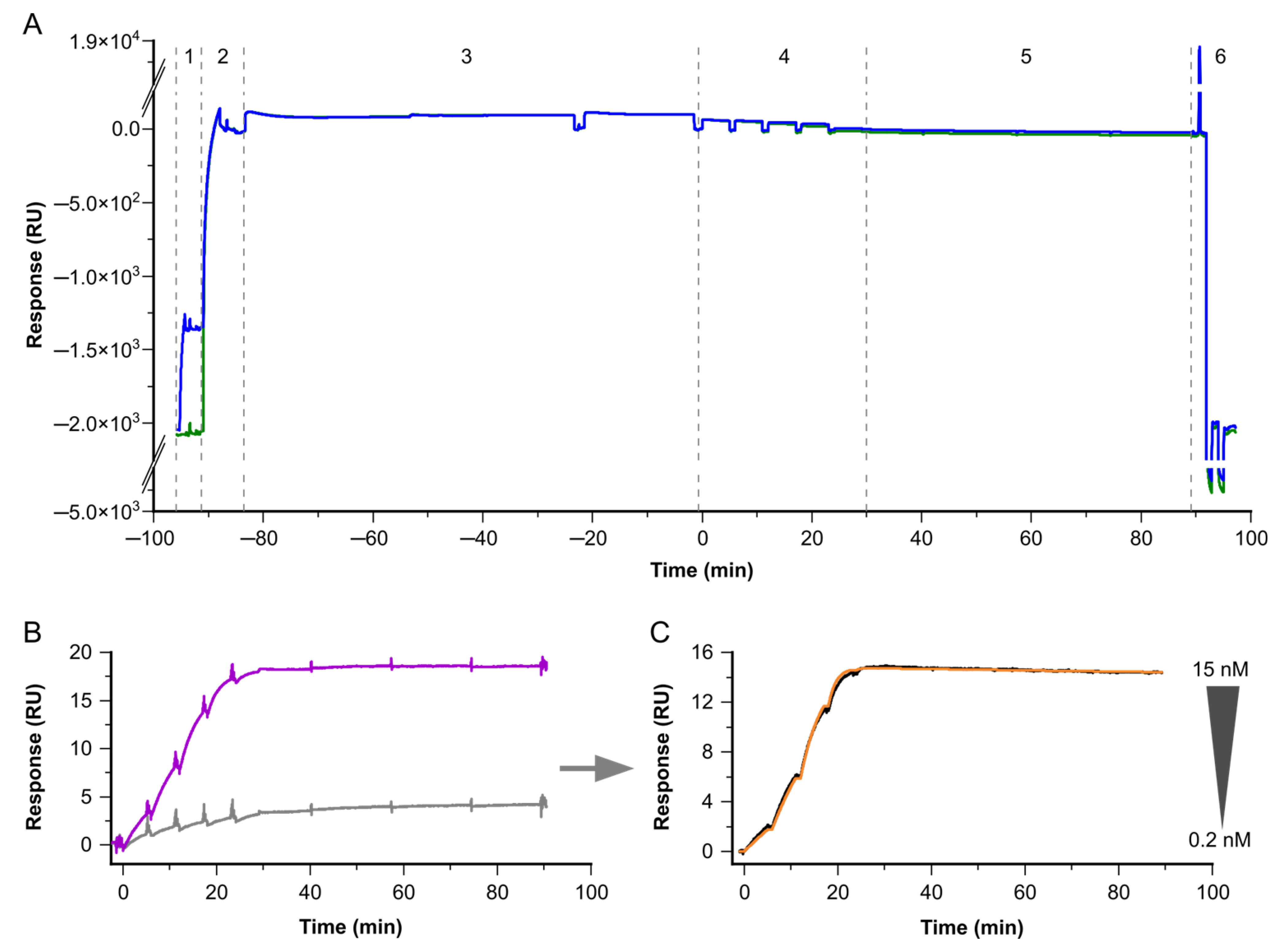


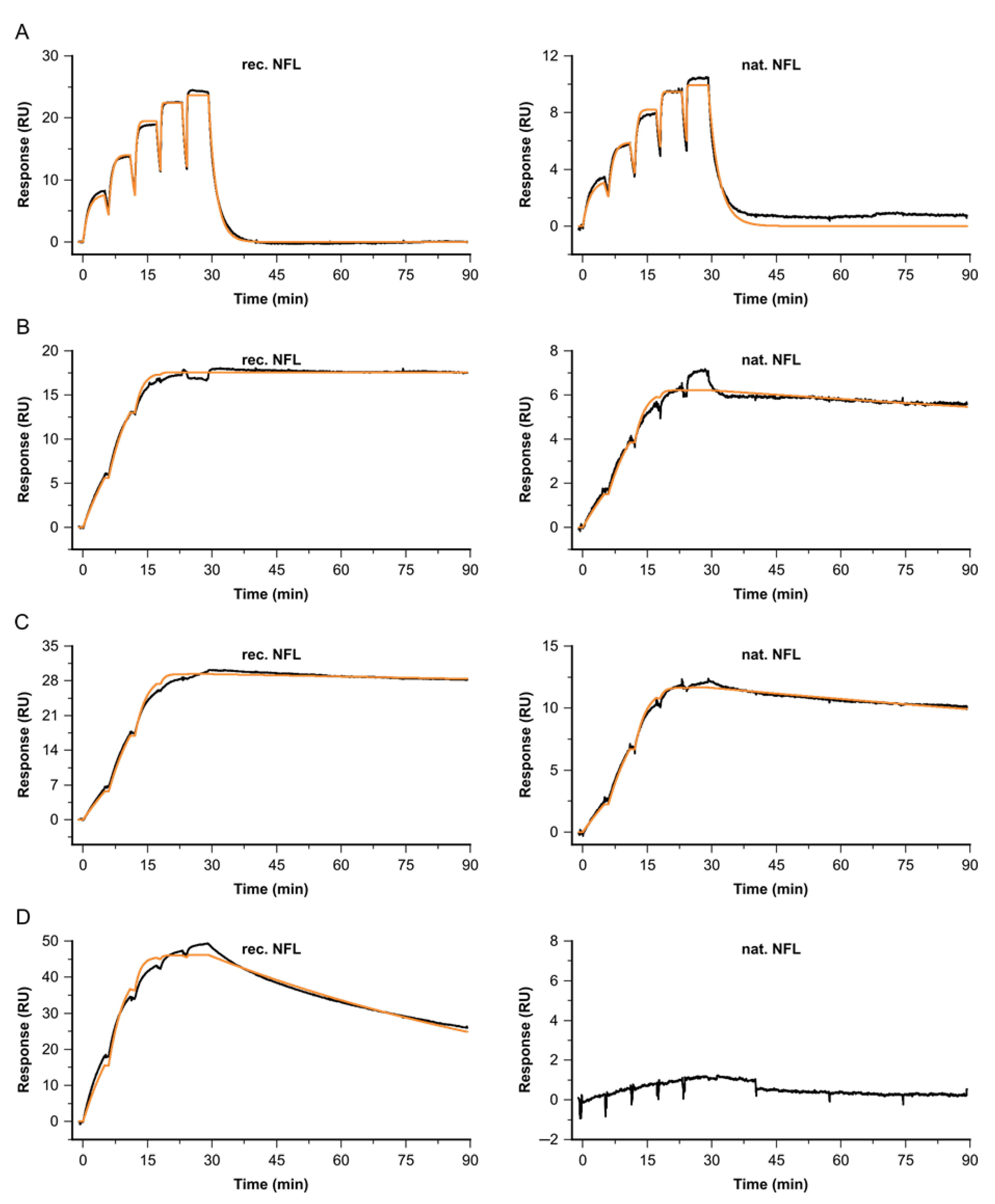
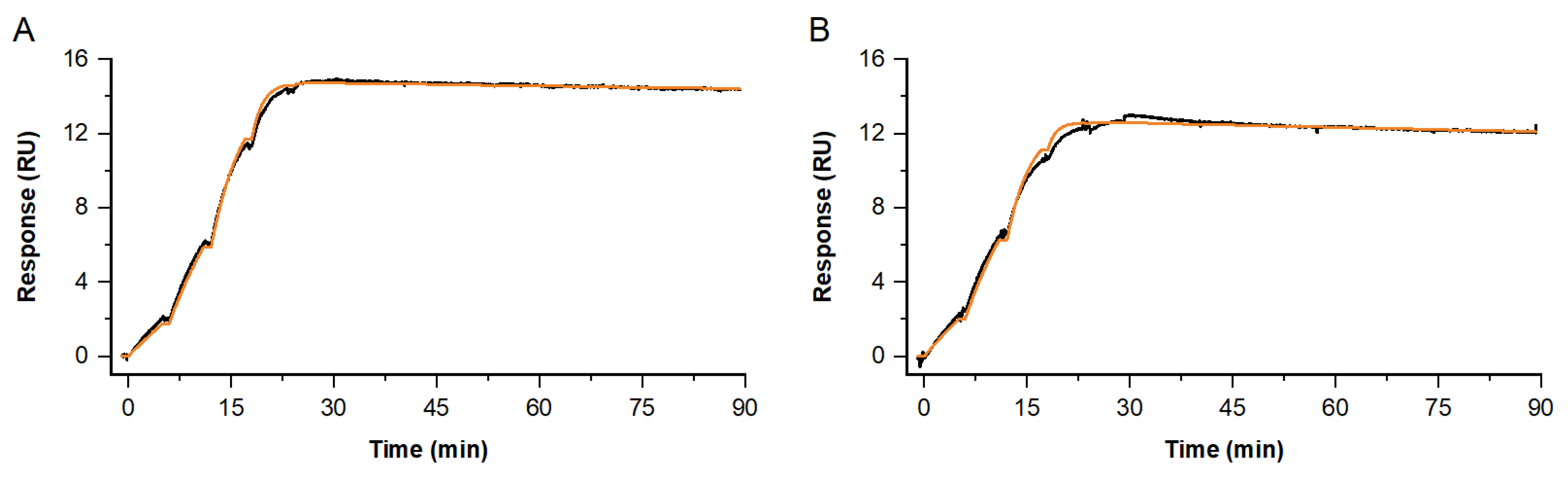

| GDF15 Origin | ka [M−1 s−1] | SE (ka) [%] | kd [s−1] | SE (kd) [%] | t/2diss [min] | KD [nM] | Rmax [RU] | U-Value |
|---|---|---|---|---|---|---|---|---|
| recombinant | 3.3 × 105 | 0.8 | 3.4 × 10−2 | 0.6 | <1 | 103 | 37.4 | 4 |
| recombinant | 3.2 × 105 | 0.9 | 3.3 × 10−2 | 0.7 | <1 | 104 | 39.7 | 4 |
| native, healthy | 3.3 × 105 | 2.3 | 2.8 × 10−2 | 1.9 | <1 | 83 | 7.6 | 12 |
| native, healthy | 2.6 × 105 | 3.2 | 2.4 × 10−2 | 2.5 | <1 | 94 | 7.9 | 15 |
| native, oncological | 4.4 × 105 | 2.0 | 4.3 × 10−2 | 1.6 | <1 | 98 | 8.8 | 7 |
| native, oncological | 2.8 × 105 | 1.3 | 3.1 × 10−2 | 1.1 | <1 | 111 | 8.6 | 5 |
| native, cardiovascular | 4.8 × 105 | 0.7 | 3.7 × 10−2 | 0.6 | <1 | 78 | 28.4 | 3 |
| native, cardiovascular | 3.7 × 105 | 1.3 | 3.5 × 10−2 | 1.2 | <1 | 96 | 33.9 | 4 |
| native, pregnant | 4.8 × 105 | 0.7 | 3.7 × 10−2 | 0.6 | <1 | 78 | 28.4 | 3 |
| native, pregnant | 3.7 × 105 | 1.3 | 3.5 × 10−2 | 1.2 | <1 | 96 | 33.9 | 4 |
| 2nd Fab | 1st Ab | Biomarker | ka [M−1 s−1] | ±SE (ka) [M−1 s−1] | kd [s−1] | ±SE (kd) [s−1] | t/2diss [min] | KD [M] | Rmax [RU] | U-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | recombinant NFL | 1.2 × 106 | 3.9 × 103 | 9.2 × 10−3 | 2.5 × 10−5 | 1 | 8.0 × 10−9 | 24 | 2 |
| A | B | native NFL from CSF | 8.4 × 105 | 1.0 × 104 | 6.8 × 10−3 | 6.5 × 10−5 | 2 | 8.0 × 10−9 | 10 | 7 |
| B | J | recombinant NFL | 3.2 × 106 | 6.9 × 103 | 1.0 × 10−5 | 2.8 × 10−8 | 1155 | 3.2 × 10−12 | 18 | 95 |
| B | J | native NFL from CSF | 2.3 × 106 | 1.2 × 104 | 3.6 × 10−5 | 5.3 × 10−7 | 322 | 1.5 × 10−11 | 6 | 5 |
| C | B | recombinant NFL | 3.6 × 106 | 9.7 × 103 | 1.0 × 10−5 | 2.7 × 10−7 | 1155 | 2.8 × 10−12 | 29 | 12 |
| C | B | native NFL from CSF | 2.9 × 106 | 1.1 × 104 | 1.1 × 10−5 | 2.4 × 10−7 | 1058 | 3.8 × 10−12 | 13 | 9 |
| C | A | native NFL from CSF | 2.1 × 106 | 2.9 × 103 | 1.0 × 10−5 | 1.5 × 10−7 | 1155 | 4.7 × 10−12 | 15 | 9 |
| I | B | recombinant NFL | 8.4 × 105 | 3.8 × 103 | 1.7 × 10−4 | 5.1 × 10−7 | 67 | 1.8 × 10−10 | 46 | 2 |
| I | B | native NFL from CSF | n.b. | n.b. | n.b. | n.b. | n.b. | n.b. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jucknischke, U.; Friebe, S.; Rehle, M.; Quast, L.; Schmidt, S.H. Antibody Profiling: Kinetics with Native Biomarkers for Diagnostic Assay and Drug Developments. Biosensors 2023, 13, 1030. https://doi.org/10.3390/bios13121030
Jucknischke U, Friebe S, Rehle M, Quast L, Schmidt SH. Antibody Profiling: Kinetics with Native Biomarkers for Diagnostic Assay and Drug Developments. Biosensors. 2023; 13(12):1030. https://doi.org/10.3390/bios13121030
Chicago/Turabian StyleJucknischke, Ute, Sebastian Friebe, Markus Rehle, Laura Quast, and Sven H. Schmidt. 2023. "Antibody Profiling: Kinetics with Native Biomarkers for Diagnostic Assay and Drug Developments" Biosensors 13, no. 12: 1030. https://doi.org/10.3390/bios13121030




